Comparison of Long Non-Coding RNA Expressions in Endometrial Polyp and Endometrial Cancer Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Selection
2.2. RNA Isolation
2.3. cDNA Synthesis
2.4. Quantitative Real-Time PCR (qRT-PCR) for Expression Analysis
2.5. Statistical Analysis
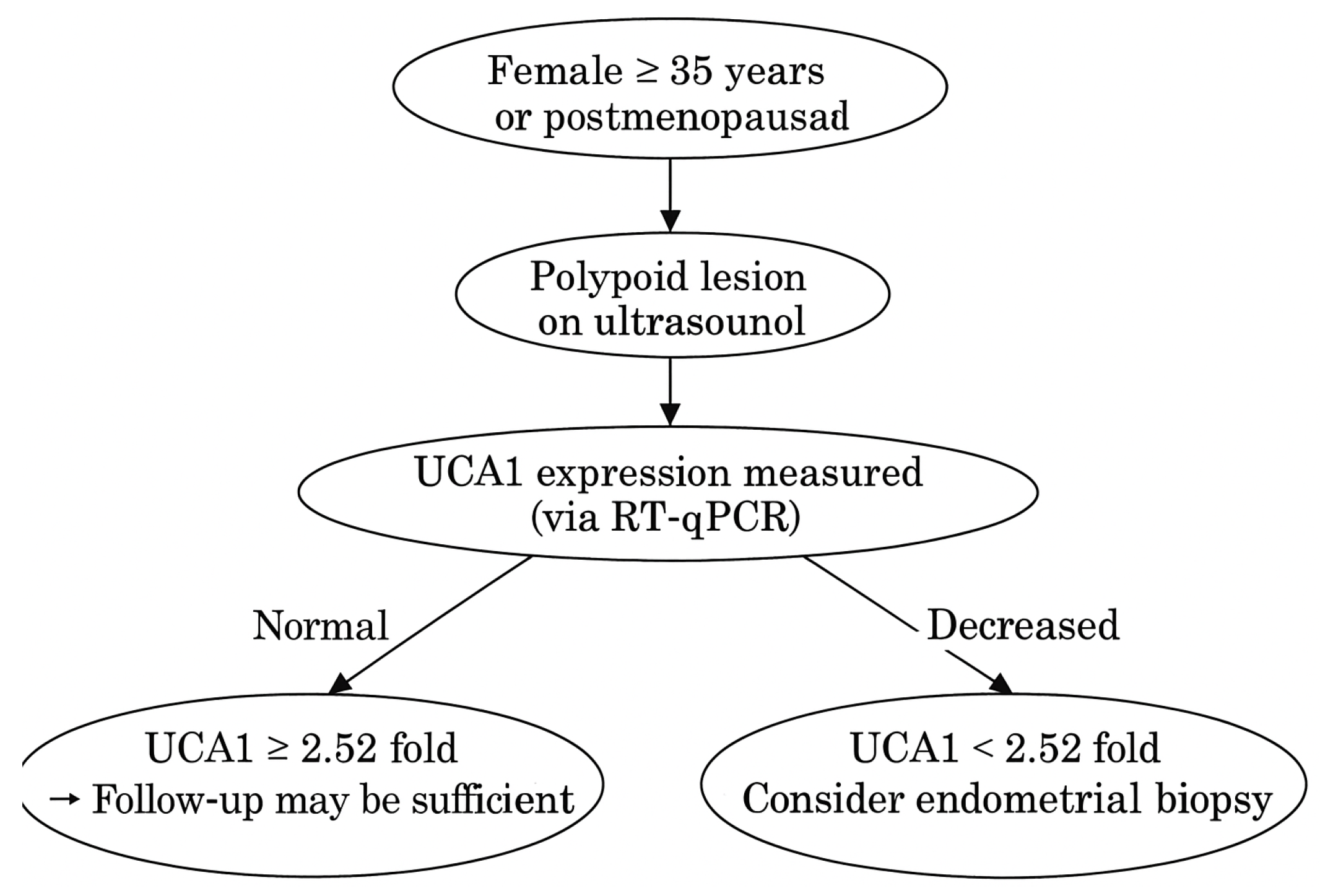
3. Results
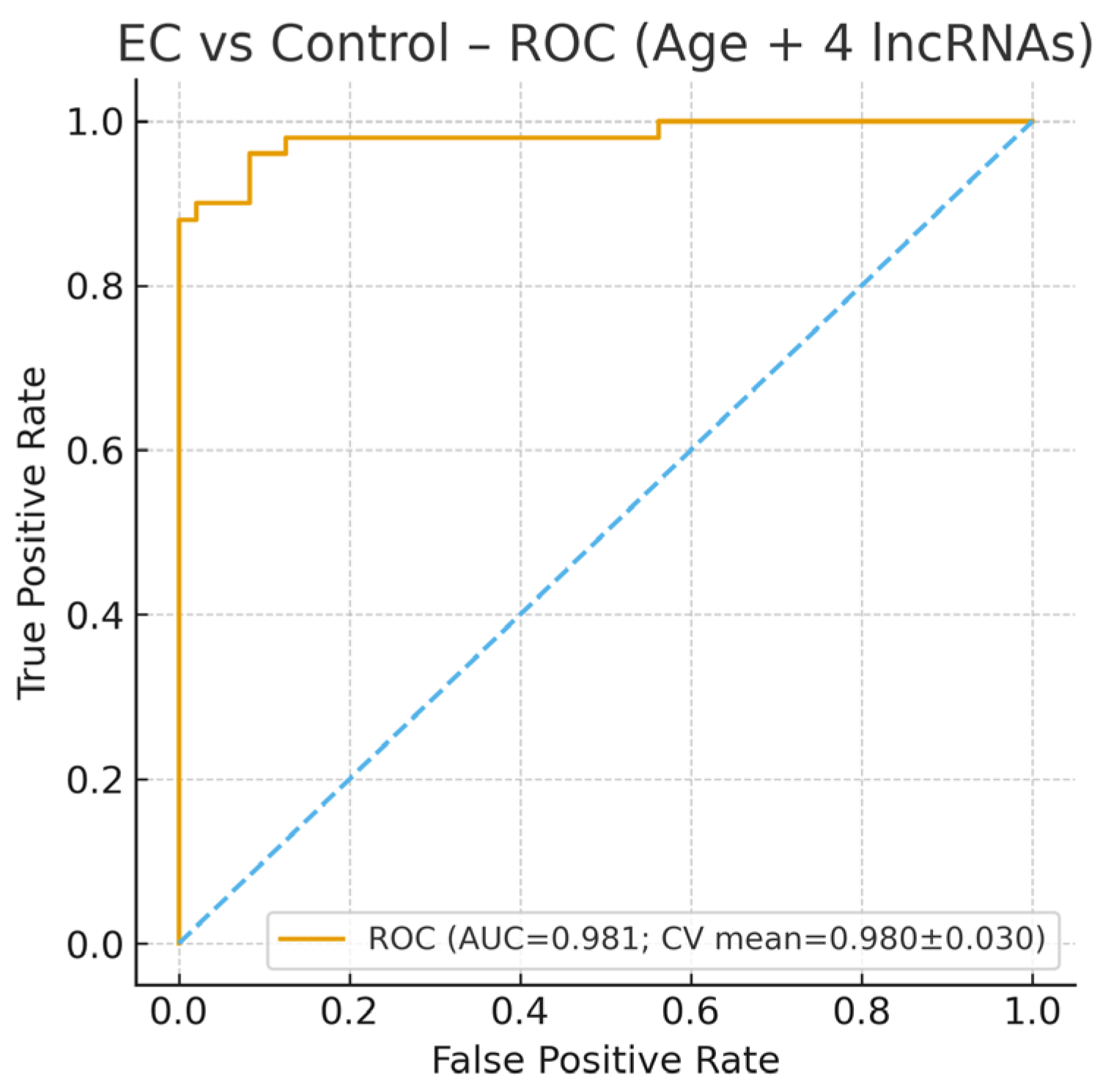
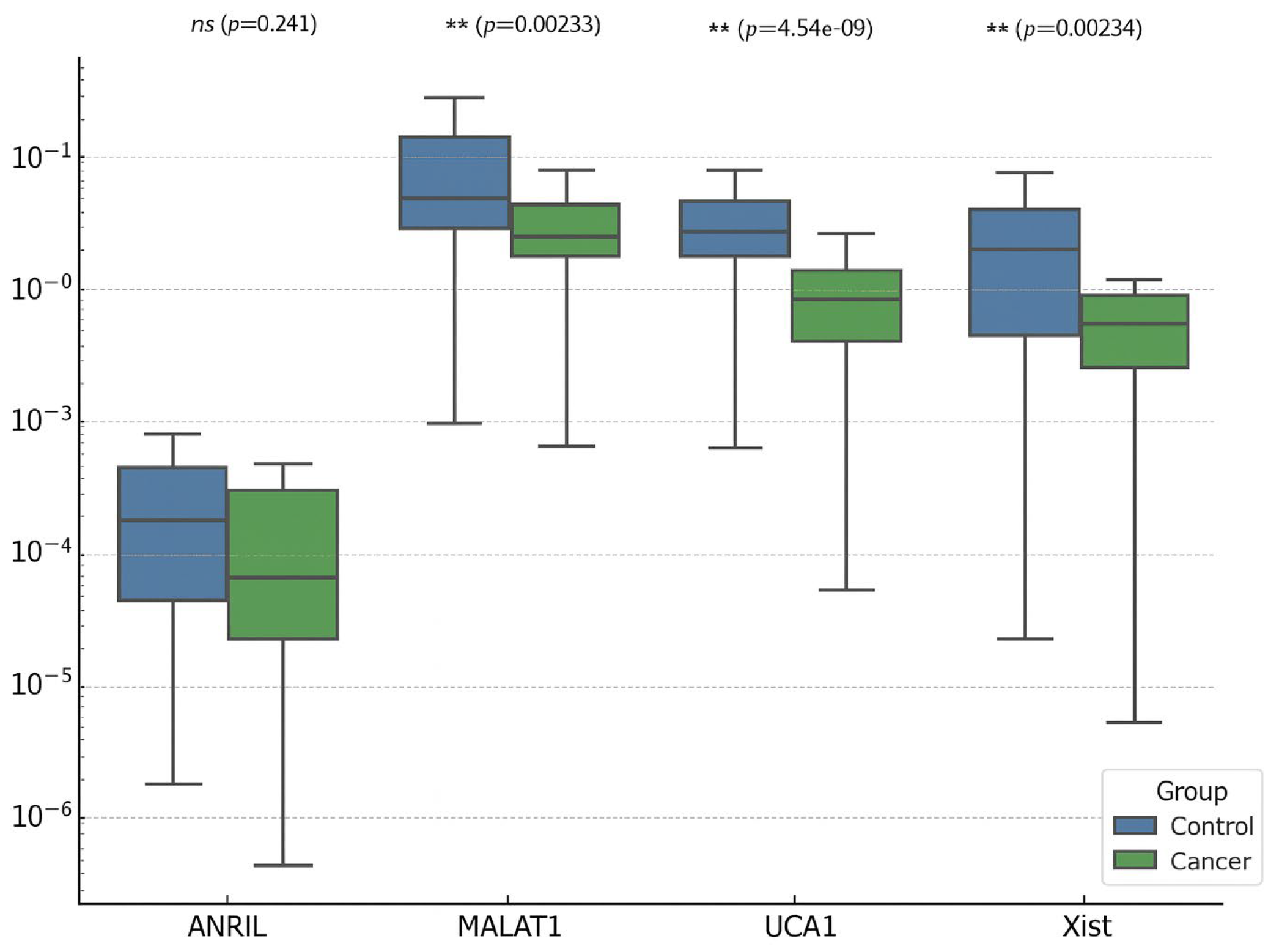
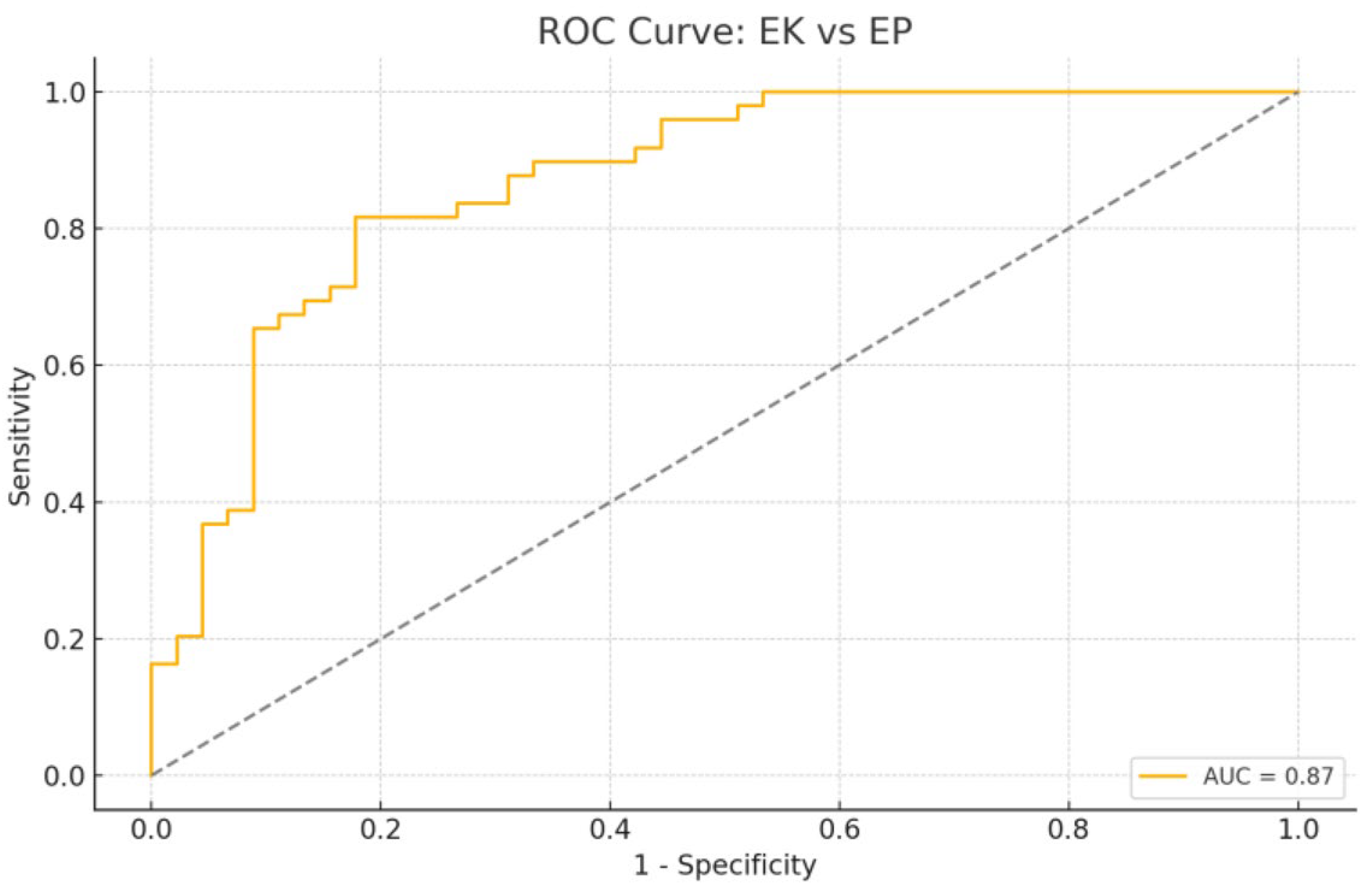
3.1. EC vs. Control
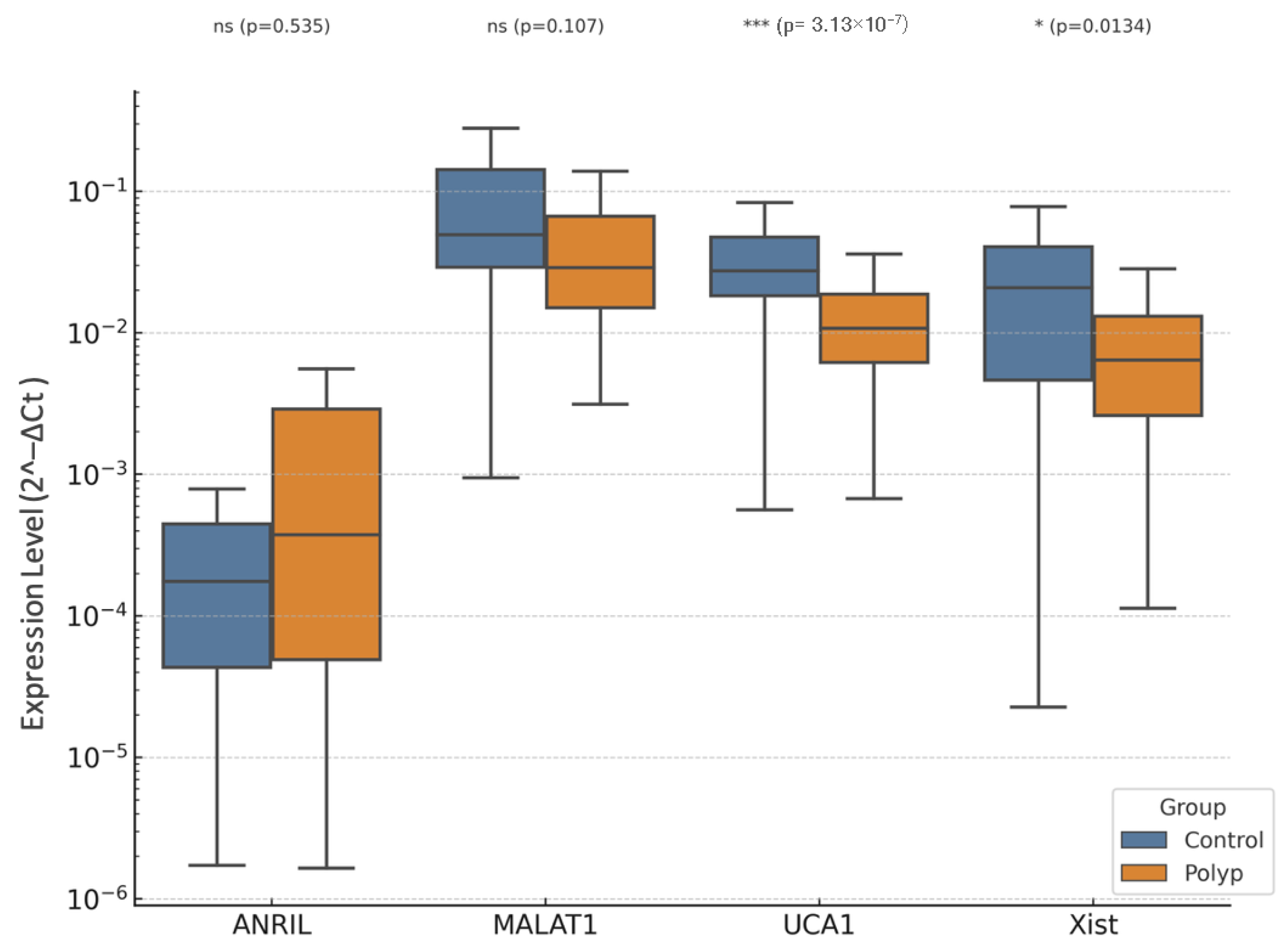
3.2. EP vs. Control
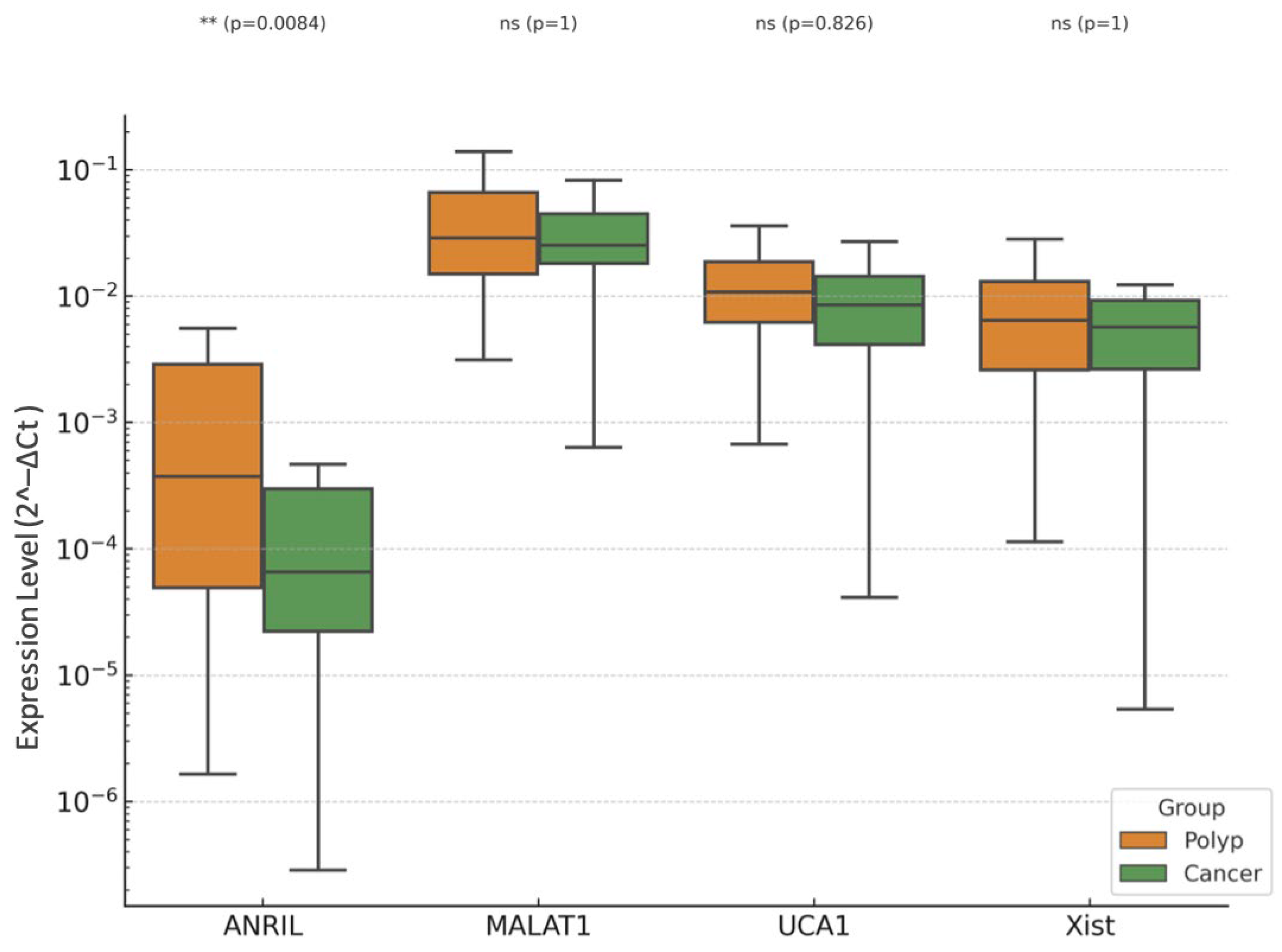
3.3. EC vs. EP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.C.; Kim, C.E.; Garcia-Alonso, L.; Vento-Tormo, R. The human endometrium: Atlases, models, and prospects. Curr. Opin. Genet. Dev. 2025, 92, 102341. [Google Scholar] [CrossRef] [PubMed]
- Golia D’Augè, T.; Cuccu, I.; Santangelo, G.; Muzii, L.; Giannini, A.; Bogani, G.; Di Donato, V. Novel Insights into Molecular Mechanisms of Endometrial Diseases. Biomolecules 2023, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Vieira, M.; Vitagliano, A.; Rossette, M.; Neto, L.; Gallo, A.; Sardo, A. Endometrial Polyps: Update Overview on Etiology, Diagnosis, Natural History and Treatment. Clin. Exp. Obstet. Gynecol. 2022, 49, 232. [Google Scholar] [CrossRef]
- Berceanu, C.; Cernea, N.; Căpitănescu, R.G.; Comănescu, A.C.; Paitici, Ş.; Rotar, I.C.; Bohîlţea, R.E.; Olinca, M.V. Endometrial polyps. Rom. J. Morphol. Embryol. 2022, 63, 323–334. [Google Scholar] [CrossRef]
