Bayesian Monte Carlo Simulation Based on Systematic Review for Personalized Risk Stratification of Contralateral Lymph Node Metastasis in Oral Squamous Cell Carcinoma †
Abstract
1. Introduction
2. Methodology
2.1. Phase I: Systematic Review
2.2. Phase II: Effect Size and Probability Estimation
2.3. Phase III: Bayesian MCS
3. Results
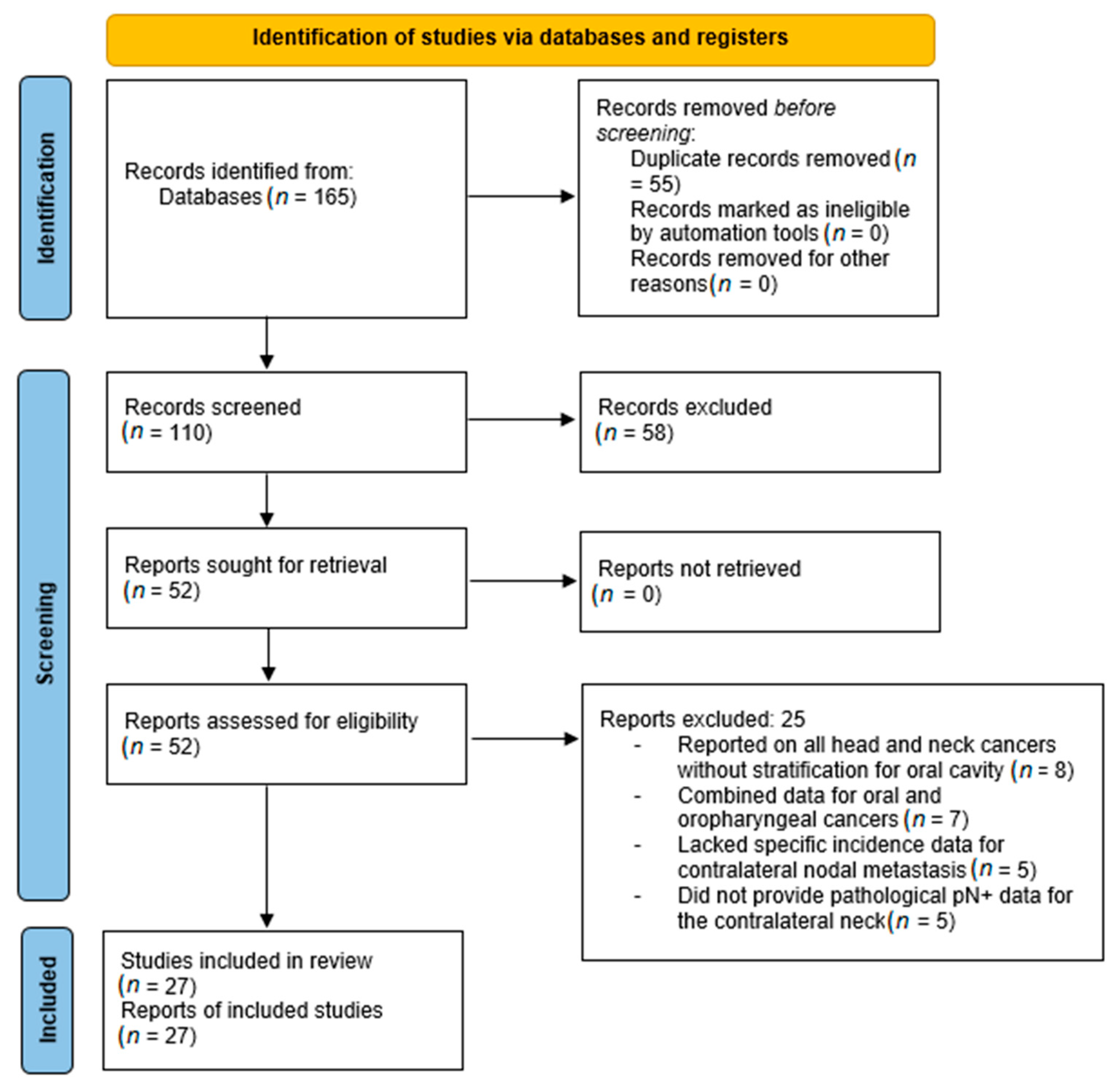
3.1. Phase I: Systematic Search Summary and Study Selection
3.2. Phase II: Systematic Review
3.3. Phase III: Bayesian MCS and Clinical Risk Stratification Framework
3.3.1. Posterior Probability Estimation
3.3.2. Bayesian Monte Carlo Simulation
3.3.3. MCS Diagnostics
3.3.4. Clinical Decision Framework
4. Discussion
4.1. Emerging Alternatives
4.2. Challenges in Preoperative Decision-Making
4.3. Current Evidence and Research Gaps
4.4. Ethical Considerations
4.5. Limitations
4.6. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Rao, K.N.; Arora, R.; Dange, P.; Nagarkar, N.; Mäkitie, A.A.; Kowalski, L.P.; Eisbruch, A.; Hamoir, M.; Civantos, F.J.; Poorten, V.V.; et al. A meta-analysis of surgical outcomes of t4a and infranotch t4b oral cancers. Oncol. Ther. 2023, 11, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Arora, R.D.; Dange, P.; Nagarkar, N.M. Standardizing the head and neck cancer treatment and research. Indian J. Surg. Oncol. 2023, 14, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Sreeram, M.P.; de Bree, R.; Mendenhall, W.M.; Strojan, P.; Stenman, G.; Mäkitie, A.; Nadal, A.; Rodrigo, J.P.; Ng, S.P.; et al. The oncological outcome of postoperative radiotherapy in patients with node-negative early-stage (t1/t2/n0) oral squamous cell carcinoma and perineural invasion: A meta-analysis. Cancers 2025, 17, 862. [Google Scholar] [CrossRef]
- Pantvaidya, G.; Rao, K.; D’Cruz, A. Management of the neck in oral cancers. Oral Oncol. 2020, 100, 104476. [Google Scholar] [CrossRef]
- Asarkar, A.A.; Chang, B.A.; de Bree, R.; Kowalski, L.P.; Guntinas-Lichius, O.; Bradley, P.J.; de Graaf, P.; Strojan, P.; Rao, K.N.; Mäkitie, A.A.; et al. Primary management of operable locally advanced oral cavity squamous cell carcinoma: Current concepts and strategies. Adv. Ther. 2024, 41, 2133–2150. [Google Scholar] [CrossRef]
- Nagarkar, N.M.; Rao, K.N.; Singh, A. Oral cavity and neck dissection. In Atlas of Head Neck and Skull-Base Surgery; Nagarkar, N.M., Mehta, R., Singh, A., Rao, K.N., Dange, P.S., Eds.; Springer Nature: Singapore, 2023; pp. 105–158. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, Y.-H.; Hsueh, W.-T.; Pao, T.-H.; Cheng, Y.-J. Contralateral Neck Nodal Outcomes and Recurrence Pattern of Small Well-Lateralized Oral Cavity Carcinoma—A Single Institution Experience. Ther. Radiol. Oncol. 2023, 7. Available online: https://tro.amegroups.org/article/view/7666 (accessed on 9 June 2025). [CrossRef]
- Struckmeier, A.-K.; Buchbender, M.; Agaimy, A.; Kesting, M. Prevalence and implications of bilateral and solely contralateral lymph node metastases in oral squamous cell carcinoma. Clin. Oral Investig. 2024, 28, 267. [Google Scholar] [CrossRef]
- Doll, C.; Mrosk, F.; Freund, L.; Neumann, F.; Kreutzer, K.; Voss, J.; Raguse, J.-D.; Beck, M.; Böhmer, D.; Rubarth, K.; et al. Management of the contralateral neck in unilateral node-positive oral squamous cell carcinoma. Cancers 2023, 15, 1088. [Google Scholar] [CrossRef]
- Kozioł-Wójcik, K.; Chloupek, A. Metastasis of tongue cancer located unilaterally, not crossing the midline, to the lymph nodes of the neck. Pol. J. Otolaryngol. 2023, 77, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, O.; Leugering, J.; Pipa, G.; Ghozzi, S.; Ullrich, A. A bayesian monte carlo approach for predicting the spread of infectious diseases. PLoS ONE 2019, 14, e0225838. [Google Scholar] [CrossRef]
- Yarandi, R.B.; Mansournia, M.A.; Zeraati, H.; Mohammad, K. An intuitive framework for Bayesian posterior simulation methods. Glob. Epidemiol. 2021, 3, 100060. [Google Scholar] [CrossRef]
