Systematic Review of Line-Field Confocal Optical Coherence Tomography for Diagnosing Pre-Malignant and Malignant Keratinocytic Lesions: Optimising the Workflow
Abstract
1. Introduction
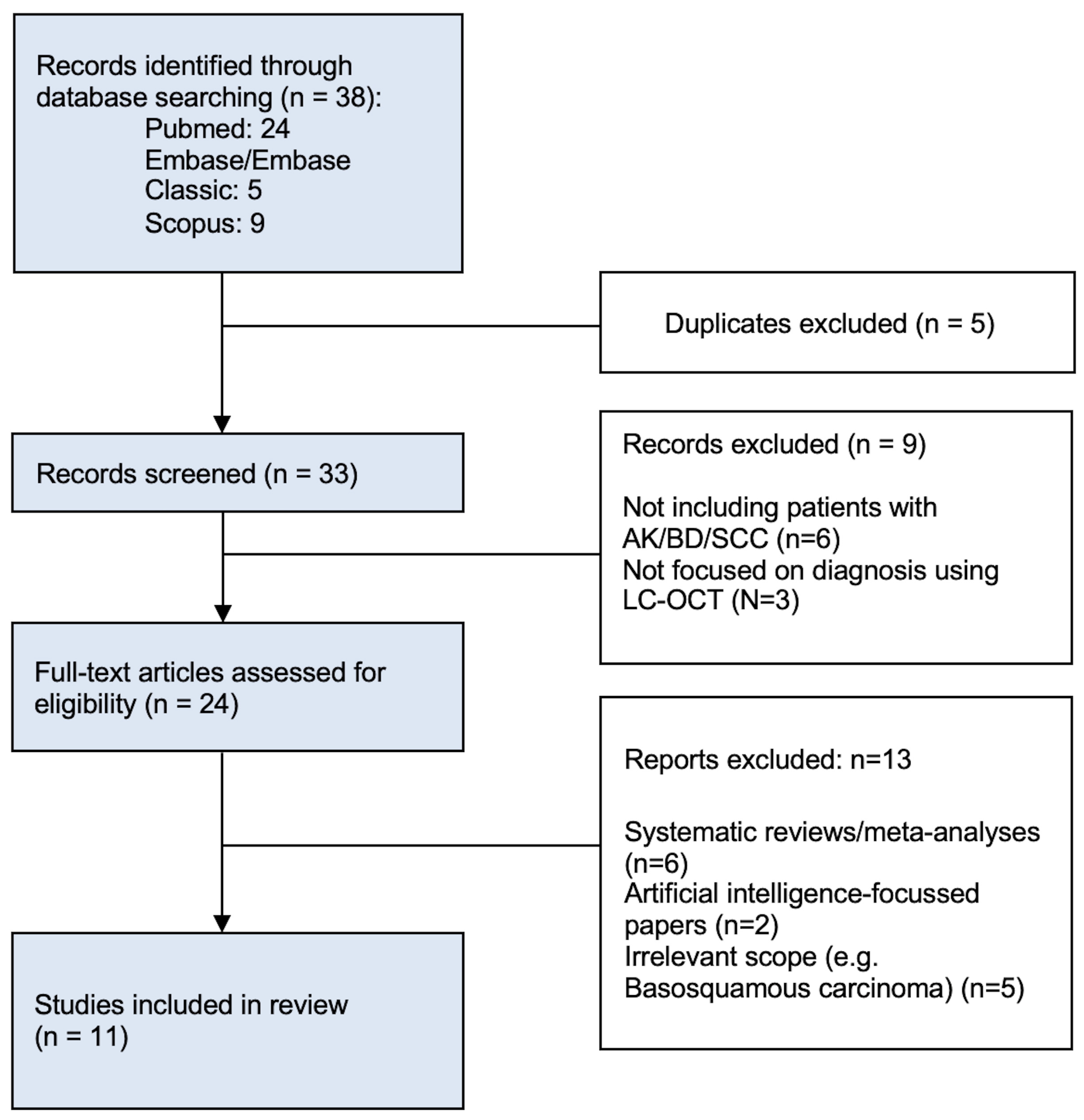
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Selection and Extraction
2.4. Quality Assessment
3. Results
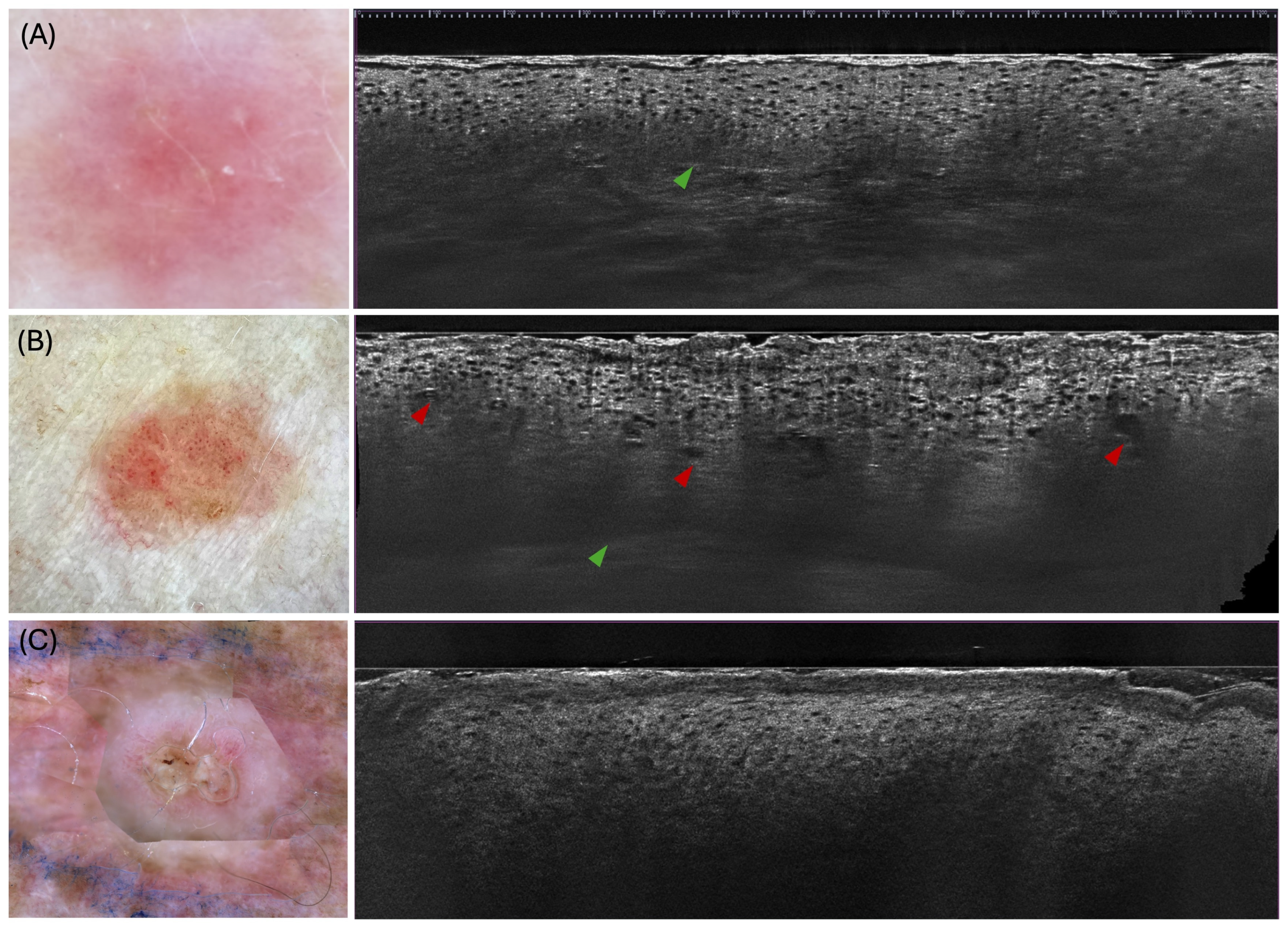
3.1. Key Criteria for Diagnosis
3.1.1. Actinic Keratosis
3.1.2. Bowen’s Disease
3.1.3. Invasive Squamous Cell Carcinoma
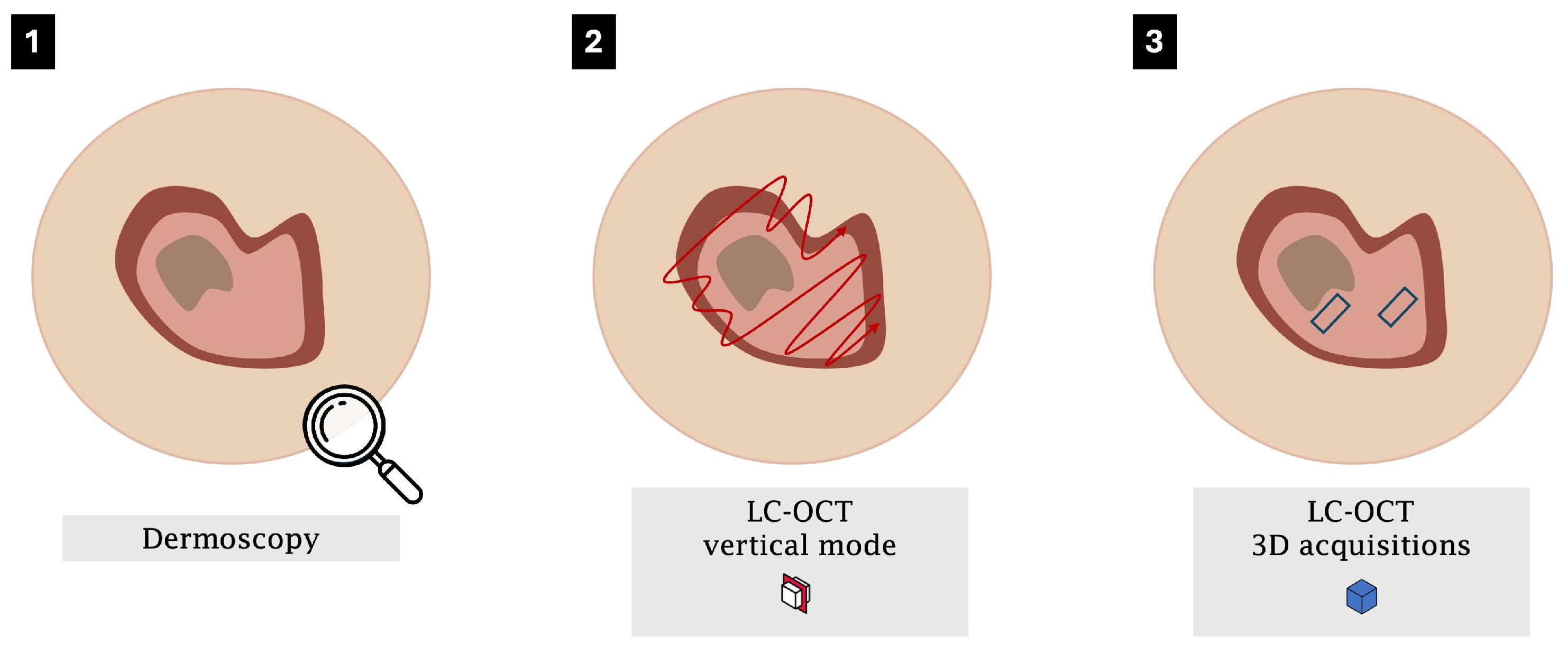
3.2. Workflow Optimisation for Keratinocytic Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cinotti, E.; Brunetti, T.; Cartocci, A.; Tognetti, L.; Suppa, M.; Malvehy, J.; Perez-Anker, J.; Puig, S.; Perrot, J.L.; Rubegni, P. Diagnostic Accuracy of Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas. Diagnostics 2023, 13, 361. [Google Scholar] [CrossRef]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-Field Confocal Optical Coherence Tomography for High-Resolution Noninvasive Imaging of Skin Tumors. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef]
- Latriglia, F.; Ogien, J.; Tavernier, C.; Fischman, S.; Suppa, M.; Perrot, J.-L.; Dubois, A. Line-Field Confocal Optical Coherence Tomography (LC-OCT) for Skin Imaging in Dermatology. Life 2023, 13, 2268. [Google Scholar] [CrossRef]
- Daxenberger, F.; Deußing, M.; Eijkenboom, Q.; Gust, C.; Thamm, J.; Hartmann, D.; French, L.E.; Welzel, J.; Schuh, S.; Sattler, E.C. Innovation in Actinic Keratosis Assessment: Artificial Intelligence-Based Approach to LC-OCT PRO Score Evaluation. Cancers 2023, 15, 4457. [Google Scholar] [CrossRef]
- Thamm, J.R.; Daxenberger, F.; Viel, T.; Gust, C.; Eijkenboom, Q.; French, L.E.; Welzel, J.; Sattler, E.C.; Schuh, S. Artificial Intelligence-Based PRO Score Assessment in Actinic Keratoses from LC-OCT Imaging Using Convolutional Neural Networks. J. Der Dtsch. Dermatol. Ges. 2023, 21, 1359–1366. [Google Scholar] [CrossRef]
- Malvehy, J.; Stratigos, A.J.; Bagot, M.; Stockfleth, E.; Ezzedine, K.; Delarue, A. Actinic Keratosis: Current Challenges and Unanswered Questions. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Marks, R. Epidemiology Non-Melanoma Skin Cancer and Solar Keratoses in Australia: A Tale of Self Immolation in Elysian Fields. Australas. J. Dermatol. 1997, 38, S26–S29. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.; Williams, G.; Green, A. High Incidence and Regression Rates of Solar Keratoses in a Queensland Community. J. Investig. Dermatol. 2000, 115, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Lim, H.W.; Margolis, D.; Weinstock, M.A.; Goodman, C.; Faulkner, E.; Gould, C.; Gemmen, E.; Dall, T. The Burden of Skin Diseases: 2004. J. Am. Acad. Dermatol. 2006, 55, 490–500. [Google Scholar] [CrossRef]
