Imaging Modalities in Craniosynostosis: A Systematic Review and Proposal of the ARCANA Protocol for Multimodal Radiation-Free Assessment
Abstract
1. Introduction
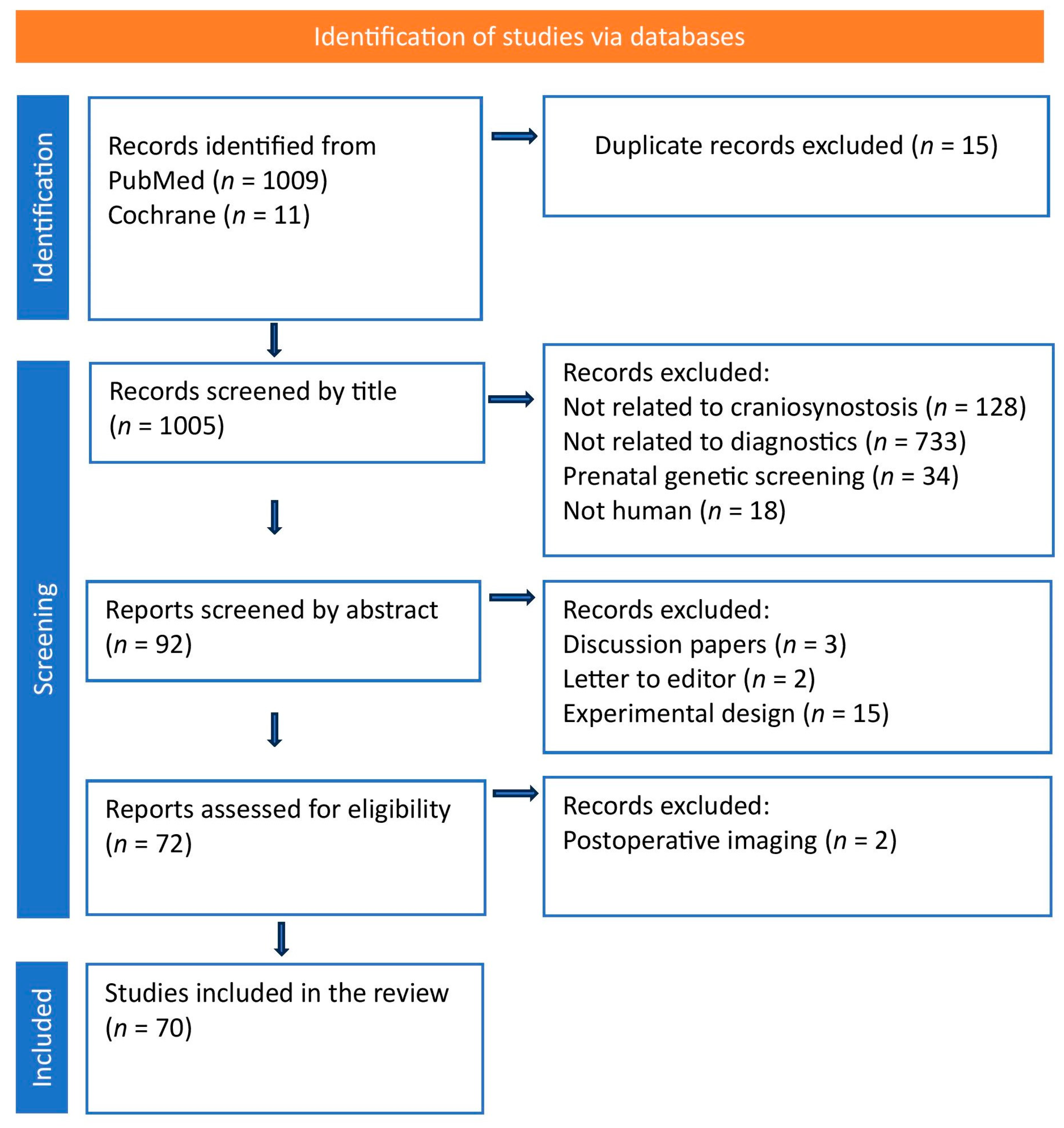
2. Methods
3. Results
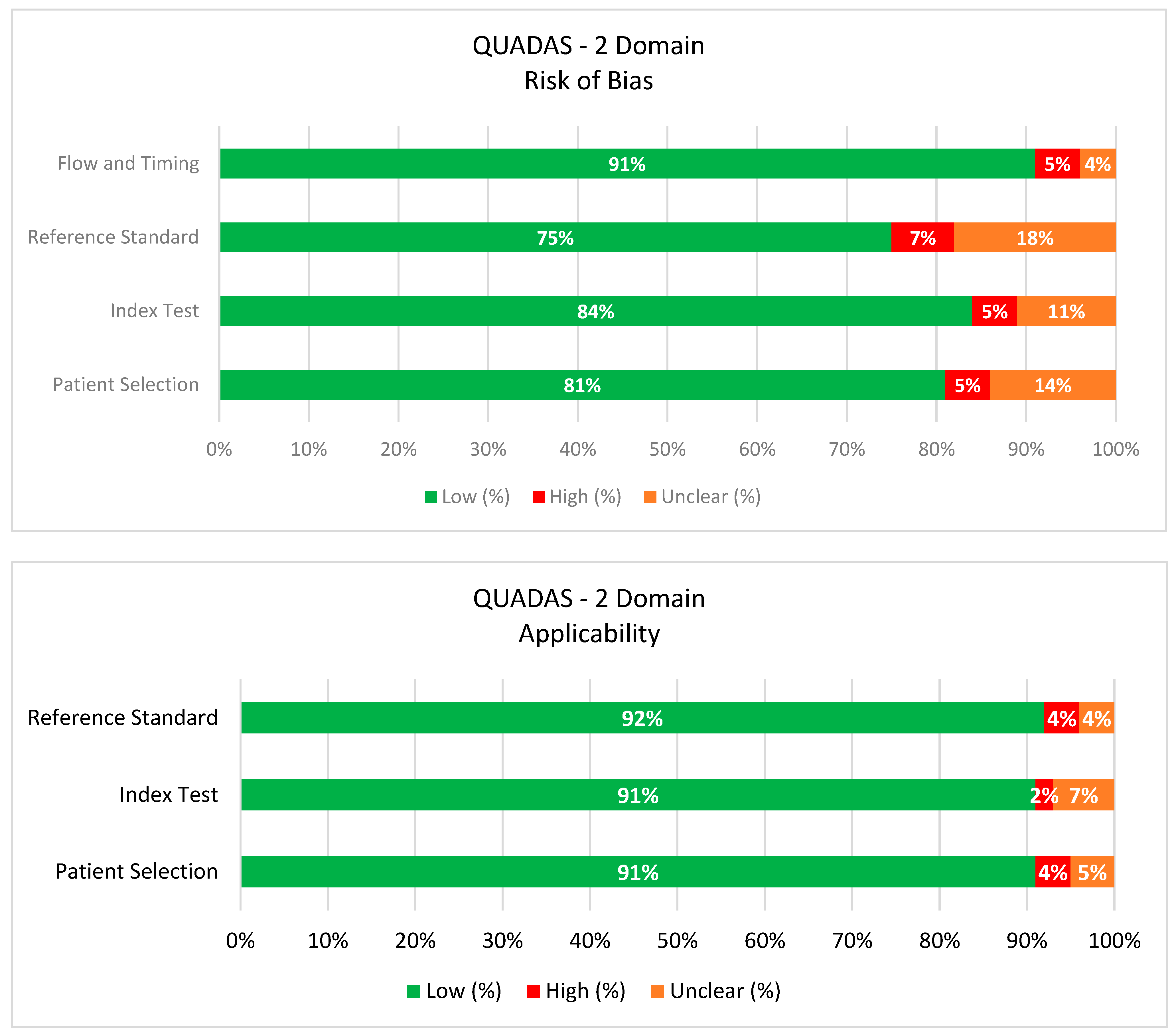
3.1. Risk of Bias Assessment
3.2. GRADE Summary of Certainty
3.3. Three-Dimensional Computed Tomography: The Reference Standard
3.4. Three-Dimensional Stereophotogrammetry
- Objective Morphometric Assessment: 3DSPG enables quantitative analysis of cranial deformities that exceeds the limitations of subjective clinical assessment and traditional anthropometric measures [50,51]. Advanced analytic techniques, such as statistical shape modeling and principal component analysis, facilitate complex shape quantification, generate patient-specific digital models, and offer continuous severity scoring systems for CS [13,52,53].
| 3DSPG Feature | Normal Finding | Abnormal in CS | Clinical Utility | Measurement Method/Tools |
|---|---|---|---|---|
| Cranial Symmetry | Bilateral, mirrored shape of cranial vault and face | Asymmetry of skull/facial contours | Quantifies degree and type of cranial asymmetry | Superimposition, symmetry analysis software, color maps |
| Suture Ridge & Depression | Smooth bone surfaces and suture lines | Visible bony ridges or suture depressions | Direct marker of fused or compensatory bone growth | 3D surface rendering, linear distance and ridge mapping |
| Cranial Vault Volume/Shape | Evenly distributed cranial volume | Regional flattening or bossing | Evaluates extent/location of vault deformity | Region-of-interest volumetry, surface deviation mapping |
| Orbital/Facial Displacement | Proportional, centered position of orbits/ears | Orbits, ears, or midface shifted/asymmetric | Detects associated orbital/ear involvement | Landmark-based measurement, 3D coordinate analysis |
3.5. Ultrasonography
- Direct Visualization: A patent suture is visualized as a hypoechoic gap between two echogenic bony plates, whereas a fused suture is identified by the loss of this gap and the presence of a continuous echogenic bony ridge [66]. The presence of acoustic shadowing (brain shadow sign) due to sound wave obstruction at closed sutures further enhances diagnostic accuracy [67].