- Loskot, P. Bayesian Methods and Monte Carlo Simulations. In Numerical Simulation-Advanced Techniques for Science and Engineering; IntechOpen: London, UK, 2022; Available online: https://www.intechopen.com/chapters/84891 (accessed on 17 July 2025).
- Zhian, T.; Monfared, S.A.H.; Rashki, M.; Azizyan, G. Enhancing decision fusion for wastewater treatment system selection using monte carlo simulation and gray analytic hierarchy process. Water 2024, 16, 1709. [Google Scholar] [CrossRef]
- Liu, H.Y.-H.; Tam, L.; Woody, N.M.; Caudell, J.; Reddy, C.A.; Ghanem, A.; Schymick, M.; Joshi, N.; Geiger, J.; Lamarre, E.; et al. Failure rate in the untreated contralateral node negative neck of small lateralized oral cavity cancers: A multi-institutional collaborative study. Oral Oncol. 2021, 115, 105190. [Google Scholar] [CrossRef]
- Udovicich, C.; Daniell, J.; Wiesenfeld, D.; Bressel, M.; Rowe, D.; Vital, D.; Cavanagh, K.; Lekgabe, E.; Wong, T.; Magarey, M.J.R.; et al. Contralateral neck failure in oral tongue cancer: Outcomes from two centers using predefined treatment criteria. Head Neck 2021, 43, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Murgasen, J.; Gao, K.; Ashford, B.; Shannon, K.; Ebrahimi, A.; Clark, J.R. Contralateral neck failure in lateralized oral squamous cell carcinoma. ANZ J. Surg. 2016, 86, 188–192. [Google Scholar] [CrossRef]
- Koo, B.S.; Lim, Y.C.; Lee, J.S.; Choi, E.C. Management of contralateral N0 neck in oral cavity squamous cell carcinoma. Head Neck 2006, 28, 896–901. [Google Scholar] [CrossRef] [PubMed]
- González-García, R.; Naval-Gías, L.; Sastre-Pérez, J.; Rodríguez-Campo, F.; Muñoz-Guerra, M.; Usandizaga, J.G.-D.; Díaz-González, F. Contralateral lymph neck node metastasis of primary squamous cell carcinoma of the tongue: A retrospective analytic study of 203 patients. Int. J. Oral Maxillofac. Surg. 2007, 36, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, L.P.; Bagietto, R.; Lara, J.R.; Santos, R.L.; Tagawa, E.K.; Santos, I.R. Factors influencing contralateral lymph node metastasis from oral carcinoma. Head Neck 1999, 21, 104–110. [Google Scholar] [CrossRef]
- Vergeer, M.R.; Doornaert, P.A.; Jonkman, A.; Kaanders, J.H.; Van den Ende, P.L.; De Jong, M.A.; Leemans, C.R.; Slotman, B.J.; Langendijk, J.A. Ipsilateral irradiation for oral and oropharyngeal carcinoma treated with primary surgery and postoperative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 682–688. [Google Scholar] [CrossRef]
- Singh, B.; Nair, S.; Nair, D.; Patil, A.; Chaturvedi, P.; D’Cruz, A.K. Ipsilateral neck nodal status as predictor of contralateral nodal metastasis in carcinoma of tongue crossing the midline. Head Neck 2013, 35, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Mair, M.; Thiagarajan, S.; Agrawal, J.; Nair, D.; Chaturvedi, P. Skin involvement and ipsilateral nodal metastasis as a predictor of contralateral nodal metastasis in buccal mucosa cancers. Indian J. Cancer 2016, 53, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, M.; Makino, T.; Morita, S.; Noda, Y.; Kariya, S.; Onoda, T.; Ando, M.; Kimata, Y.; Nishizaki, K.; Okano, M.; et al. Midline involvement and perineural invasion predict contralateral neck metastasis that affects overall and disease-free survival in locally advanced oral tongue squamous cell carcinoma. Front. Oncol. 2022, 12, 1010252. [Google Scholar] [CrossRef]
- Knopf, A.; Jacob, S.; Bier, H.; Scherer, E.Q. Bilateral versus ipsilateral neck dissection in oral and oropharyngeal cancer with contralateral cN0 neck. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 3161–3168. [Google Scholar] [CrossRef]
- Ho, A.S.; Kim, S.; Tighiouart, M.; Gudino, C.; Mita, A.; Scher, K.S.; Laury, A.; Prasad, R.; Shiao, S.L.; Van Eyk, J.E.; et al. Metastatic lymph node burden and survival in oral cavity cancer. J. Clin. Oncol. 2017, 35, 3601–3609. [Google Scholar] [CrossRef] [PubMed]