- Schaefer, I.; Augustin, M.; Spehr, C.; Reusch, M.; Kornek, T. Prevalence and Risk Factors of Actinic Keratoses in Germany—Analysis of Multisource Data. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 309–313. [Google Scholar] [CrossRef]
- Harvey, I.; Frankel, S.; Marks, R.; Shalom, D.; Nolan-Farrell, M. Non-Melanoma Skin Cancer and Solar Keratoses. I. Methods and Descriptive Results of the South Wales Skin Cancer Study. Br. J. Cancer 1996, 74, 1302–1307. [Google Scholar] [CrossRef]
- Criscione, V.D.; Weinstock, M.A.; Naylor, M.F.; Luque, C.; Eide, M.J.; Bingham, S.F.; The Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group. Actinic Keratoses: Natural History and Risk of Malignant Transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009, 115, 2523–2530. [Google Scholar] [CrossRef]
- Guorgis, G.; Anderson, C.; Lyth, J.; Falk, M. Actinic Keratosis Diagnosis and Increased Risk of Developing Skin Cancer: A 10-Year Cohort Study of 17,651 Patients in Sweden. Acta Derm.-Venereol. 2020, 100, adv00128. [Google Scholar] [CrossRef] [PubMed]
- Euvrard, S.; Ulrich, C.; Lefrancois, N. Immunosuppressants and Skin Cancer in Transplant Patients: Focus on Rapamycin. Dermatol. Surg. 2004, 30, 628–633. [Google Scholar] [CrossRef]
- Rosen, T.; Lebwohl, M.G. Prevalence and Awareness of Actinic Keratosis: Barriers and Opportunities. J. Am. Acad. Dermatol. 2013, 68, S2–S9. [Google Scholar] [CrossRef]
- Mohandas, P.; Lowden, M.; Varma, S. Bowen’s Disease. BMJ 2020, 368, m813. [Google Scholar] [CrossRef]
- Ding, S.; Hu, L.; Rao, Y.; Ren, R.; Tong, X.; Guo, A.; Huang, J.; Tang, Z. Application of in Vivo Reflectance Confocal Microscopy in the Diagnosis of Bowen’s Disease. Microsc. Res. Tech. 2024, 87, 2842–2849. [Google Scholar] [CrossRef] [PubMed]
- Mazzilli, S.; Gamo-Villegas, R.; Pampin-Franco, A.; Estebaran, J.L.L.; Pinedo, F.; Vollono, L.; Di Prete, M.; Campione, E.; Gonzalez, S. Reflectance Confocal Microscopy of Pigmented Bowen’s Disease: A Case Series of Difficult to Diagnose Lesions. Case Rep. Dermatol. 2020, 12, 98–106. [Google Scholar] [CrossRef]
- Petzold, A.; Wessely, A.; Steeb, T.; Berking, C.; Heppt, M.V. Efficacy of Interventions for Cutaneous Squamous Cell Carcinoma in Situ (Bowen’s Disease): A Systematic Review and Meta-Analysis of Proportions. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Venables, Z.; Nijsten, T.; Wong, K.; Autier, P.; Broggio, J.; Deas, A.; Harwood, C.; Hollestein, L.; Langan, S.; Morgan, E.; et al. Epidemiology of Basal and Cutaneous Squamous Cell Carcinoma in the U.K. 2013–15: A Cohort Study. Br. J. Dermatol. 2019, 181, 474–482. [Google Scholar] [CrossRef]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef]