- Clinical Strengths: US is portable, does not require sedation, and can be performed bedside, which minimizes stress for the child [70]. This modality proves particularly valuable for differentiating deformational plagiocephaly from true synostosis, potentially eliminating the need for additional imaging when suture patency is confirmed [71].
| US Feature | Normal Finding | Abnormal in CS | Clinical Utility | Imaging Plane/View or Technique |
|---|---|---|---|---|
| Suture Patency | Open, hypoechoic sutures | Absent, fused, or hyperechoic/narrowed line | Directly identifies fused sutures | Coronal or sagittal view over the suture |
| Suture Morphology | Thin, regular echogenic lines (<3 mm) | Thickened, irregular, or discontinuous suture | Suggests abnormal fusion or bone reaction | High-frequency linear probe, zoomed scan |
| Bone Edges/Overriding | Smooth, aligned cranial bones | Step-off, overriding bone at suture | Supports diagnosis, shows compensatory bone changes | Oblique sweep across suture lines |
| Cranial Contour | Regular, symmetric skull shape | Abnormal head shape (ridge, asymmetry, flattening) | Assesses severity and surgical indication | Panoramic scan or composite sweep |
3.6. Magnetic Resonance Imaging
- Advanced Bone Imaging Capabilities: Traditional MRI limitations in cortical bone visualization stemmed from extremely low free-water content and short T2 relaxation times [73]. Novel MRI sequences have overcome this drawback through several approaches:
- ○
- Zero Echo Time (ZTE) and Ultrashort Echo Time (UTE) sequences capture signals within microseconds after frequency excitation, capturing rapidly decaying signals from bound water in cortical bone [74]. These techniques enable high-contrast bone visualization producing 3DCT-comparable images of skull anatomy [19,20,21].
- ○
- Golden Angle Volumetric Interpolated Breath-hold Examination (GA-VIBE) offers motion-robust cranial bone imaging with reported sensitivity of 97% and specificity of 96% for detecting suture closure compared to standard 3DCT [20].
- ○
- Black Bone MRI (BBMRI) utilizes conventional sequences with parameter modifications, making it more widely available on existing MRI systems than specialized UTE/ZTE sequences [75]. This approach provides high-fidelity cranial reconstructions adequate for distinguishing craniosynostosis from positional deformities [76,77].