- Capote-Moreno, A.; Naval, L.; Muñoz-Guerra, M.F.; Sastre, J.; Rodríguez-Campo, F.J. Prognostic Factors Influencing contralateral neck lymph node metastases in oral and oropharyngeal carcinoma. J. Oral Maxillofac. Surg. 2010, 68, 268–275. [Google Scholar] [CrossRef]
- Kurita, H.; Koike, T.; Narikawa, J.-N.; Sakai, H.; Nakatsuka, A.; Uehara, S.; Kobayashi, H.; Kurashina, K. Clinical predictors for contralateral neck lymph node metastasis from unilateral squamous cell carcinoma in the oral cavity. Oral Oncol. 2004, 40, 898–903. [Google Scholar] [CrossRef]
- Donaduzzi, L.; De-Conto, F.; Kuze, L.; Rovani, G.; Flores, M.; Pasqualotti, A. Occurrence of contralateral lymph neck node metastasis in patients with squamous cell carcinoma of the oral cavity. J. Clin. Exp. Dent. 2014, 6, e209–e213. [Google Scholar] [CrossRef]
- Tseng, J.R.; Ho, T.Y.; Lin, C.Y.; Lee, L.Y.; Wang, H.M.; Liao, C.T.; Yen, T.C. Clinical outcomes of patients with oral cavity squamous cell carcinoma and retropharyngeal lymph node metastasis identified by fdg pet/ct. PLoS ONE 2013, 8, e79766. [Google Scholar] [CrossRef]
- Ganly, I.; Goldstein, D.; Carlson, D.L.; Patel, S.G.; O’Sullivan, B.; Lee, N.; Gullane, P.; Shah, J.P. Long-term regional control and survival in patients with “low-risk,” early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: The importance of tumor thickness. Cancer 2013, 119, 1168–1176. [Google Scholar] [CrossRef]
- Singhavi, H.R.; Nair, S.; Mair, M.; Mathur, R.; Singh, A.; Pai, A.; Patil, A.; Nair, D.; Chaturvedi, P. Addressing the contralateral neck for ipsilateral disease recurrence in oral cavity cancers. Eur. J. Surg. Oncol. 2021, 47, 1384–1388. [Google Scholar] [CrossRef]
- Yang, T.; Wang, C.; Ko, J.; Lin, C.; Lou, P. Association of tumor satellite distance with prognosis and contralateral neck recurrence of tongue squamous cell carcinoma. Head Neck 2008, 30, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.; Budrukkar, A.; Murthy, V.; Pai, P.; Kanoja, A.; Ghosh-Laskar, S.; Deshmukh, A.; Pantvaidya, G.; Kannan, S.; Patil, V.; et al. Contralateral nodal relapse in well-lateralised oral cavity cancers treated uniformly with ipsilateral surgery and adjuvant radiotherapy with or without concurrent chemotherapy: A retrospective study. Clin. Oncol. 2024, 36, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Otsuru, M.; Hasegawa, T.; Akashi, M.; Yamada, S.-I.; Kurita, H.; Okura, M.; Yamakawa, N.; Kirita, T.; Yanamoto, S.; et al. Treatment and prognosis of oral cancer patients with confirmed contralateral neck metastasis: A multicenter retrospective analysis. Int. J. Environ. Res. Public Health 2022, 19, 9229. [Google Scholar] [CrossRef] [PubMed]
- González-García, R.; Naval-Gías, L.; Rodríguez-Campo, F.J.; Sastre-Pérez, J.; Muñoz-Guerra, M.F.; Usandizaga, J.L.G.-D. Contralateral lymph neck node metastasis of squamous cell carcinoma of the oral cavity: A retrospective analytic study in 315 patients. J. Oral Maxillofac. Surg. 2008, 66, 1390–1398. [Google Scholar] [CrossRef]
- Flörke, C.; Gülses, A.; Altmann, C.-R.; Wiltfang, J.; Wieker, H.; Naujokat, H. Clinicopathological risk factors for contralateral lymph node metastases in intraoral squamous cell carcinoma: A study of 331 cases. Curr. Oncol. 2021, 28, 1886–1898. [Google Scholar] [CrossRef]
- Gao, W.; Tian, Z.; Fang, X.; Xue, J.; Li, Z.; Yang, C.; Ma, C. Regional metastasis to anatomies beyond traditional neck dissection boundaries: A multi-institutional analysis focused on unconventional metastases in oral cancer patients. World J. Surg. Oncol. 2020, 18, 281. [Google Scholar] [CrossRef]
- Nobis, C.-P.; Otto, S.; Grigorieva, T.; Alnaqbi, M.; Troeltzsch, M.; Schöpe, J.; Wagenpfeil, S.; Ehrenfeld, M.; Wolff, K.-D.; Kesting, M.R. Elective neck dissection in unilateral carcinomas of the tongue: Unilateral versus bilateral approach. J. Cranio-Maxillofac. Surg. 2017, 45, 579–584. [Google Scholar] [CrossRef]