- Kolitz, E.; Lopes, F.C.P.S.; Arffa, M.; Pineider, J.; Bogucka, R.; Adamson, A.S. UV Exposure and the Risk of Keratinocyte Carcinoma in Skin of Color: A Systematic Review. JAMA Dermatol. 2022, 158, 542. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Mahler, V. The Epidemiology of Skin Cancer. Br. J. Dermatol. 2002, 146, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Beyond the Obvious: Environmental Health Implications of Polar Polycyclic Aromatic Hydrocarbons. Environ. Int. 2019, 123, 543–557. [Google Scholar] [CrossRef]
- Álvarez-Salafranca, M.; Zaballos, P. Dermoscopy of Squamous Cell Carcinoma: From Actinic Keratosis to Invasive Forms. Actas Dermo-Sifiliográficas 2024, 115, T883–T895. [Google Scholar] [CrossRef]
- Azimi, A.; Jabbour, S.; Patrick, E.; Fernandez-Penas, P. Non-Invasive Diagnosis of Early Cutaneous Squamous Cell Carcinoma. Exp. Dermatol. 2023, 32, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Suppa, M.; Fontaine, M.; Dejonckheere, G.; Cinotti, E.; Yélamos, O.; Diet, G.; Tognetti, L.; Miyamoto, M.; Cano, C.O.; Perez-Anker, J.; et al. Line-Field Confocal Optical Coherence Tomography of Basal Cell Carcinoma: A Descriptive Study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Lacarrubba, F.; Verzì, A.E.; Polita, M.; Aleo, A.; Micali, G. Line-Field Confocal Optical Coherence Tomography in the Treatment Monitoring of Actinic Keratosis with Tirbanibulin: A Pilot Study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1131–e1133. [Google Scholar] [CrossRef]
- Orte Cano, C.; Suppa, M.; Del Marmol, V. Where Artificial Intelligence Can Take Us in the Management and Understanding of Cancerization Fields. Cancers 2023, 15, 5264. [Google Scholar] [CrossRef]
- Cinotti, E.; Bertello, M.; Cartocci, A.; Fiorani, D.; Tognetti, L.; Solmi, V.; Cappilli, S.; Peris, K.; Perrot, J.L.; Suppa, M.; et al. Comparison of Reflectance Confocal Microscopy and Line-Field Optical Coherence Tomography for the Identification of Keratinocyte Skin Tumours. Ski. Res. Technol. 2023, 29, e13215. [Google Scholar] [CrossRef]
- Pathak, G.N.; Sanabria, B.; Caetano, V.D.; Rafiq, B.; Rao, B.K. Evaluation and Treatment Monitoring of Actinic Keratosis Using Line-Field Confocal Optical Coherence Tomography. Ski. Res. Technol. 2024, 30, e13526. [Google Scholar] [CrossRef]
- Jacobsen, K.; Ortner, V.K.; Wenande, E.; Fredman, G.; Untracht, G.R.; Wolswijk, T.; Cruts, E.; Mosterd, K.; Nielsen, K.; Philipsen, P.A.; et al. Interobserver Agreement on Line-Field Confocal Optical Coherence Tomography Image Markers in Keratinocyte Carcinomas and Precursor Lesions. Arch. Dermatol. Res. 2024, 316, 608. [Google Scholar] [CrossRef]