- Intracranial Assessment Advantages: MRI’s superior soft tissue contrast enables comprehensive evaluation of intracranial anatomy, revealing pathological details that other imaging modalities may not detect. This includes assessment of associated anomalies such as ventriculomegaly, Chiari malformation, and other developmental abnormalities commonly observed in CS [78,79,80]. These findings can significantly alter surgical planning and patient management strategies [41].
- Advanced Neuroimaging Capabilities: Diffusion Tensor Imaging (DTI) provides assessment of white matter tract integrity, organization, and development [4,47]. This MRI technique offers objective evaluation of the potential neurodevelopmental impact of cranial constraint by measuring the physical properties of neural pathways [4].
- Comprehensive Single-Session Assessment: The integration of advanced osseous imaging sequences with standard brain imaging protocols enables extensive evaluation of both skeletal and neural anatomy in a single examination session [84]. This approach updates diagnostic workflows while providing more complete anatomical information than traditional single-modality approaches [85,86]. The development of sophisticated machine learning frameworks can even synthesize high-resolution pseudo-CT images directly from MRI data, demonstrating excellent bone segmentation accuracy [87,88,89].
- Availability: Advanced MRI sequences for bone visualization (ZTE, UTE, GA-VIBE, BBMRI) are not yet widely available across all imaging centers, limiting their routine clinical implementation.
| MRI Feature | UTE | ZTE | GA-VIBE | BBMRI |
|---|---|---|---|---|
| Core Principle | Captures signal from tissues with ultra-short T2WI (bone) by using echo times < 1 ms | Uses effectively zero echo time to image tissues with extremely short T2WI (bone) | 3D T1-GRE with golden-angle radial sampling for improved motion robustness and bone contrast | 3D T1-GRE with short TE, low flip-angle for pronounced bone/soft tissue contrast |
| Bone Visualization | Excellent; shows cortical bone, high contrast vs. soft tissue | Excellent; shows bone with high contrast, “CT-like” appearance | Excellent; BBMRI-like, strong delineation of cortical bone | Very Good; bone edges and surfaces are highlighted, though less sensitive than UTE/ZTE |
| Soft Tissue Contrast | Moderate; focus is on bone/tendons, not ideal for soft tissue | Moderate; same as UTE | Good; provides usable soft tissue images along with bone | Good; can visualize soft tissue, but main use is for bone |
| 3D Isotropic Imaging | Yes, multiplanar and 3D modeling | Yes, 3D reconstructions | Yes, 3D surface renderings/virtual models | Yes, thin slices, 3D reconstructions easily obtained |
| Motion Robustness | Good; fast acquisition, but may still be susceptible to motion | Good; very fast acquisition, less motion sensitivity | Excellent; radial sampling is highly motion-resistant | Good; fast acquisition times reduce motion artifacts |
| Availability | Limited; needs dedicated hardware/software on MRI systems | Limited; mostly on high-end, research or new MRI systems | Variable; available on newer platforms, research, and pediatric centers | Increasing; can be implemented on many current MRI scanners |
| Limitations | Hardware/sequence availability; may produce noise if not optimized | Not widely available; complex post-processing often required | Mainly motion-resistance improvement and surface bone, less inner bone | Slightly lower bone detail vs. UTE/ZTE; parameter tuning required |