- Sheng, K.K.; Lyons, S.D. To treat or not to treat? An evidence-based practice guide for the management of endometrial polyps. Climacteric 2020, 23, 336–342. [Google Scholar] [CrossRef]
- Van Den Bosch, T.; Verbakel, J.Y.; Valentin, L.; Wynants, L.; De Cock, B.; Pascual, M.A.; Leone, F.P.G.; Sladkevicius, P.; Alcazar, J.L.; Votino, A.; et al. Typical ultrasound features of various endometrial pathologies described using International Endometrial Tumor Analysis (IETA) terminology in women with abnormal uterine bleeding. Ultrasound Obstet. Gynecol. 2021, 57, 164–172. [Google Scholar] [CrossRef]
- de Rijk, S.R.; Steenbergen, M.E.; Nieboer, T.E.; Coppus, S.F. Atypical Endometrial Polyps and Concurrent Endometrial Cancer: A Systematic Review. Obstet. Gynecol. 2016, 128, 519. [Google Scholar] [CrossRef]
- Savelli, L.; De Iaco, P.; Santini, D.; Rosati, F.; Ghi, T.; Pignotti, E.; Bovicelli, L. Histopathologic features and risk factors for benignity, hyperplasia, and cancer in endometrial polyps. Am. J. Obs. Gynecol. 2003, 188, 927–931. [Google Scholar] [CrossRef]
- Namazov, A.; Gemer, O.; Ben-Arie, A.; Israeli, O.; Bart, O.; Saphier, O.; Mahler, N.; Kapustian, V.; Silberstein, T. Endometrial Polyp Size and the Risk of Malignancy in Asymptomatic Postmenopausal Women. J. Obs. Gynaecol. Can. 2019, 41, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Aljubran, F.; Nothnick, W.B. Long non-coding RNAs in endometrial physiology and pathophysiology. Mol. Cell. Endocrinol. 2021, 525, 111190. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Chang, H.J.; Heo, J.-H.; Yum, S.H.; Jo, E.; Kim, M.; Lee, S.