- Donelli, C.; Suppa, M.; Tognetti, L.; Perrot, J.L.; Calabrese, L.; Pérez-Anker, J.; Malvehy, J.; Rubegni, P.; Cinotti, E. Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas: Real-Life Data over Three Years. Curr. Oncol. 2023, 30, 8853–8864. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Hartmann, D.; French, L.E.; Sattler, E.C.; Welzel, J. In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers 2021, 13, 2856. [Google Scholar] [CrossRef]
- Cinotti, E.; Tognetti, L.; Cartocci, A.; Lamberti, A.; Gherbassi, S.; Cano, C.O.; Lenoir, C.; Dejonckheere, G.; Diet, G.; Fontaine, M.; et al. Line-Field Confocal Optical Coherence Tomography for Actinic Keratosis and Squamous Cell Carcinoma: A Descriptive Study. Clin. Exp. Dermatol. 2021, 46, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E.C. Line-Field Confocal Optical Coherence Tomography for the in Vivo Real-Time Diagnosis of Different Stages of Keratinocyte Skin Cancer: A Preliminary Study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, C.; Cinotti, E.; Tognetti, L.; Cano, C.O.; Diet, G.; Miyamoto, M.; Rocq, L.; Trépant, A.; Perez-Anker, J.; Puig, S.; et al. Line-Field Confocal Optical Coherence Tomography of Actinic Keratosis: A Case Series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, E900–E902. [Google Scholar] [CrossRef]
- Di Stefani, A.; Cappilli, S.; Cuffaro, G.; Fionda, B.; Pagliara, M.M.; Paradisi, A.; Ricci, C.; Rossi, E.; Sammarco, M.G.; Schinzari, G.; et al. Line-Field Confocal Optical Coherence Tomography Evaluation of Eyelid Skin Lesions. Diagnostics 2023, 13, 3590. [Google Scholar] [CrossRef] [PubMed]
- Verzì, A.E.; Russo, A.; Castellino, N.; Caltabiano, R.; Fallico, M.; Cappellani, F.; Micali, G.; Lacarrubba, F. Line-Field Confocal Optical Coherence Tomography of Eyelid Margin Growths: A Case Series. Ski. Res. Technol. 2024, 30, e13559. [Google Scholar] [CrossRef]
| Study | Study Design | Country | Methodology | Item Generation Method (e.g., Instrument Used to Measure Items) | Number of Patients and Lesions | Total Group Characteristics (Age, Gender, Type/Location) |
|---|---|---|---|---|---|---|
| Lacarrubba et al., 2023 [28] | Prospective pilot study | ITA | Ten immunocompetent patients with Olsen I actinic keratosis were enrolled; tirbanibulin 1% ointment was applied once daily for 5 consecutive days to a 25-cm2 area containing 4–8 AKs; clinical and LC-OCT evaluations were performed at baseline, Day 8, and Day 57 to monitor treatment response; histopathology confirmed diagnosis. | LC-OCT (DeepLive™, DAMAE Medical, Paris, France) in vertical and horizontal modes | Patients (n = 10) AK lesions (n = 55) | 2 females (20%) and 8 males (80%); Mean age: 74 years (range: 62–85); Lesion locations: Scalp (80%) and face (20%). |