4. Discussion
5. The Arcana Protocol: An Integrated Clinical Algorithm
5.1. Protocol Structure and Clinical Algorithm
5.1.1. STEP 1: Universal Radiation-Free Screening
Applied to All Children with Suspected Craniosynostosis
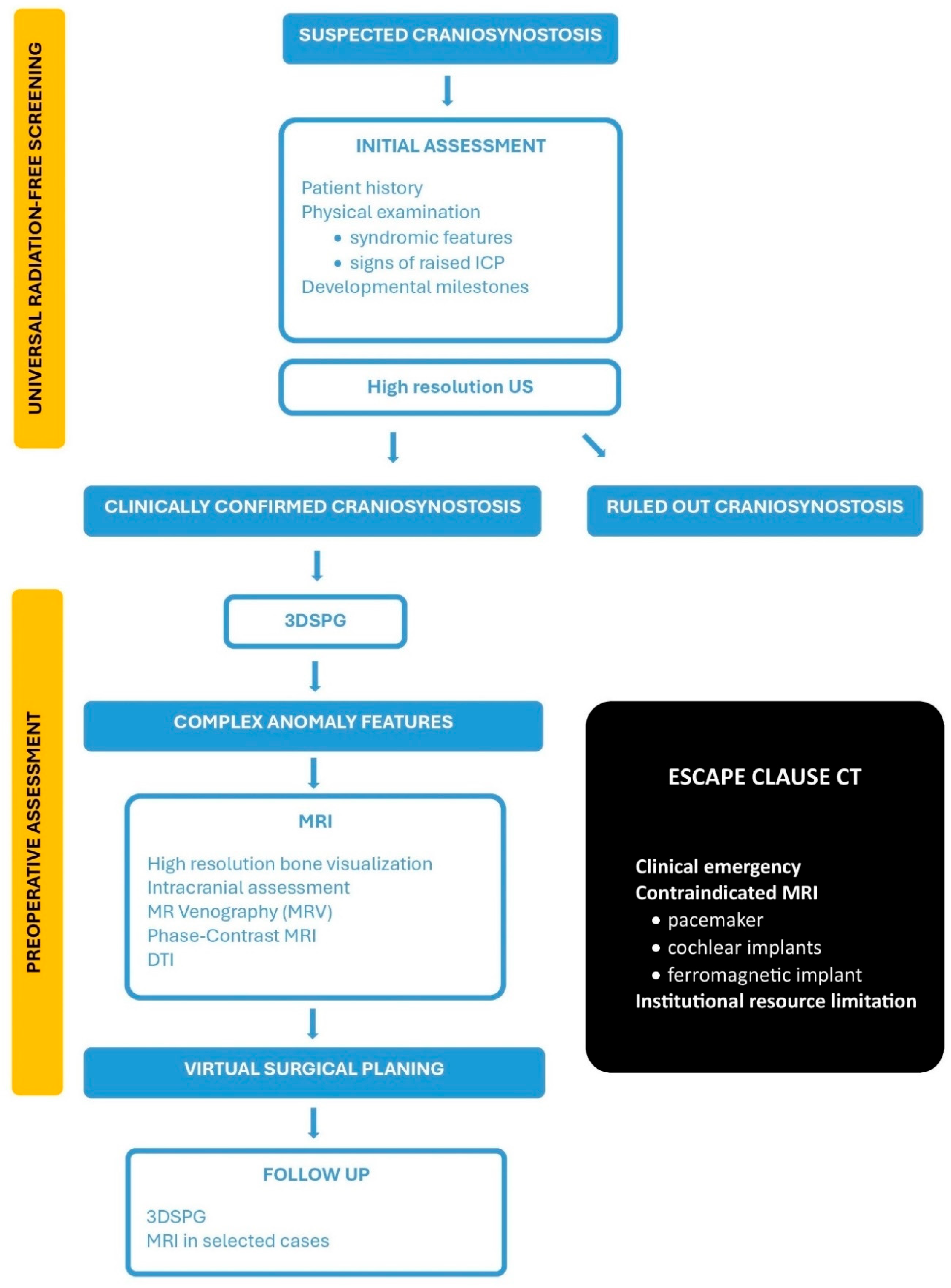
5.1.2. The Escape Clause: When 3DCT Is Justified?
- Complex Surgical Scenarios with Diagnostic UncertaintyThese scenarios are rare and involve patients whose comprehensive, radiation-free imaging yields inconclusive results, leading to significant diagnostic uncertainty and surgical risk. All available radiation-free alternatives must have been exhausted, and the information potentially gained from 3DCT must be both essential and unobtainable through other means.
- Absolute MRI ContraindicationsThese are patients with life-threatening contraindications to MRI, such as those with incompatible implanted devices (e.g., specific pacemakers, some cochlear implants, or other ferromagnetic devices) that preclude safe MRI scanning. Such cases are exceptionally rare in pediatric craniosynostosis but require comprehensive pre-scan screening and device compatibility verification.
- Institutional Resource LimitationsIn settings where advanced MRI sequences or specialized neuroradiological expertise are genuinely unavailable, 3DCT may be needed. This situation should be viewed as a temporary institutional limitation that is not acceptable in the long-term clinical practice. Educational initiatives must be established to provide specialized training for radiologists, neurosurgeons, and technical personnel, enabling them to master advanced MRI interpretation techniques and radiation-free diagnostic approaches.
5.1.3. Future Horizons for the Proposed ARCANA Protocol
5.1.4. Limitations of the Study and ARCANA Protocol
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lattanzi, W.; Barba, M.; Di Pietro, L.; Boyadjiev, S.A. Genetic advances in craniosynostosis. Am. J. Med. Genet. Part A 2017, 173, 1406–1429. [Google Scholar] [CrossRef]
- Massimi, L.; Bianchi, F.; Frassanito, P.; Calandrelli, R.; Tamburrini, G.; Caldarelli, M. Imaging in craniosynostosis: When and what? Child’s Nerv. Syst. 2019, 35, 2055–2069. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.D.; Yang, J.; Beckett, J.S.; Lacadie, C.; Scheinost, D.; Persing, S.; Zellner, E.G.; Oosting, D.; Keifer, C.; Friedman, H.E.; et al. Normalization of brain morphology after surgery in sagittal craniosynostosis. J. Neurosurg. Pediatr. 2016, 17, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Moscarelli, J.; Almeida, M.N.; Lacadie, C.; Hu, K.G.; Ihnat, J.M.H.; Parikh, N.; Persing, J.A.; Alperovich, M. A diffusion tensor imaging comparison of white matter development in nonsyndromic craniosynostosis to neurotypical infants. Child’s Nerv. Syst. 2024, 40, 1477–1487. [Google Scholar] [CrossRef]
- Brenner, D.J.; Elliston, C.D.; Hall, E.J.; Berdon, W.E. Estimated Risks of Radiation-Induced Fatal Cancer from Pediatric CT. Am. J. Roentgenol. 2001, 176, 289–296. [Google Scholar] [CrossRef]
- Kharbanda, A.B.; Krause, E.; Lu, Y.; Blumberg, K. Analysis of radiation dose to pediatric patients during computed tomography examinations. Acad. Emerg. Med. 2015, 22, 670–675. [Google Scholar] [CrossRef]
- Obara, H.; Takahashi, M.; Kudou, K.; Mariya, Y.; Takai, Y.; Kashiwakura, I. Estimation of effective doses in pediatric X-ray computed tomography examination. Exp. Ther. Med. 2017, 14, 4515–4520. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Bosch de Basea, M.; Thierry-Chef, I.; Harbron, R.; Hauptmann, M.; Byrnes, G.; Bernier, M.O.; Le Cornet, L.; Dabin, J.; Ferro, G.; Istad, T.S.; et al. Risk of hematological malignancies from CT radiation exposure in children, adolescents and young adults. Nat. Med. 2023, 29, 3111–3119. [Google Scholar] [CrossRef]
- Yeung, A.W.K. The “As Low As Reasonably Achievable” (ALARA) principle: A brief historical overview and a bibliometric analysis of the most cited publications. Radioprotection 2019, 54, 103–109. [Google Scholar] [CrossRef]
- Duncan, C.; Pears, N.E.; Dai, H.; Smith, W.A.P.; O’Higgins, P. Applications of 3D Photography in Craniofacial Surgery. J. Pediatr. Neurosci. 2022, 17 (Suppl. 1), S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Görg, C.; Elkhill, C.; Chaij, J.; Royalty, K.; Nguyen, P.D.; French, B.; Cruz-Guerrero, I.A.; Porras, A.R. SHAPE: A visual computing pipeline for interactive landmarking of 3D photograms and patient reporting for assessing craniosynostosis. Comput. Graph. 2024, 125, 104056. [Google Scholar] [CrossRef] [PubMed]