- Mahieu, R.; Toom, I.J.D.; Boeve, K.; Lobeek, D.; Bloemena, E.; Donswijk, M.L.; de Keizer, B.; Klop, W.M.C.; Leemans, C.R.; Willems, S.M.; et al. Contralateral regional recurrence in lateralized or paramedian early-stage oral cancer undergoing sentinel lymph node biopsy—Comparison to a historic elective neck dissection cohort. Front. Oncol. 2021, 11, 644306. [Google Scholar] [CrossRef]
- Toom, I.J.D.; Boeve, K.; van Weert, S.; Bloemena, E.; Brouwers, A.H.; Hoekstra, O.S.; de Keizer, B.; van der Vegt, B.; Willems, S.M.; Leemans, C.R.; et al. High rate of unexpected lymphatic drainage patterns and a high accuracy of the sentinel lymph node biopsy in oral cancer after previous neck treatment. Oral Oncol. 2019, 94, 68–72. [Google Scholar] [CrossRef]
- Flach, G.B.; Broglie, M.A.; van Schie, A.; Bloemena, E.; Leemans, C.R.; de Bree, R.; Stoeckli, S.J. Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma in the previously treated neck. Oral Oncol. 2012, 48, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Earnest-Noble, L.B.; Lipsky, R.S.; Kuhel, W.I.; Kutler, D.I. Identification of occult metastatic disease via lymphoscintigraphy-guided neck dissection in N0 oral squamous cell carcinoma. Head Neck 2022, 44, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Yuan, J.; Huang, T. Contralateral neck metastasis in lateralized cT3/4N0 oral squamous cell carcinoma. J. Cranio-Maxillofac. Surg. 2025, 53, 222–227. [Google Scholar] [CrossRef]
- Noorlag, R.; Nulent, T.J.K.; Delwel, V.E.; Pameijer, F.A.; Willems, S.M.; de Bree, R.; van Es, R.J. Assessment of tumour depth in early tongue cancer: Accuracy of MRI and intraoral ultrasound. Oral Oncol. 2020, 110, 104895. [Google Scholar] [CrossRef]
- Park, S.I.; Guenette, J.P.; Suh, C.H.; Hanna, G.J.; Chung, S.R.; Baek, J.H.; Lee, J.H.; Choi, Y.J. The diagnostic performance of CT and MRI for detecting extranodal extension in patients with head and neck squamous cell carcinoma: A systematic review and diagnostic meta-analysis. Eur. Radiol. 2021, 31, 2048–2061. [Google Scholar] [CrossRef]
- Lorusso, G.; Maggialetti, N.; Laugello, F.; Garofalo, A.; Villanova, I.; Greco, S.; Morelli, C.; Pignataro, P.; Lucarelli, N.M.; Ianora, A.A.S. Diagnostic performance of magnetic resonance sequences in staging lymph node involvement and extranodal extension in head and neck squamous cell carcinoma. Diagnostics 2025, 15, 1251. [Google Scholar] [CrossRef] [PubMed]
- Pakkanen, A.; Marttila, E.; Apajalahti, S.; Snäll, J.; Wilkman, T. Reliability of the pre-operative imaging to assess neck nodal involvement in oral cancer patients, a single-center study. Med. Oral Patol. Oral Cir. Bucal 2022, 27, e191–e197. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; Scaravilli, A.; Ugga, L.; Ponsiglione, A.; Stanzione, A.; D’arco, F.; D’anna, G.; Cuocolo, R. Radiomics Applications in head and neck tumor imaging: A narrative review. Cancers 2023, 15, 1174. [Google Scholar] [CrossRef]
- Kann, B.H.; Likitlersuang, J.; Bontempi, D.; Ye, Z.; Aneja, S.; Bakst, R.; Kelly, H.R.; Juliano, A.F.; Payabvash, S.; Guenette, J.P.; et al. Screening for extranodal extension in HPV-associated oropharyngeal carcinoma: Evaluation of a CT-based deep learning algorithm in patient data from a multicentre, randomised de-escalation trial. Lancet Digit. Health 2023, 5, e360–e369. [Google Scholar] [CrossRef]


| Author | Publication Year | Country | Sample Size | Type of Study | Newcastle–Ottawa Scale | Subsite in Oral Cavity | Parameter Assessed | Comments |
|---|---|---|---|---|---|---|---|---|
| Swain et al. [35] | 2024 | India | 208 | Retrospective | 7 | Tongue, lower gingiva, upper gingiva/hard palate, floor of the mouth, and buccal mucosa | Lateralized lesion | The study found that 21.3% of patients with positive lymph nodes developed contralateral nodal relapse (CLNR). The most common site for CLNR was level IB lymph nodes. Factors associated with CLNR included the number of positive lymph nodes, involvement of specific lymph node levels, and the presence of extranodal extension, lymphatic invasion, and perineural invasion. Having two or more positive lymph nodes was an independent risk factor for CLNR, and the risk increased with each additional positive node. |