| Cianotti et al., 2023 [30] | Retrospective multicenter study | BEL and ITA | Retrospective collection of SCC and AK lesions that were imaged with both RCM and LC-OCT before surgery; three blinded observers evaluated predefined LC-OCT criteria; histopathology served as the gold standard; statistical analyses included descriptive analysis, proportion tests, and Gwet’s AC1 for agreement. | LC-OCT (CE-marked prototype, DAMAE Medical, Paris, France); ≥4 images and 1 video per lesion. RCM: VivaScope 3000 (MAVIG GmbH, Munich, Germany) ≥1 image of different depths and 1 stack per lesion | Patients (n = 52) Histologically proven tumors (n = 52): −33 SCCs (23 in situ and 10 invasive) −19 AKs | 26 females (50%) and 26 males (50%); Mean age: 66.6 ± 13.7 years; SCC locations: Head/neck (54.5%), upper extremities (24.2%), trunk (12.1%), lower extremities (9.1%); AK locations: Head/neck (57.9%), upper extremities (26.3%), trunk (10.5%), lower extremities (5.3%). |
| Pathak et al., 2024 [31] | Descriptive observational study | USA | LC-OCT imaging of AK lesions before cryotherapy with re-evaluation at 4-week follow-up; an intention-to-treat analysis was conducted. | LC-OCT (DeepLive™, DAMAE Medical, Paris, France) | Patients (n = 6) Total lesions (n = 8) | 4 females and 2 males; Mean age: 69.5 years; Lesion locations: upper arm, chest, cheek, hand, forehead, and scalp. |
| Jacobsen et al., 2024 [32] | Interobserver agreement study | DNK; NLD; SWE | Six evaluators blinded to histopathology independently assessed 75 LC-OCT images for the presence/absence of 10 predefined markers, also reporting confidence and artifact impact; interobserver agreement was determined using Conger’s kappa coefficient. | LC-OCT (DeepLive™, DAMAE Medical, Paris, France); high-resolution video acquisitions (cross-section and en-face views) | Total lesions (n = 75) 21 SCC 21 BCC (including 6 superficial and 15 nodular) 12 BD 21 AK (including 6 grade I, 9 grade II, and 6 grade III) | NR |
| Donelli et al., 2023 [33] | Prospective observational monocentric study | BEL; ESP; FRA; ITA | Included all cutaneous lesions with uncertain clinical/dermoscopic diagnoses of possible malignant skin tumors (excluding lesions on the eyelid margin, internal cantus, and upper eyelid); dermoscopic and LC-OCT diagnoses were entered prospectively into software; histopathology was used as the gold standard. | LC-OCT (DeepLive™, DAMAE Medical, Paris, France) providing vertical, horizontal, and 3D images. Dermoscopy using VivaCam D200 and Dermlite DL200 Hybrid handheld | Lesions analyzed: 1481 total; final analysis included 466 lesions (312 excised, 149 biopsied, 5 followed up). BCC (n = 152), AK (n = 20), Bowen’s disease (n = 14), SCC (n = 22), intradermal nevi (n = 4), seborrheic keratosis (n = 14), sebaceous hyperplasia (n = 1), inflammatory lesions (n = 15), other lesions (n = 70) | NR |