- Ho, O.A.; Saber, N.; Stephens, D.; Clausen, A.; Drake, J.; Forrest, C.; Phillips, J. Comparing the Use of 3D Photogrammetry and Computed Tomography in Assessing the Severity of Single-Suture Nonsyndromic Craniosynostosis. Plast. Surg. 2017, 25, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Mertens, C.; Wessel, E.; Berger, M.; Ristow, O.; Hoffmann, J.; Kansy, K.; Freudlsperger, C.; Bächli, H.; Engel, M. The value of three-dimensional photogrammetry in isolated sagittal synostosis: Impact of age and surgical technique on intracranial volume and cephalic index—a retrospective cohort study. J. Cranio-Maxillofac. Surg. 2017, 45, 2010–2016. [Google Scholar] [CrossRef]
- Rückschloß, T.; Zittel, S.; Hassanein, E.; El Damaty, A.; Krieg, S.M.; Ristow, O.; Hoffmann, J.; Engel, M. Photogrammetric evaluation of extended midline strip craniectomy with bilateral parietal osteotomies on frontal morphology in patients with isolated sagittal synostosis. Neurosurg. Focus. 2025, 58, E7. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, L.; Cao, D.; Izadikhah, I.; Gao, P.; Zhao, Y.; Yan, B. Accuracy of three-dimensional photogrammetry and cone beam computed tomography based on linear measurements in patients with facial deformities. Dentomaxillofacial Radiol. 2021, 50, 20200001. [Google Scholar] [CrossRef]
- Pogliani, L.M.; Zuccotti, G.V.; Reggiori, M.; Erbetta, A.; Lacerenza, M.; Prada, F.; Furlanetto, M.; Vetrano, I.G.; Valentini, L.G. Surface Cranial Ultrasound: The Natural Heir to X-Ray for the Screening of Skull Deformities in Infants. Ultraschall Der Med. Eur. J. Ultrasound 2022, 44, 503–511. [Google Scholar] [CrossRef]
- Rozovsky, K.; Udjus, K.; Wilson, N.; Barrowman, N.J.; Simanovsky, N.; Miller, E. Cranial Ultrasound as a First-Line Imaging Examination for Craniosynostosis. Pediatrics 2016, 137, e20152230. [Google Scholar] [CrossRef]
- Kamona, N.; Jones, B.C.; Lee, H.; Song, H.K.; Rajapakse, C.S.; Wagner, C.S.; Bartlett, S.P.; Wehrli, F.W. Cranial bone imaging using ultrashort echo-time bone-selective MRI as an alternative to gradient-echo based “black-bone” techniques. Magn. Reson. Mater. Phys. Biol. Med. 2023, 37, 83–92. [Google Scholar] [CrossRef]
- Patel, K.B.; Eldeniz, C.; Skolnick, G.B.; Jammalamadaka, U.; Commean, P.K.; Goyal, M.S.; Smyth, M.D.; An, H. 3D pediatric cranial bone imaging using high-resolution MRI for visualizing cranial sutures: A pilot study. J. Neurosurg. Pediatr. 2020, 26, 311–317. [Google Scholar] [CrossRef]
- Saarikko, A.; Mellanen, E.; Kuusela, L.; Leikola, J.; Karppinen, A.; Autti, T.; Virtanen, P.; Brandstack, N. Comparison of Black Bone MRI and 3D-CT in the preoperative evaluation of patients with craniosynostosis. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mathijssen, I.M.J. Updated Guideline on Treatment and Management of Craniosynostosis. J. Craniofacial Surg. 2021, 32, 371–450. [Google Scholar] [CrossRef] [PubMed]
- Bhalodia, R.; Dvoracek, L.A.; Ayyash, A.M.; Kavan, L.; Whitaker, R.; Goldstein, J.A. Quantifying the Severity of Metopic Craniosynostosis: A Pilot Study Application of Machine Learning in Craniofacial Surgery. J. Craniofacial Surg. 2020, 31, 697–701. [Google Scholar] [CrossRef]
- Kronig, S.A.J.; Kronig, O.D.M.; Vrooman, H.A.; Veenland, J.F.; Van Adrichem, L.N.A. New diagnostic approach of the different types of isolated craniosynostosis. Eur. J. Pediatr. 2020, 180, 1211–1217. [Google Scholar] [CrossRef]
- Fahradyan, A.; Daneshgaran, G.; Hoffman, T.L.; Wexler, A.; Francis, S.H. Challenging the Norm: Is Routine Use of Cranial CT in Evaluation of Craniosynostosis Necessary? J. Craniofacial Surg. 2021, 32, 2496–2499. [Google Scholar] [CrossRef]
- Meulepas, J.M.; Ronckers, C.M.; Smets, A.M.J.B.; Nievelstein, R.A.J.; Gradowska, P.; Lee, C.; Jahnen, A.; van Straten, M.; de Wit, M.-C.Y.; Zonnenberg, B.; et al. Radiation Exposure From Pediatric CT Scans and Subsequent Cancer Risk in The Netherlands. JNCI J. Natl. Cancer Inst. 2019, 111, 256–263. [Google Scholar] [CrossRef]
- Nakai, Y.; Miyazaki, O.; Kitamura, M.; Imai, R.; Okamoto, R.; Tsutsumi, Y.; Miyasaka, M.; Ogiwara, H.; Miura, H.; Yamada, K.; et al. Evaluation of radiation dose reduction in head CT using the half-dose method. Jpn. J. Radiol. 2023, 41, 872–881. [Google Scholar] [CrossRef]
- Zarella, C.; Didier, R.; Bergquist, C.; Bardo, D.M.E.; Selden, N.R.; Kuang, A.A. A Reduction in Radiation Exposure During Pediatric Craniofacial Computed Tomography. J. Craniofacial Surg. 2016, 27, 331–333. [Google Scholar] [CrossRef]
- Dindaroglu, F.; Yetkiner, E. Cone Beam Computed Tomography in Orthodontics. Turk. J. Orthod. 2016, 29, 16–21. [Google Scholar] [CrossRef]