| Struckmeier et al. [9] | 2024 | Germany | 420 | Retrospective | 7 | Oral cavity | Lateralized lesion | Isolated contralateral metastases in 0.95%, bilateral metastases in 7.13%, and occult bilateral metastases in 3.81%. Higher tumour stages, localization at the upper jaw or floor of mouth, and ipsilateral LNMs were associated with increased contralateral LNM risk. |
| Midline reaching | ||||||||
| Kozioł-Wójcik et al. [11] | 2023 | Poland | 106 | Retrospective | 6 | Tongue | Lateralized lesion | 13% contralateral lymph node metastasis. |
| Doll et al. [10] | 2023 | Germany | 65 | Retrospective | 7 | Oral cavity | Lateralized lesion | 9.2% developed recurrent contralateral CLNM. END showed no significant benefit in overall survival (OS) or recurrence-free survival (RFS). Increased ipsilateral lymph node ratio was associated with contralateral CLNM. |
| Sakamoto et al. [36] | 2022 | Japan | 388 | Retrospective | 7 | Tongue, lower gingiva, upper gingiva/hard palate, floor of the mouth, buccal mucosa and others | Lateralized lesion | pN2c oral cancer patients revealed that advanced T-stage significantly correlated with poor overall survival (OS) and disease-specific survival (DSS). Contralateral elective neck dissection was not recommended, and no significant difference in outcomes between ipsilateral and bilateral neck dissection. |
| Lesion crossing midline | ||||||||
| Extranodal spread in the ipsilateral neck, level of metastasis in the ipsilateral neck, extranodal spread in the contralateral neck | ||||||||
| Akamatsu et al. [25] | 2022 | Japan | 32 | Retrospective | 7 | Oral tongue | T3 and T4a oral tongue | Midline involvement and PNI were independent predictors of CLNM. |
| Liu et al. [16] | 2021 | Australia | 176 | Retrospective | 6 | Oral tongue and floor of mouth | Lateralized lesion | 5% of patients had contralateral neck failure. Pathological predictors for contralateral neck failure was depth of invasion (DOI) > 10 mm and ipsilateral neck node positive status. |
| Udovicich et al. [17] | 2021 | Australia | 258 | Retrospective | 7 | Oral tongue | Lateralized lesion | 15.2% of cases had pathological involvement of the contralateral neck. Rates of contralateral neck failure increased with higher N classification, DOI ≥ 6 mm, extracapsular extension (ECE), and perineural invasion (PNI). |
| Lesion crossing midline | ||||||||
| Tumour factors–Depth of invasion, Tumour thickness, perineural invasion, lymphovascular invasion | ||||||||
| Flörke et al. [38] | 2021 | Germany | 350 | Retrospective | 7 | Floor of the mouth, tongue, alveolar process, hard palate, inner cheek, oropharynx, lip and extraoral extension | Lateralized lesion | The contralateral neck node metastasis seen in 35 cases (8.6%). Floor of the mouth was the region where contralateral metastases (51.43%) were most commonly found. No contralateral neck node metastasis in the buccal plane, lower lip, and oropharynx. |
| Lesion crossing midline | ||||||||
| Rajendra et al. [33] | 2021 | India | 78 | Retrospective | 6 | Tongue and buccal mucosa | Recurrent oral cancer on the same side of the previous malignancy | Recurrent oral cavity SCC found a 23.1% incidence of contralateral nodal metastasis (CNM), with a significant association to a depth of invasion (DOI) greater than 10 mm. PET-CECT demonstrated high negative predictive value (NPV), emphasizing the importance of addressing the contralateral neck in tumours with higher DOI. |
| Knopf et al. [26] | 2020 | Germany | 471 | Retrospective | 7 | Oral cavity—cheek, bucco alveolar sulcus, mouth floor, tongue oropharynx—tonsil, soft palate, uvula, tongue base, lat. pharyngeal wall, dorsal pharyngeal wall, vallecula | Lateral | Bilateral neck dissection of the node-negative contralateral neck did not improve overall survival or the recurrence free survival in OC and OPC patients. |