-R. Expression Profiling of Coding and Noncoding RNAs in the Endometrium of Patients with Endometriosis. Int. J. Mol. Sci. 2024, 25, 10581. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Y.; Zhao, Y.; Chen, D.; Fang, T.; Ding, M. Risk factors of endometrial cancer in patients with endometrial hyperplasia: Implication for clinical treatments. BMC Women’s Health 2021, 21, 312. [Google Scholar] [CrossRef]
- Corr, B.; Cosgrove, C.; Spinosa, D.; Guntupalli, S. Endometrial cancer: Molecular classification and future treatments. BMJ Med. 2022, 1, e000152. [Google Scholar] [CrossRef]
- La Ferlita, A.; Battaglia, R.; Andronico, F.; Caruso, S.; Cianci, A.; Purrello, M.; Di Pietro, C. Non-Coding RNAs in Endometrial Physiopathology. Int. J. Mol. Sci. 2018, 19, 2120. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Kumar, A.; Girisa, S.; Alqahtani, M.S.; Abbas, M.; Hegde, M.; Sethi, G.; Kunnumakkara, A.B. Targeting Autophagy Using Long Non-Coding RNAs (LncRNAs): New Landscapes in the Arena of Cancer Therapeutics. Cells 2023, 12, 810. [Google Scholar] [CrossRef]
- Wong, M.; Thanatsis, N.; Nardelli, F.; Amin, T.; Jurkovic, D. Risk of Pre-Malignancy or Malignancy in Postmenopausal Endometrial Polyps: A CHAID Decision Tree Analysis. Diagnostics 2021, 11, 1094. [Google Scholar] [CrossRef]
- Chen, S.; Liang, Y.; Shen, Y.; Wang, X. lncRNA XIST/miR-129-2-3p axis targets CCP110 to regulate the proliferation, invasion and migration of endometrial cancer cells. Exp. Ther. Med. 2023, 25, 159. [Google Scholar] [CrossRef]