- O’Sullivan, H.; Bracken, S.; Doyle, J.; Twomey, E.; Murray, D.J.; Kyne, L. X-rays had little value in diagnosing children’s abnormal skull shapes, and primary care clinicians should refer concerns to specialist teams. Acta Paediatr. 2020, 110, 1330–1334. [Google Scholar] [CrossRef]
- Alnaif, N.; Zhou, M.; Galli, R.; Azzi, A.J.; Alamri, A.; Gilardino, M. The Role of Preoperative Computed Tomography in Nonsyndromic Craniosynostosis. J. Craniofacial Surg. 2019, 30, 424–428. [Google Scholar] [CrossRef]
- Barreto, I.L.; Tuna, I.S.; Rajderkar, D.A.; Ching, J.A.; Governale, L.S. Pediatric craniosynostosis computed tomography: An institutional experience in reducing radiation dose while maintaining diagnostic image quality. Pediatr. Radiol. 2021, 52, 85–96. [Google Scholar] [CrossRef]
- Bruce, M.K.; Mittal, A.M.; Whitt, D.S.; Flom, L.L.; Pfaff, M.J.; Losee, J.E.; Goldstein, J.A. Computed tomography associated radiation exposure in children with craniosynostosis. Child’s Nerv. Syst. 2021, 37, 2635–2641. [Google Scholar] [CrossRef] [PubMed]
- da Silva Freitas, R.; de Freitas Azzolini, T.; Shin, J.H.; Persing, J.A. Associated (Parallel) Tomographic Findings in Patients With Single-Sutural Synostosis. J. Craniofacial Surg. 2010, 21, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, F.; Garcia-Gonzalez, O.; Szathmari, A.; Chauvel-Picard, J.; Beuriat, P.A.; Paulus, C.; Gleizal, A.; Mottolese, C. Emissary veins and pericerebral cerebrospinal fluid in trigonocephaly: Do they define a specific subtype? Child’s Nerv. Syst. 2021, 37, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.W.; Hulstaert, T.L.; Belsack, D.; Buls, N.; Van Gompel, G.; Nieboer, K.H.; Buyl, R.; Verhelle, F.; De Maeseneer, M.; de Mey, J. Dedicated sub 0.1 mSv 3DCT using MBIR in children with suspected craniosynostosis: Quality assessment. Eur. Radiol. 2015, 26, 892–899. [Google Scholar] [CrossRef]
- Fearon, J.A.; Singh, D.J.; Beals, S.P.; Yu, J.C. The Diagnosis and Treatment of Single-Sutural Synostoses: Are Computed Tomographic Scans Necessary? Plast. Reconstr. Surg. 2007, 120, 1327–1331. [Google Scholar] [CrossRef]
- He, K.; Boukind, A.; Sanka, A.S.; Ribaudo, J.G.; Chryssofos, S.; Skolnick, G.B.; Yaeger, L.B.; Thomas, A.M.; Mian, A.Y.; Patel, K.B. Systematic Review and Meta-Analysis of Radiation Dose Reduction Studies in Pediatric Head CT. Am. J. Neuroradiol. 2025, 46, 1875–1883. [Google Scholar] [CrossRef]
- Montoya, J.C.; Eckel, L.J.; DeLone, D.R.; Kotsenas, A.L.; Diehn, F.E.; Yu, L.; Bartley, A.C.; Carter, R.E.; McCollough, C.H.; Fletcher, J.G. Low-Dose CT for Craniosynostosis: Preserving Diagnostic Benefit with Substantial Radiation Dose Reduction. Am. J. Neuroradiol. 2017, 38, 672–677. [Google Scholar] [CrossRef]
- Ravindra, V.M.; Awad, A.-W.; Baker, C.M.; Lee, A.; Anderson, R.C.E.; Gociman, B.; Patel, K.B.; Smyth, M.D.; Birgfeld, C.; Pollack, I.F.; et al. Preoperative imaging patterns and intracranial findings in single-suture craniosynostosis: A study from the Synostosis Research Group. J. Neurosurg. Pediatr. 2021, 28, 344–350. [Google Scholar] [CrossRef]
- Sheppard, J.P.; Nguyen, T.; Alkhalid, Y.; Beckett, J.S.; Salamon, N.; Yang, I. Risk of Brain Tumor Induction from Pediatric Head CT Procedures: A Systematic Literature Review. Brain Tumor Res. Treat. 2018, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Goetti, R. Evaluation of ultra-low-dose CT with tin filter for craniosynostosis. J. Med. Imaging Radiat. Oncol. 2024, 69, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Vannier, M.W.; Hildebolt, C.F.; Marsh, J.L.; Pilgram, T.K.; McAlister, W.H.; Shackelford, G.D.; Offutt, C.J.; Knapp, R.H. Craniosynostosis: Diagnostic value of three-dimensional CT reconstruction. Radiology 1989, 173, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, Y.; Choi, Y.H.; Lee, S.B.; Lee, S.; Cho, Y.J.; Shin, S.-M.; Phi, J.H.; Kim, S.K.; Cheon, J.-E. Ultra-low-dose computed tomography with deep learning reconstruction for craniosynostosis at radiation doses comparable to skull radiographs: A pilot study. Pediatr. Radiol. 2023, 53, 2260–2268. [Google Scholar] [CrossRef]
- Uffmann, M.; Schaefer-Prokop, C. Digital radiography: The balance between image quality and required radiation dose. Eur. J. Radiol. 2009, 72, 202–208. [Google Scholar] [CrossRef]
- Russo, C.; Aliberti, F.; Ferrara, U.P.; Russo, C.; De Gennaro, D.V.; Cristofano, A.; Nastro, A.; Cicala, D.; Spennato, P.; Quarantelli, M.; et al. Neuroimaging in Nonsyndromic Craniosynostosis: Key Concepts to Unlock Innovation. Diagnostics 2024, 14, 1842. [Google Scholar] [CrossRef]
- Abdel-Alim, T.; Kurniawan, M.; Mathijssen, I.; Dremmen, M.; Dirven, C.; Niessen, W.; Roshchupkin, G.; van Veelen, M.-L. Sagittal Craniosynostosis: Comparing Surgical Techniques Using 3D Photogrammetry. Plast. Reconstr. Surg. 2023, 152, 675e–688e. [Google Scholar] [CrossRef]