| midline reaching | ||||||||
| Midline-crossing | ||||||||
| Gao et al. [39] | 2020 | China | 89 | Retrospective | 6 | Buccal mucosa, upper gingiva, lower gingiva, floor of mouth, tongue, retromolar trigone, lip, hard palate | Well-lateralized and lesions crossing midline | Discusses the unconventional metastatic lymph nodes (UMLNs) with varied primary or recurrent sites. Recurrences were predominant causes of death, with an overall survival rate of 38.2%. Sublingual UMLNs were associated with an increased risk of simultaneous contralateral metastasis. |
| Ho et al. [27] | 2017 | USA | 14,554 NCDB | Retrospective | 7 | Oral tongue, upper/lower gum, floor of mouth, hard palate, and other parts of the mouth (e.g., buccal mucosa, retromolar trigone | T-stage, number of lymph nodes, size of lymph node, lower-level lymph node involvement presence of ENE | Increasing metastatic nodes were associated with worse overall survival (OS), with a significant impact up to four nodes. Examining more lymph nodes improved OS up to 35 nodes, with no added benefit beyond. Features like extranodal extension and lower neck involvement independently increased mortality risk. Contralateral neck involvement did not significantly impact survival. |
| Nobis et al. [40] | 2017 | Germany | 150 | Retrospective | 6 | Oral tongue | T1 and T2 lateralized lesion | Contralateral neck node metastasis was present in 2.7% of patients. |
| Habib et al. [18] | 2016 | Australia | 481 | Retrospective | 7 | Oral tongue, floor of mouth, alveolus, buccal, retromolar trigone, hard palate, unspecified | Well-lateralized (more than 1 cm from midline) T1 to T4 primary lesion | 2.9% patients developed isolated contralateral neck failure. Most contralateral failures occurred in patients with oral tongue primaries. Poorly differentiated tumours or pathologically proven ipsilateral nodal metastases were at significantly higher risk of contralateral failure. Presence of both conferred a 10% risk of contralateral failure. |
| Mair et al. [24] | 2016 | India | 125 | Retrospective | 6 | Buccal mucosa | Lesions reaching midline | 20.8% had bilateral neck node metastasis, and 1.6% had isolated contralateral nodal metastasis. |
| Lesions crossing midline | ||||||||
| Donaduzzi et al. [30] | 2014 | Brazil | 303 | Retrospective | 6 | Floor of mouth, tongue, vestibule, palate and other locations | Lateralized lesion | 18.8% of patients had contralateral neck node metastasis during presentation. |
| Lesion involving midline | ||||||||
| Singh et al. [23] | 2013 | India | 243 | Retrospective | 7 | Tongue | Lesion reaching midline | 28.4% of patients had bilateral, and 0.8% had isolated contralateral nodal metastases. Ipsilateral nodal metastasis is the most significant factor influencing contralateral nodal metastasis, with pathological T classification also as an independent risk factor. |
| Lesion crossing midline | ||||||||
| Tseng et al. [31] | 2013 | Tiwan | 36 | Retrospective | 6 | Alveolar ridge, hard palate, mouth floor, buccal mucosa, retromolar trigone, tongue | Stage I to IVb oral cancer with retropharyngeal lymph node metastasis | Survival analysis of retropharyngeal lymph nodes was performed (RPLN). 50% of patients with primary RPLN had contralateral neck node metastasis, 3.8% of recurrent RPLN had contralateral neck node metastasis. OSCC patients with RPLN involvement have poor outcomes. |
| Ganly et al. [32] | 2013 | USA | 164 | Retrospective | 7 | Oral tongue | Well-lateralized T1- T2N0 lesions | Contralateral neck failure was seen in 39% of patients. Most important factor for neck failure was tumour thickness ≥4 mm. |