| Ruini et al., 2021 (second) [34] | Pilot observational study | DEU | Fifty facial AKs clinically suspected were imaged with LC-OCT (vertical mode) before biopsy (within 0–7 days); observers classified the basal proliferation pattern (PRO I, II, III) using LC-OCT vertical images; histopathology served as the gold standard. | LC-OCT (CE-marked prototype, DAMAE Medical, Paris, France); vertical mode | Patients (n = 43) Histopathologically confirmed AK lesions (n = 50); 5 excluded due to insufficient image quality | 17 females (40%) and 26 males (60%); Mean age: 73.8 years (range: 57–86); Lesion location: face (100%). |
| Cianotti et al., 2021 [35] | Retrospective observational multicentre study | BEL and ITA | Lesions clinically suspected of AK or SCC were imaged with LC-OCT before surgical excision and then examined histopathologically; three blinded observers evaluated LC-OCT image quality and criteria; univariate and multivariate analyses were performed. | LC-OCT (CE-marked prototype, DAMAE Medical, Paris, France); ≥4 images and 2 videos per lesion | Patients (n = 158) Histologically confirmed lesions (n = 158): 108 SCC (62 in situ, 46 invasive) and 50 AK | 83 females (52.5%) and 75 males (47.5%); Mean age: 69.8 ± 12.7 years; SCC: Head/neck (54.6%), legs (17.6%), arms (14.8%), trunk (13%); AK: Head/neck (62%), legs (14%), arms (12%), trunk (12%). |
| Ruini et al., 2021 (first) [36] | Prospective observational study | DEU | Clinical, dermoscopic, and LC-OCT images were prospectively collected and analyzed for lesions suspected of keratinocyte skin cancer; histopathology was used as the gold standard; exemplary lesions were additionally investigated with conventional OCT and RCM for comparison. | LC-OCT (CE-marked prototype, DAMAE Medical, Paris, France); Vertical, horizontal, and 3D modes. Dermoscopy (FotoFinder/DermoGenius) OCT: VivoSight device (Michelson Diagnostics, Orpington, UK) RCM: VivaScope 3000 camera (MAVIG GmbH, Munich, Germany) | Histopathologically confirmed lesions (n = 73) −46 AKs (10 hypertrophic, 5 atrophic, 3 bowenoid) −11 Bowen’s disease −16 SCCs | 25 females (34%) and 48 males (66%); Mean age: 74.8 years; Lesion locations: Head/neck (52.1%), scalp (28.8%), upper limbs (9.6%), lower limbs (5.5%), trunk (2.7%), genital area (1.4%). |