- Xu, R.; Zhu, X.; Chen, F.; Huang, C.; Ai, K.; Wu, H.; Zhang, L.; Zhao, X. LncRNA XIST/miR-200c regulates the stemness properties and tumourigenicity of human bladder cancer stem cell-like cells. Cancer Cell Int. 2018, 18, 41. [Google Scholar] [CrossRef]
- Nousiopoulou, E.; Vrettou, K.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Tsikouras, P.; Nikolettos, N.; Nikolettos, K.; Psilopatis, I. The Role of Urothelial Cancer-Associated 1 in Gynecological Cancers. Curr. Issues Mol. Biol. 2024, 46, 2772–2797. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Zhai, J.; Wang, Q.; Zhang, B. Long Noncoding RNA UCA1 Facilitates Endometrial Cancer Development by Regulating KLF5 and RXFP1 Gene Expressions. Cancer Biother. Radiopharm. 2021, 36, 521–533. [Google Scholar] [CrossRef]
- Thapa, R.; Afzal, O.; Afzal, M.; Gupta, G.; Bhat, A.A.; Hassan almalki, W.; Kazmi, I.; Alzarea, S.I.; Saleem, S.; Arora, P.; et al. From LncRNA to metastasis: The MALAT1-EMT axis in cancer progression. Pathol. Res. Pract. 2024, 253, 154959. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, M.; Najafi, S.; Dari, M.A.G.; Sheykhi-Sabzehpoush, M.; Dayer, D.; Cheraghzadeh, M.; Azizidoost, S. Functional roles of long noncoding RNA MALAT1 in gynecologic cancers. Clin. Transl. Oncol. 2023, 25, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.; Valinezhad, K.; Adel, E.; Munirathinam, G. MALAT-1 Is a Key Regulator of Epithelial–Mesenchymal Transition in Cancer: A Potential Therapeutic Target for Metastasis. Cancers 2024, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.H.; Tu, M.; Liu, H.Y. Role of MALAT1 in gynecological cancers: Pathologic and therapeutic aspects (Review). Oncol. Lett. 2021, 21, 333. [Google Scholar] [CrossRef]