| Capote-Moreno et al. [28] | 2010 | Spain | 402 | Retrospective | 7 | Oral cavity—tongue, floor of the mouth, buccal mucosa, gum, hard palate oropharynx-tongue base, tonsil | Lesions crossing midline | 5.1% had primary positive contralateral metastases in neck dissection specimens and 4.8% had contralateral recurrences at follow-up. Homolateral lymph node metastases and extension across the midline were the most important predictors of contralateral metastases. Contralateral neck node metastasis was associated with poor prognosis. |
| Lesions not crossing midline | ||||||||
| Yang et al. [34] | 2008 | Tiwan | 119 | Retrospective | 6 | Oral tongue | Stage I to IV oral tongue lesion | The incidence of contralateral neck lymph node metastasis was significantly higher in cases with TSD > 0.5 mm. |
| Tumour satellite distance (TSD) | ||||||||
| González-García et al. [37] | 2008 | Spain | 315 | Retrospective | 7 | Anterior two thirds of the tongue, lateral floor of the mouth, lateral gingiva, and buccal mucosa | Lateralized lesion (more than 1 cm from midline) | 5.69% patients developed contralateral neck recurrence (CLNR). Delay in diagnosis 12 or more months is associated with increased CLNR. Presence of ipsilateral neck metastasis at the time of diagnosis is associated with an augmented incidence of CLNR in SCC of the oral cavity. |
| González-García et al. [20] | 2007 | Spain | 203 | Retrospective | 7 | Anterior oral tongue | Lesions not crossing midline | 4.96% of patients had contralateral neck node relapse. Histopathological grading, and peritumoural inflammation had statistically significant association with contralateral neck node relapse. |
| Lesions crossing midline | ||||||||
| Koo et al. [19] | 2006 | Korea | 66 | Retrospective | 6 | Oral cavity | Lesions not crossing midline | Clinically negative but pathologically positive contralateral lymph nodes occurred in 11%. Of the 11 cases with a clinically positive ipsilateral node neck, contralateral occult lymph node metastases developed in 36%, in contrast with 5% in the cases with clinically N0 ipsilateral necks. |
| Lesions crossing midline | ||||||||
| Kurita et al. [29] | 2004 | Japan | 129 | Prospective | 7 | Tongue, lower gingiva, the buccal mucosa, the upper gingiva, and the floor of the mouth | Lateralized lesion | Contralateral neck metastasis (CLNM) was diagnosed in 19 patients. The correlation with CLNM included factors such as T-stage, number of ipsilateral lymph node metastases, level of ipsilateral lymph node metastases, histopathological grading, mode of invasion, and midline extension. Multivariate analysis identified T-stage, number of ipsilateral lymph node metastases, and histopathological grading as significant independent predictors for CLNM. |
| Kowalski et al. [21] | 1999 | Brazil | 513 | Prospective | 7 | Tongue, Floor of mouth, Inferior gingiva, Retromolar trigone and Others | Lateralized lesion (>1 cm from midline) | Contralateral neck metastases were identified in 38 cases, with five having initially undergone bilateral cervical dissection. Risk factors for contralateral metastases encompassed primary tumour location, midline involvement, clinical stage, vascular embolization, and perineural infiltration. Risks ranging from 1.8 to 9.6 times higher in Stage II, III, and IV compared to Stage I cases. |
| Reaching midline (<1 cm from midline) | ||||||||
| Midline | ||||||||
| Crossing midline (<1 cm from midline) | ||||||||
| Crossing midline (>1 cm from midline) |
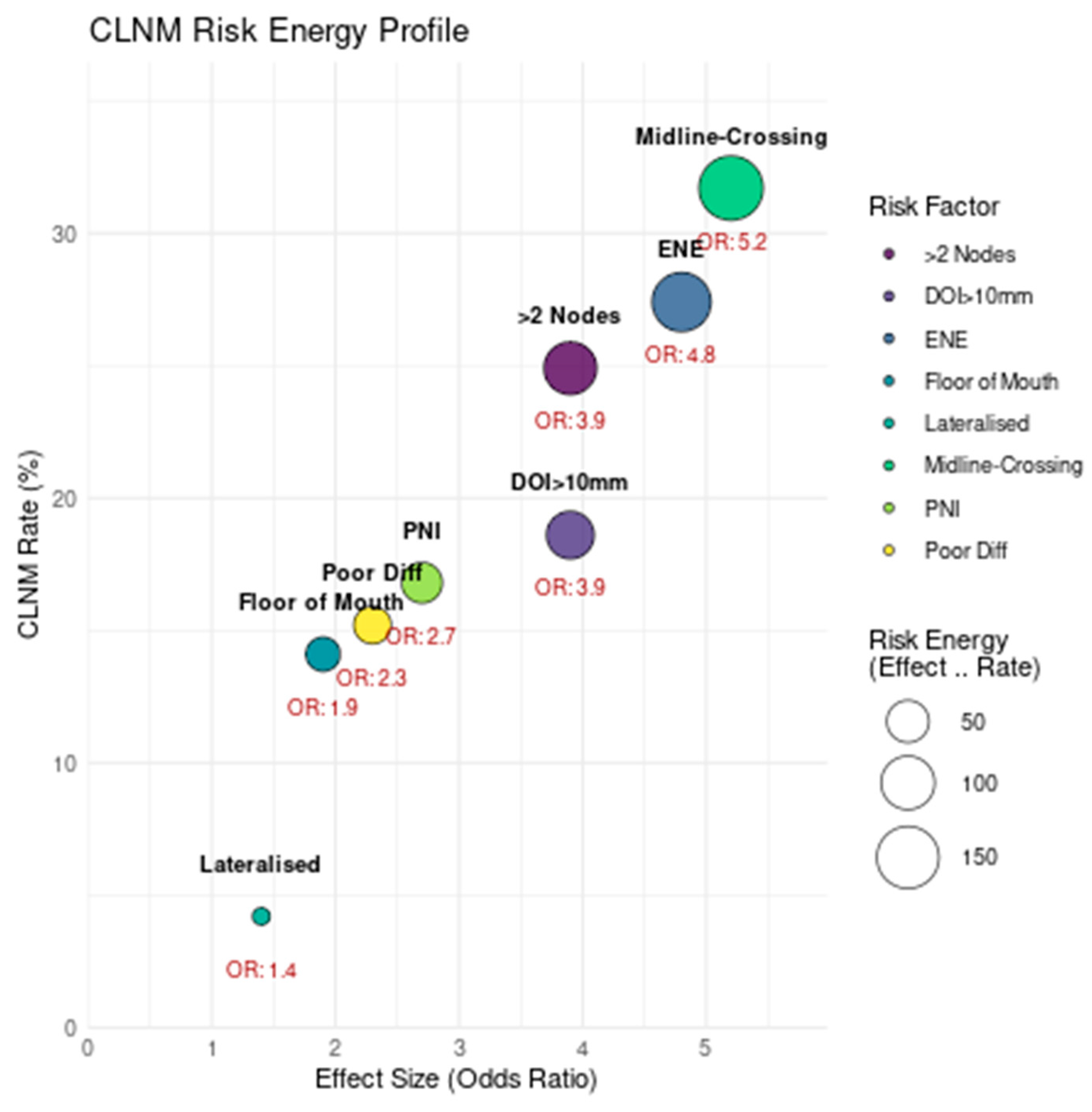
| Risk Factor | Avg CLNM Rate (Range) | Effect Size (95% CI) | Calculation Methodology | Supporting Studies |
|---|---|---|---|---|
| Midline-crossing tumour * | 32.7% (18.8–51.4%) | OR 5.2 (4.1–6.6) |
| Struckmeier et al. [9], Sakamoto et al. [36], Akamatsu et al. [25], Udovicich et al. [17], Flörke et al. [38], Mair et al. [24], Donaduzzi et al. [30], Singh et al. [23], Capote-Moreno et al. [28], González-García et al. (2007) [20], Koo et al. [19], Kurita et al. [29], Kowalski et al. [21] |
| Extranodal extension */** | 29.8% (15.2–51.4%) | OR 4.8 (3.7–6.2) |
| Swain et al. [35], Sakamoto et al. [36], Ho et al. [27], Kowalski et al. [21] |