| Lenoir et al., 2021 [37] | Case series | BEL; ITA; FRA; ESP | Lesions clinically and dermoscopically suggestive of actinic keratosis (AK) were included; AK subtypes (atrophic, hypertrophic, proliferative, acantholytic) were characterized based on LC-OCT findings; histopathology served as the gold standard for diagnostic confirmation. | LC-OCT (CE-marked prototype, DAMAE Medical, Paris, France); vertical, horizontal, and 3D modes | Histopathologically confirmed AK lesions (n = 16) | NR |
| Di Stefani et al., 2023 [38] | Retrospective observational study | ITA | Patients with biopsy-proven equivocal eyelid skin lesions underwent in vivo LC-OCT imaging before surgical excision; images were evaluated by two investigators (with a third for discrepancies); histopathological examination was used as the gold standard. | LC-OCT (DeepLive™, DAMAE Medical, Paris, France) in vertical and horizontal modes | Patients (n = 51) Histopathologically confirmed lesions (n = 51) | 28 females (55%) and 23 males (45%); Mean age: 66.4 years (range: 34–88); Lesion locations: Lower eyelid (39%), medial canthus (27%), upper eyelid (18%), lateral canthus (16%). |
| Verzì et al., 2024 [39] | Case series | ITA | Eyelid margin lesions with a challenging clinical appearance (onset ≤ 12 months) were imaged before surgical excision; histopathological examination was used as the gold standard. | LC-OCT (DeepLive™, DAMAE Medical, Paris, France) | Patients (n = 28) Histopathologically confirmed lesions (n = 31) | 13 females (46%) and 15 males (54%); Mean age: 64.7 years (range: 44–87); Lesion locations: Upper eyelid (13 cases) and lower eyelid (18 cases). |
| Keratinocytic Lesions | Studies That Contributed | Methodological Limitations (No/Minor/Moderate/Serious Limitations) | Coherence | Adequacy | Assessment of Confidence (High/Moderate/Low/Very Low) |
|---|---|---|---|---|---|
| Actinic keratosis | [28,30,31,32,33,34,35,36,37,38] | Minor (consistent reporting across multiple studies with histopathological validation) | High (features align strongly across studies with consistent percentages and descriptions) | Rich (detailed quantitative measurements and qualitative descriptions from multiple studies) | High |
| Bowen’s Disease | [32,35,36] | Moderate (fewer studies focusing specifically on Bowen’s) | High (features align between studies despite varying terminology) | Moderate (detailed descriptions but from limited number of studies) | Moderate |
| Invasive SCC | [30,32,35,36,37,38,39] | Minor (consistent reporting with histopathological validation across multiple studies) | High (features align strongly across studies with complementary descriptions) | Rich (detailed quantitative measurements with multiple supporting studies) | High |
| Keratinocytic Lesions | Studies That Contributed | Epidermal | Structural | Vascular/Dermal |
|---|---|---|---|---|