- Nakaoka, H.; Gurumurthy, A.; Hayano, T.; Ahmadloo, S.; Omer, W.H.; Yoshihara, K.; Yamamoto, A.; Kurose, K.; Enomoto, T.; Akira, S.; et al. Allelic Imbalance in Regulation of ANRIL through Chromatin Interaction at 9p21 Endometriosis Risk Locus. PLoS Genet. 2016, 12, e1005893. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, Y.Y.; Ding, J.X.; Feng, W.W.; Jin, H.Y.; Hua, K.Q. Long non-coding RNA ANRIL predicts poor prognosis and promotes invasion/metastasis in serous ovarian cancer. Int. J. Oncol. 2015, 46, 2497–2505. [Google Scholar] [CrossRef]
- Sanchez, A.; Lhuillier, J.; Grosjean, G.; Ayadi, L.; Maenner, S. The Long Non-Coding RNA ANRIL in Cancers. Cancers 2023, 15, 4160. [Google Scholar] [CrossRef]
- Vitale, S.G.; Haimovich, S.; Laganà, A.S.; Alonso, L.; Di Spiezio Sardo, A.; Carugno, J. Endometrial polyps. An evidence-based diagnosis and management guide. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Cetin, F.; Kayar, İ.; Birge, Ö.; Goc, G. Malignancy risk factors based on endometrial polyp. BMC Women’s Health 2024, 24, 567. [Google Scholar] [CrossRef] [PubMed]
- Perevalova, A.M.; Kobelev, V.S.; Sisakyan, V.G.; Gulyaeva, L.F.; Pustylnyak, V.O. Role of Tumor Suppressor PTEN and Its Regulation in Malignant Transformation of Endometrium. Biochem. Mosc. 2022, 87, 1310–1326. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Konno, Y.; Ihira, K.; Kobayashi, N.; Yue, J.; Watari, H. Long non-coding RNA DLEU2 drives EMT and glycolysis in endometrial cancer through HK2 by competitively binding with miR-455 and by modulating the EZH2/miR-181a pathway. J. Exp. Clin. Cancer Res. 2021, 40, 216. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Ma, M.; Wang, Q.; Shi, J.; Wu, C. Analysis of long non-coding RNAs associated with disulfidptosis for prognostic signature and immunotherapy response in uterine corpus endometrial carcinoma. Sci. Rep. 2023, 13, 22220. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y. Identification of autophagy-related long non-coding RNAs in endometrial cancer via comprehensive bioinformatics analysis. BMC Women’s Health 2022, 22, 85. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The Nuclear-Retained Noncoding RNA MALAT1 Regulates Alternative Splicing by Modulating SR Splicing Factor Phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Xiong, W.; Peng, Y.; Li, X.; Long, X.; Jin, J.; Liang, J.; Weng, R.; Liu, J.; et al. A novel mechanism regulating pyroptosis-induced fibrosis in endometriosis via lnc-MALAT1/miR-141-3p/NLRP3 pathway. Biol. Reprod. 2023, 109, 156–171. [Google Scholar] [CrossRef]
- Hosseini, N.F.; Manoochehri, H.; Khoei, S.G.; Sheykhhasan, M. The Functional Role of Long Non-coding RNA UCA1 in Human Multiple Cancers: A Review Study. Curr. Mol. Med. 2021, 21, 96–110. [Google Scholar] [CrossRef]

| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Enzyme activation | 95 °C | 3 min | 1 |
| Denaturation | 95 °C | 15 s | 40 |
| Annealing/Extension | 60 °C | 1 min | 40 |
| Variable | EP | EC | Control | p-Value |
|---|---|---|---|---|
| Age (years) * | 47.76 ± 9.35 | 59.18 ± 9.2 | 44.24 ± 5.12 | <0.001 |
| BMI (kg/m2) | 27.85 ± 3.74 | 29.12 ± 3.45 | 27.37 ± 2.48 | 0.0127 |