- Abdel-Alim, T.; Iping, R.; Wolvius, E.B.; Mathijssen, I.M.J.; Dirven, C.M.F.; Niessen, W.J.; van Veelen, M.-L.C.; Roshchupkin, G.V. Three-Dimensional Stereophotogrammetry in the Evaluation of Craniosynostosis: Current and Potential Use Cases. J. Craniofacial Surg. 2021, 32, 956–963. [Google Scholar] [CrossRef]
- de Jong, G.; Bijlsma, E.; Meulstee, J.; Wennen, M.; van Lindert, E.; Maal, T.; Aquarius, R.; Delye, H. Combining deep learning with 3D stereophotogrammetry for craniosynostosis diagnosis. Sci. Rep. 2020, 10, 15346. [Google Scholar] [CrossRef]
- Ramsey, J.A.; Stevens, P.M.; Coats, B.; Dixon, T.J.; Chaker, S.C.; Bonfield, C.M.; Golinko, M.S. Comprehensive craniometry for sagittal synostosis. Neurosurg. Focus. 2025, 58, E8. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.K.; Tao, W.; Beiriger, J.; Christensen, C.; Pfaff, M.J.; Whitaker, R.; Goldstein, J.A. 3D Photography to Quantify the Severity of Metopic Craniosynostosis. Cleft Palate Craniofacial J. 2022, 60, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Somorin, T.J.; Kueper, J.; Dixon, A.; Kass, N.; Khan, N.; Iyer, K.; Wagoner, J.; Rogers, A.; Whitaker, R.; et al. Quantifying Sagittal Craniosynostosis Severity: A Machine Learning Approach With CranioRate. Cleft Palate Craniofacial J. 2025. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Alim, T.; Tapia Chaca, F.; Mathijssen, I.M.J.; Dirven, C.M.F.; Niessen, W.J.; Wolvius, E.B.; van Veelen, M.L.C.; Roshchupkin, G.V. Quantifying dysmorphologies of the neurocranium using artificial neural networks. J. Anat. 2024, 245, 903–913. [Google Scholar] [CrossRef]
- Porras, A.R.; Tu, L.; Tsering, D.; Mantilla, E.; Oh, A.; Enquobahrie, A.; Keating, R.; Rogers, G.F.; Linguraru, M.G. Quantification of Head Shape from Three-Dimensional Photography for Presurgical and Postsurgical Evaluation of Craniosynostosis. Plast. Reconstr. Surg. 2019, 144, 1051e–1060e. [Google Scholar] [CrossRef]
- Meulstee, J.W.; Verhamme, L.M.; Borstlap, W.A.; Van der Heijden, F.; De Jong, G.A.; Xi, T.; Bergé, S.J.; Delye, H.; Maal, T.J.J. A new method for three-dimensional evaluation of the cranial shape and the automatic identification of craniosynostosis using 3D stereophotogrammetry. Int. J. Oral. Maxillofac. Surg. 2017, 46, 819–826. [Google Scholar] [CrossRef]
- Petrides, G.; Clark, J.R.; Low, H.; Lovell, N.; Eviston, T.J. Three-dimensional scanners for soft-tissue facial assessment in clinical practice. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 605–614. [Google Scholar] [CrossRef]
- Azimi, N.; Talebi Rafsanjan, K.; Khanmohammadi Khorami, M.M.; Ebadifar, A.; Azadi, A. Applications of Machine Learning in Image Analysis to Identify Craniosynostosis: A Systematic Review and Meta-Analysis. Orthod. Craniofacial Res. 2025, 28, 733–751. [Google Scholar] [CrossRef]
- Elkhill, C.; Liu, J.; Linguraru, M.G.; LeBeau, S.; Khechoyan, D.; French, B.; Porras, A.R. Geometric learning and statistical modeling for surgical outcomes evaluation in craniosynostosis using 3D photogrammetry. Comput. Methods Programs Biomed. 2023, 240, 107689. [Google Scholar] [CrossRef]
- Kuehle, R.; Ringwald, F.; Bouffleur, F.; Hagen, N.; Schaufelberger, M.; Nahm, W.; Hoffmann, J.; Freudlsperger, C.; Engel, M.; Eisenmann, U. The Use of Artificial Intelligence for the Classification of Craniofacial Deformities. J. Clin. Med. 2023, 12, 7082. [Google Scholar] [CrossRef]
- Barbero-García, I.; Lerma, J.L.; Marqués-Mateu, Á.; Miranda, P. Low-Cost Smartphone-Based Photogrammetry for the Analysis of Cranial Deformation in Infants. World Neurosurg. 2017, 102, 545–554. [Google Scholar] [CrossRef]
- Beiriger, J.W.; Tao, W.; Bruce, M.K.; Anstadt, E.; Christensen, C.; Smetona, J.; Whitaker, R.; Goldstein, J.A. CranioRate: An Image-Based, Deep-Phenotyping Analysis Toolset and Online Clinician Interface for Metopic Craniosynostosis. Plast. Reconstr. Surg. 2023, 153, 112e–119e. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.M.; Edison, R.L.; Hallac, R.R. Artificial Intelligence Applications in Pediatric Craniofacial Surgery. Diagnostics 2025, 15, 829. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.M.; Zhurov, A.; Playle, R.; Ong, E.; Richmond, S. Reproducibility of facial soft tissue landmarks on 3D laser-scanned facial images. Orthod. Craniofacial Res. 2009, 12, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Proisy, M.; Bruneau, B.; Riffaud, L. How ultrasonography can contribute to diagnosis of craniosynostosis. Neurochirurgie 2019, 65, 228–231. [Google Scholar] [CrossRef]