| Actinic keratosis | [28,30,31,32,33,34,35,36,37,38] | Hyperkeratosis (42–100%) Parakeratosis (64–83%) Acanthosis (71–76%) Keratinocytic atypia (82–100%) | Mean epidermal thickness 105–126.2 μm Well-preserved/visible DEJ (48–70%) Architectural disorganisation (77.8–97%) Tumour budding (45–58%) | Dilated vessels (57.1–89.5%) Elastosis/collagen alterations (26.2–61%) |
| Bowen’s Disease | [32,35,36] | Hyperkeratosis (69.4–100%) Parakeratosis (63–100%) Keratinocytic atypia (73.3–100%) | Mean epidermal thickness 141–168.5 μm DEJ well-defined (24–80%), Bowenoid pattern (90%) Architectural disorganisation (>90%) Tumour budding (33.3–49%) Broad strands (9.7%) | Dilated vessels (44–70%) Glomerular vessels (20–61.9%) Elastosis/collagen alterations (10–70%) |
| Invasive SCC | [30,32,35,36,37,38,39] | Hyperkeratosis (72.7–100%) Parakeratosis (62–93.8%) Acanthosis (75–93.8%) Marked keratinocytic atypia (70–84%) Atypical nuclei (95%) Erosion/ulceration (63–68%) | Mean epidermal thickness 154–232 μm DEJ well-defined (18.8–57%) Architectural disorganisation (93–100%) Tumour budding (44–45%) Broad strands (29–63%) | Dilated vessels (55–78.6%) Glomerular vessels (21.4–80%) Elastosis/collagen alterations (7.4–75%) Keratin pearls (0–81%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos e Silva Caldeira Marques, M.L.; Hero, J.; el-Sharouni, M.-A.; García Bustínduy, M.; Guitera, P. Systematic Review of Line-Field Confocal Optical Coherence Tomography for Diagnosing Pre-Malignant and Malignant Keratinocytic Lesions: Optimising the Workflow. Diagnostics 2025, 15, 2746. https://doi.org/10.3390/diagnostics15212746
Santos e Silva Caldeira Marques ML, Hero J, el-Sharouni M-A, García Bustínduy M, Guitera P. Systematic Review of Line-Field Confocal Optical Coherence Tomography for Diagnosing Pre-Malignant and Malignant Keratinocytic Lesions: Optimising the Workflow. Diagnostics. 2025; 15(21):2746. https://doi.org/10.3390/diagnostics15212746
Chicago/Turabian StyleSantos e Silva Caldeira Marques, Maria Luísa, Justin Hero, Mary-Ann el-Sharouni, Marta García Bustínduy, and Pascale Guitera. 2025. "Systematic Review of Line-Field Confocal Optical Coherence Tomography for Diagnosing Pre-Malignant and Malignant Keratinocytic Lesions: Optimising the Workflow" Diagnostics 15, no. 21: 2746. https://doi.org/10.3390/diagnostics15212746
APA StyleSantos e Silva Caldeira Marques, M. L., Hero, J., el-Sharouni, M.-A., García Bustínduy, M., & Guitera, P. (2025). Systematic Review of Line-Field Confocal Optical Coherence Tomography for Diagnosing Pre-Malignant and Malignant Keratinocytic Lesions: Optimising the Workflow. Diagnostics, 15(21), 2746. https://doi.org/10.3390/diagnostics15212746