| Gravida (n) | 1.68 ± 0.84 | 2.26 ± 1.27 | 1.78 ± 0.93 | 0.0117 |
| Parity (n) | 1.42 ± 0.84 | 1.86 ± 1.13 | 1.52 ± 0.84 | 0.0626 |
| Smoking (%) | 36% | 40% | 60% | 0.0361 |
| Variable | EP | EC | Control | p-Value |
|---|---|---|---|---|
| Ferritin (ng/mL) | 58.73 ± 82.97 | 37.27 ± 40.72 | 51.13 ± 56.97 | 0.9152 |
| Glucose (mg/dL) | 81.53 ± 6.75 | 78.26 ± 10.84 | 87.12 ± 15.4 | 0.0123 |
| Haemoglobin (g/dL) | 12.11 ± 1.17 | 11.33 ± 1.23 | 11.76 ± 1.28 | 0.007 |
| WBC (K/μL) | 7.73 ± 1.83 | 7.59 ± 2.39 | 7.39 ± 1.92 | 0.6837 |
| Lymphocyte (K/μL) * | 2.12 ± 0.46 | 1.99 ± 0.58 | 1.89 ± 0.42 | 0.0674 |
| Neutrophil (K/μL) | 6.63 ± 1.81 | 7.31 ± 2.18 | 7.16 ± 1.77 | 0.1615 |
| Platelet (×103) | 225.62 ± 76.74 | 231.34 ± 57.37 | 202.48 ± 60.3 | 0.0139 |
| Total Cholesterol (mg/dL) | 177.67 ± 34.46 | 201.05 ± 43.21 | 212.6 ± 71.59 | 0.060 |
| HDL (mg/dL) | 63.17 ± 12.82 | 56.29 ± 9.37 | 55.74 ± 15.5 | 0.1046 |
| LDL (mg/dL) | 93.77 ± 35.48 | 112.16 ± 34.06 | 105.71 ± 41.32 | 0.0874 |
| Triglyceride (mg/dL) | 137.43 ± 63.76 | 168.11 ± 92.5 | 207.37 ± 96.1 | 0.0063 |
| Free T3 (pg/mL) * | 3.3 ± 0.8 | 3.14 ± 0.72 | 3.17 ± 0.69 | 0.7322 |
| Free T4 (ng/dL) | 1.35 ± 0.35 | 1.19 ± 0.25 | 1.31 ± 0.3 | 0.2432 |
| TSH (uIU/mL) | 3.42 ± 2.17 | 3.58 ± 2.26 | 3.31 ± 2.74 | 0.7425 |
| Creatinin (mg/dL) | 1.32 ± 0.49 | 1.24 ± 0.5 | 1.09 ± 0.36 | 0.0638 |
| Urea (mg/dL) | 31.08 ± 13.2 | 26.8 ± 12.6 | 25.66 ± 10.28 | 0.0851 |
| Vitamin D | 15.83 ± 25.45 | 15.27 ± 29.06 | 19.50 ± 27.46 | 0.8112 |
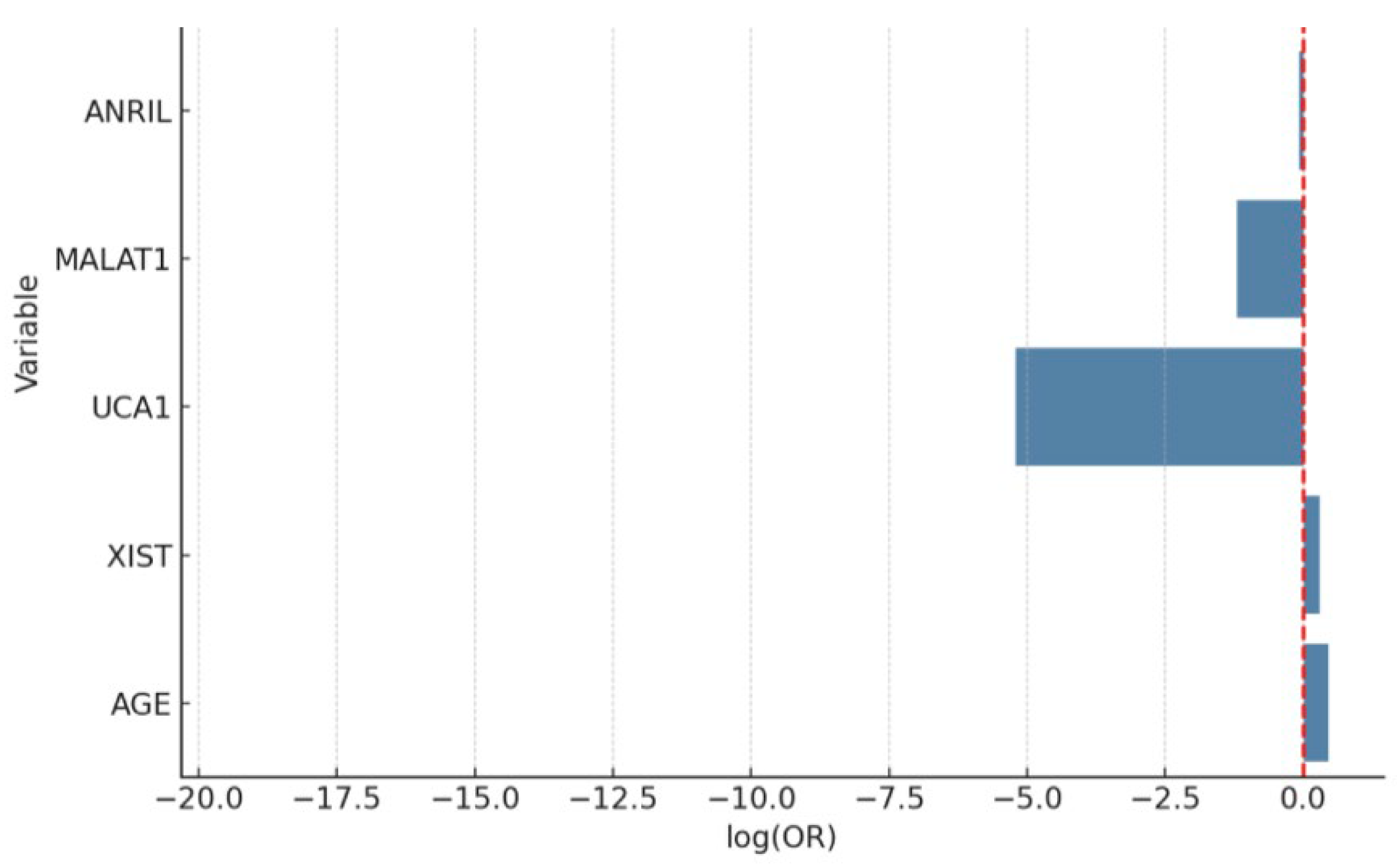
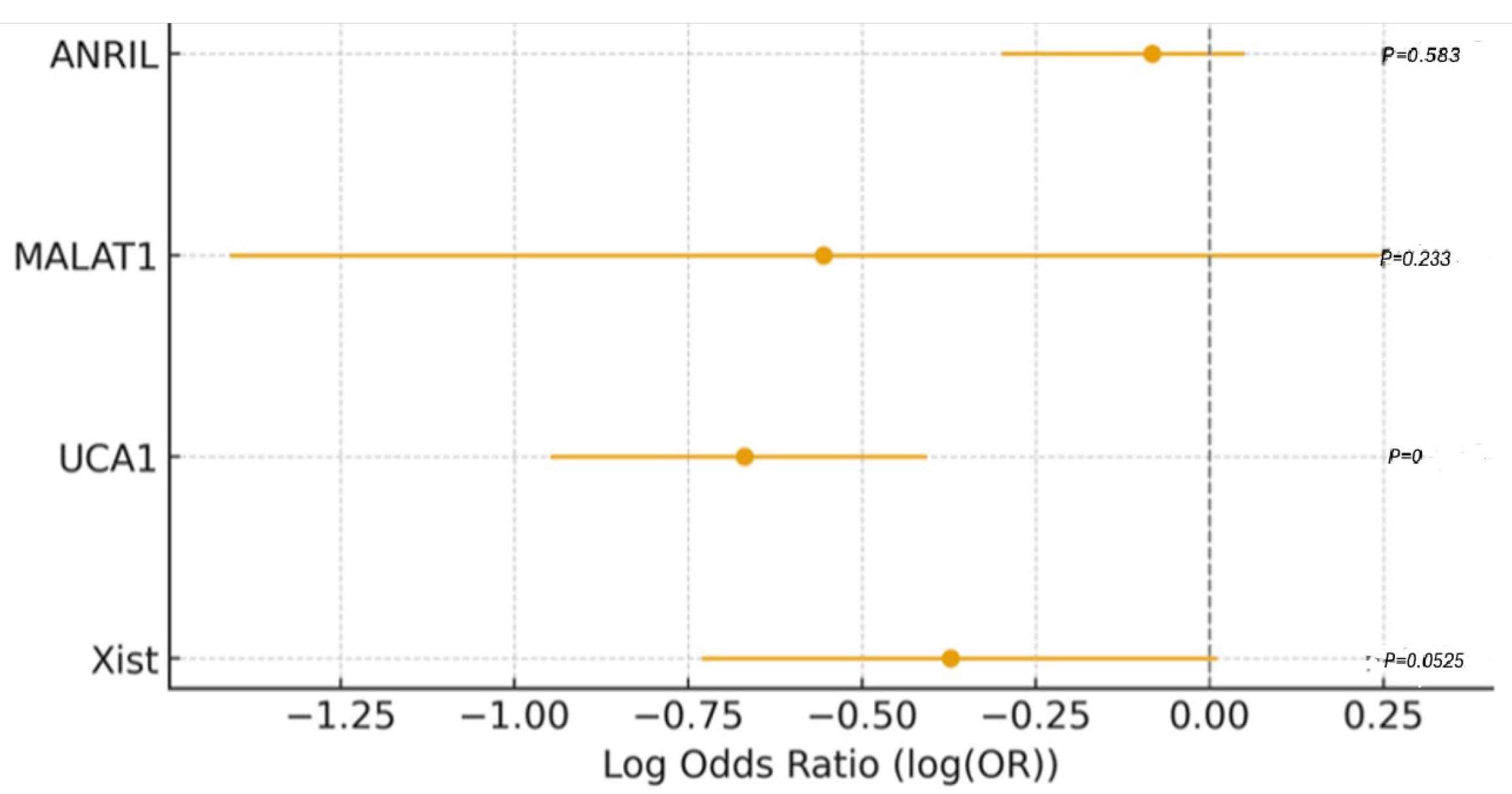