| ≥2 ipsilateral nodes */** | 28.4% (21.3–50%) | OR 3.9 (3.0–5.1) |
| Swain et al. [35], Doll et al. [10], Ho et al. [27], Habib et al. [18], Kurita et al. [29] |
| DOI >10 mm */** | 19.1% (5–39%) | Cohen’s d = 0.81 (0.65–0.97) |
| Liu et al. [16], Udovicich et al. [17], Rajendra et al. [33], Ganly et al. [32] |
| PNI present ** | 20.3% (5–38%) | OR 2.7 (2.0–3.6) |
| Swain et al. [35], Akamatsu et al. [25], Udovicich et al. [17], Kowalski et al. [21] |
| Poor differentiation */** | 18.6% (10–28.4%) | OR 2.3 (1.8–3.0) |
| Habib et al. [18], González-García et al. (2007) [20], González-García et al. (2008) [37], Kurita et al. [29] |
| Floor of mouth subsite * | 17.9% (5–51.4%) | OR 1.9 (1.5–2.4) |
| Struckmeier et al. [9], Flörke et al. [38] |
| Lateralized lesions * | 8.5% (2.7–15.2%) | OR 1.4 (1.1–1.8) |
| Swain et al. [35], Struckmeier et al. [9], Kozioł-Wójcik et al. [11], Doll et al. [10], Sakamoto et al. [36], Liu et al. [16], Udovicich et al. [17], Flörke et al. [38], Habib et al. [18], Donaduzzi et al. [30], González-García et al. (2007) [20], Kurita et al. [29], Kowalski et al. [21] |
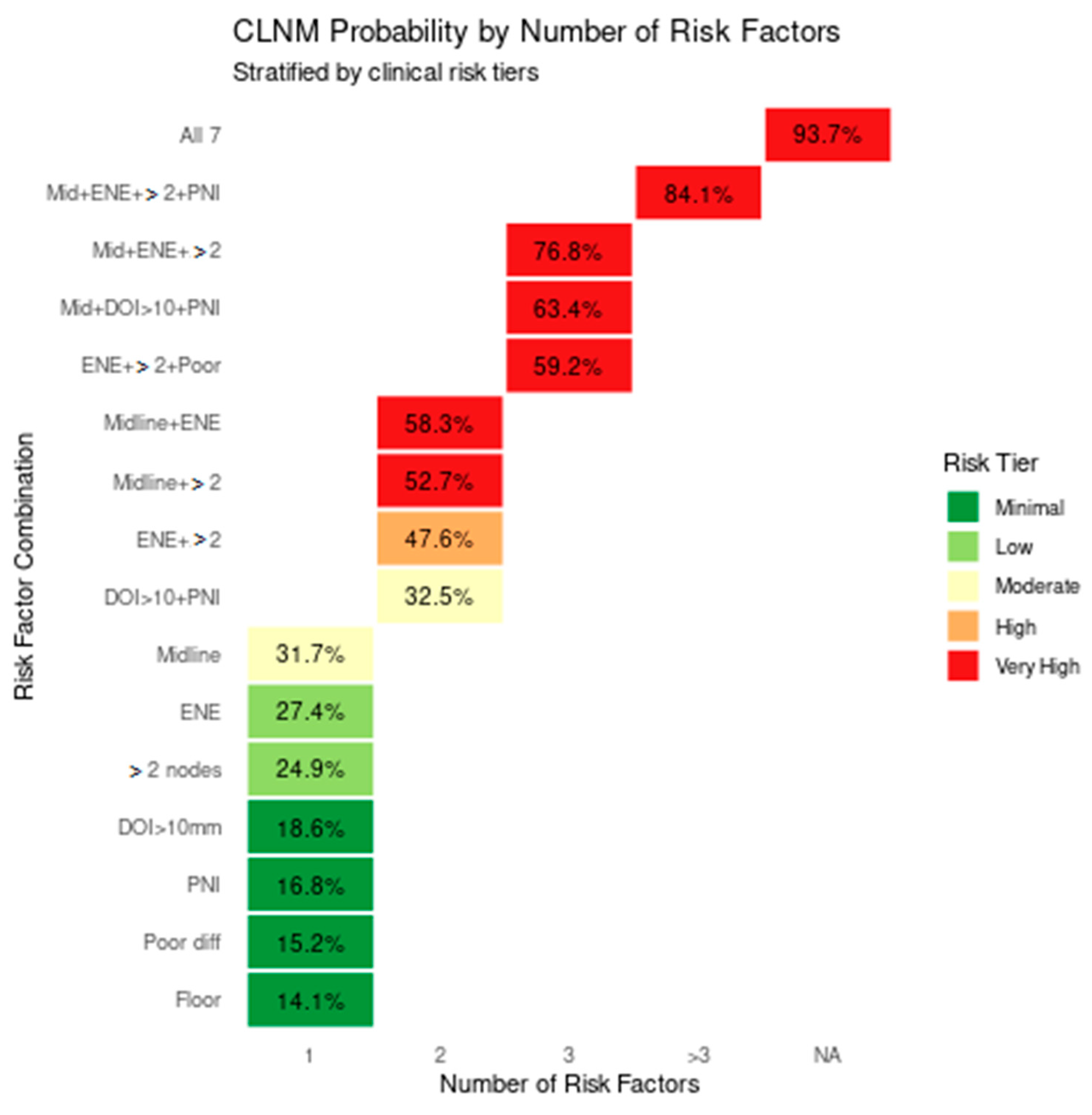
| Risk Factor Combination | Posterior CLNM Probability | 95% Credible Interval | Risk Multiplier vs. Baseline |
|---|---|---|---|
| Baseline (lateralized, no other risks) | 4.2% | 3.8–4.6% | 1.0× (reference) |
| Single Factors | |||
| 31.7% | 30.2–33.3% | 7.5× |
| 27.4% | 26.0–28.9% | 6.5× |
| 24.9% | 23.5–26.3% | 5.9× |
| 18.6% | 17.4–19.9% | 4.4× |
| 16.8% | 15.6–18.0% | 4.0× |
| 15.2% | 14.1–16.4% | 3.6× |
| 14.1% | 13.0–15.3% | 3.4× |
| 2 Risk Factors | |||
| 58.3% | 56.5–60.1% | 13.9× |
| 52.7% | 50.9–54.5% | 12.5× |
| 47.6% | 45.8–49.4% | 11.3× |
| 32.5% | 30.9–34.1% | 7.7× |
| 3 Risk Factors | |||
| 76.8% | 75.3–78.3% | 18.3× |
| 63.4% | 61.7–65.1% | 15.1× |
| 59.2% | 57.4–61.0% | 14.1× |
| >3 Risk Factors | |||
| 84.1% | 82.8–85.4% | 20.0× |
| 93.7% | 92.6–94.8% | 22.3× |
| Risk Tier | CLNM Probability | Dominant Risk Factors |
|---|---|---|
| Minimal Risk | <20% |
|
| Low Risk | 20–30% |
|
| Moderate Risk | 30–40% |
|
| High Risk | 40–50% |
|
| Very High Risk | >50% |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, K.N.; Sreeram, M.P.; Dange, P.; Pelaz, A.C.; Piazza, C.; Bree, R.d.; Lopez, F.; Guntinas-Lichius, O.; Kowalski, L.P.; Robbins, K.T.; et al. Bayesian Monte Carlo Simulation Based on Systematic Review for Personalized Risk Stratification of Contralateral Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Diagnostics 2025, 15, 2668. https://doi.org/10.3390/diagnostics15212668
Rao KN, Sreeram MP, Dange P, Pelaz AC, Piazza C, Bree Rd, Lopez F, Guntinas-Lichius O, Kowalski LP, Robbins KT, et al. Bayesian Monte Carlo Simulation Based on Systematic Review for Personalized Risk Stratification of Contralateral Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Diagnostics. 2025; 15(21):2668. https://doi.org/10.3390/diagnostics15212668
Chicago/Turabian StyleRao, Karthik N., M. P. Sreeram, Prajwal Dange, Andres Coca Pelaz, Cesare Piazza, Remco de Bree, Fernando Lopez, Orlando Guntinas-Lichius, Luiz Paulo Kowalski, Kevin T. Robbins, and et al. 2025. "Bayesian Monte Carlo Simulation Based on Systematic Review for Personalized Risk Stratification of Contralateral Lymph Node Metastasis in Oral Squamous Cell Carcinoma" Diagnostics 15, no. 21: 2668. https://doi.org/10.3390/diagnostics15212668
APA StyleRao, K. N., Sreeram, M. P., Dange, P., Pelaz, A. C., Piazza, C., Bree, R. d., Lopez, F., Guntinas-Lichius, O., Kowalski, L. P., Robbins, K. T., Strojan, P., Suárez, C., Homma, A., Takes, R., Rodrigo, J. P., Hamoir, M., Eisbruch, A., Civantos, F., Araújo, A. L. D., ... Ferlito, A. (2025). Bayesian Monte Carlo Simulation Based on Systematic Review for Personalized Risk Stratification of Contralateral Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Diagnostics, 15(21), 2668. https://doi.org/10.3390/diagnostics15212668