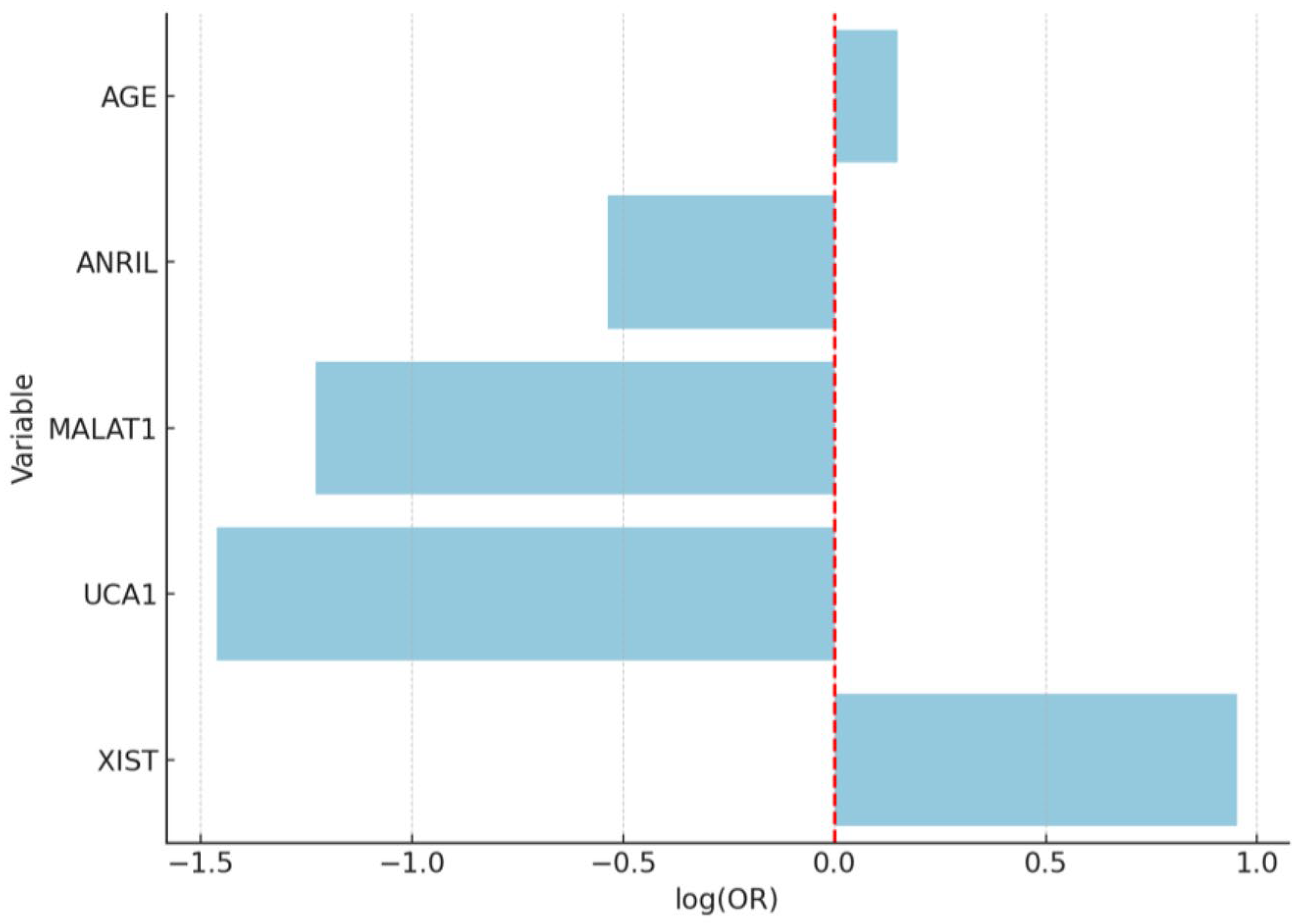
| Variable | Coefficient (Log-Odds) | Std. Error | Wald p-Value | OR | 95% CI (Low) | 95% CI (High) |
|---|---|---|---|---|---|---|
| Intercept | −0.0834 | 0.1711 | 0.620 | — | — | — |
| UCA1 | 1.3945 | 0.3257 | <0.001 | 4.03 | 2.13 | 7.64 |
| XIST | 0.8721 | 0.3493 | 0.012 | 2.39 | 1.21 | 4.74 |
| MALAT1 | 0.6517 | 0.2912 | 0.025 | 1.92 | 1.09 | 3.38 |
| ANRIL | 0.3178 | 0.2845 | 0.264 | 1.37 | 0.78 | 2.41 |
| Age | 1.0124 | 0.3025 | 0.001 | 2.75 | 1.52 | 4.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulbul, C.B.; Solmaz Avcikurt, A.; Kayabasi, C.; Dalmizrak, A.; Erol, Z. Comparison of Long Non-Coding RNA Expressions in Endometrial Polyp and Endometrial Cancer Cases. Diagnostics 2025, 15, 2741. https://doi.org/10.3390/diagnostics15212741
Bulbul CB, Solmaz Avcikurt A, Kayabasi C, Dalmizrak A, Erol Z. Comparison of Long Non-Coding RNA Expressions in Endometrial Polyp and Endometrial Cancer Cases. Diagnostics. 2025; 15(21):2741. https://doi.org/10.3390/diagnostics15212741
Chicago/Turabian StyleBulbul, Cagla Bahar, Ayla Solmaz Avcikurt, Cagla Kayabasi, Aysegul Dalmizrak, and Zafer Erol. 2025. "Comparison of Long Non-Coding RNA Expressions in Endometrial Polyp and Endometrial Cancer Cases" Diagnostics 15, no. 21: 2741. https://doi.org/10.3390/diagnostics15212741
APA StyleBulbul, C. B., Solmaz Avcikurt, A., Kayabasi, C., Dalmizrak, A., & Erol, Z. (2025). Comparison of Long Non-Coding RNA Expressions in Endometrial Polyp and Endometrial Cancer Cases. Diagnostics, 15(21), 2741. https://doi.org/10.3390/diagnostics15212741