- Pogliani, L.; Zuccotti, G.V.; Furlanetto, M.; Giudici, V.; Erbetta, A.; Chiapparini, L.; Valentini, L. Cranial ultrasound is a reliable first step imaging in children with suspected craniosynostosis. Child’s Nerv. Syst. 2017, 33, 1545–1552. [Google Scholar] [CrossRef]
- Krajden Haratz, K.; Leibovitz, Z.; Svirsky, R.; Drummond, C.L.; Lev, D.; Gindes, L.; Lerman-Sagie, T.; Malinger, G. The ‘Brain Shadowing Sign’: A Novel Marker of Fetal Craniosynostosis. Fetal Diagn. Ther. 2016, 40, 277–284. [Google Scholar] [CrossRef]
- Hall, K.M.; Besachio, D.A.; Moore, M.D.; Mora, A.J.; Carter, W.R. Effectiveness of screening for craniosynostosis with ultrasound: A retrospective review. Pediatr. Radiol. 2017, 47, 606–612. [Google Scholar] [CrossRef]
- Whittall, I.; Lambert, W.A.; Moote, D.J.; Bookland, M.J.; Martin, J.E.; Hughes, C.D.; Hersh, D.S. Postnatal diagnosis of single-suture craniosynostosis with cranial ultrasound: A systematic review. Child’s Nerv. Syst. 2021, 37, 3705–3714. [Google Scholar] [CrossRef]
- Proisy, M.; Riffaud, L.; Chouklati, K.; Tréguier, C.; Bruneau, B. Ultrasonography for the diagnosis of craniosynostosis. Eur. J. Radiol. 2017, 90, 250–255. [Google Scholar] [CrossRef]
- Marino, S.; Ruggieri, M.; Marino, L.; Falsaperla, R. Sutures ultrasound: Useful diagnostic screening for posterior plagiocephaly. Child’s Nerv. Syst. 2021, 37, 3715–3720. [Google Scholar] [CrossRef]
- Soboleski, D.; Mussari, B.; McCloskey, D.; Sauerbrei, E.; Espinosa, F.; Fletcher, A. High-resolution sonography of the abnormal cranial suture. Pediatr. Radiol. 1998, 28, 79–82. [Google Scholar] [CrossRef] [PubMed]
- More, S.S.; Zhang, X. Ultrashort Echo Time and Zero Echo Time MRI and Their Applications at High Magnetic Fields: A Literature Survey. Investig. Magn. Reson. Imaging 2024, 28, 153. [Google Scholar] [CrossRef]
- Kobayashi, N.; Bambach, S.; Ho, M.-L. Ultrashort Echo-Time MR Imaging of the Pediatric Head and Neck. Magn. Reson. Imaging Clin. North. Am. 2021, 29, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Syrmos, N.C.; Magdum, S.A. Imaging in Craniofacial Disorders With Special Emphasis on Gradient Echo Black-Bone and Zero Time Echo MRI Sequences. J. Pediatr. Neurosci. 2022, 17 (Suppl. 1), S14–S20. [Google Scholar] [CrossRef]
- Kuusela, L.; Hukki, A.; Brandstack, N.; Autti, T.; Leikola, J.; Saarikko, A. Use of black-bone MRI in the diagnosis of the patients with posterior plagiocephaly. Child’s Nerv. Syst. 2018, 34, 1383–1389. [Google Scholar] [CrossRef]
- Valeggia, S.; Dremmen, M.H.G.; Mathijssen, I.M.J.; Gaillard, L.; Manara, R.; Ceccato, R.; van Hattem, M.; Gahrmann, R. Black Bone MRI vs. CT in temporal bone assessment in craniosynostosis: A radiation-free alternative. Neuroradiology 2024, 67, 257–267. [Google Scholar] [CrossRef]
- Cuthbert, H.; Gallo, P.; Galloway, L.; Goel, A.; Afshari, F.T.; Solanki, G.A.; Rodrigues, D.; Gagen, R.; Pepper, J. Occult craniosynostosis in normocephalic children with Chiari I malformation. J. Neuroradiol. 2025, 52, 101336. [Google Scholar] [CrossRef]
- Strahle, J.; Muraszko, K.M.; Buchman, S.R.; Kapurch, J.; Garton, H.J.L.; Maher, C.O. Chiari malformation associated with craniosynostosis. Neurosurg. Focus. 2011, 31, E2. [Google Scholar] [CrossRef]
- Zavala, C.A.; Zima, L.A.; Greives, M.R.; Fletcher, S.A.; Shah, M.N.; Miller, B.A.; Sandberg, D.I.; Nguyen, P.D. Can Craniosynostosis be Diagnosed on Physical Examination? A Retrospective Review. J. Craniofacial Surg. 2023, 34, 2046–2050. [Google Scholar] [CrossRef]
- De Martino, L.; Mirabelli, P.; Quaglietta, L.; Ferrara, U.P.; Picariello, S.; De Gennaro, D.V.; Aiello, M.; Smaldone, G.; Aliberti, F.; Spennato, P.; et al. Biobank for craniosynostosis and faciocraniosynostosis, rare pediatric congenital craniofacial disorders: A study protocol. Child’s Nerv. Syst. 2024, 40, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Cinalli, G.; Russo, C.; Vitulli, F.; Parlato, R.S.; Spennato, P.; Imperato, A.; Quarantelli, M.; Covelli, E.; Aliberti, F. Changes in venous drainage after posterior cranial vault distraction and foramen magnum decompression in syndromic craniosynostosis. J. Neurosurg. Pediatr. 2022, 30, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Doerga, P.N.; Lequin, M.H.; Dremmen, M.H.G.; den Ottelander, B.K.; Mauff, K.A.L.; Wagner, M.W.; Hernandez-Tamames, J.A.; Versnel, S.L.; Joosten, K.F.M.; van Veelen, M.-L.C.; et al. Cerebral blood flow in children with syndromic craniosynostosis: Cohort arterial spin labeling studies. J. Neurosurg. Pediatr. 2020, 25, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Florkow, M.C.; Willemsen, K.; Mascarenhas, V.V.; Oei, E.H.G.; van Stralen, M.; Seevinck, P.R. Magnetic Resonance Imaging Versus Computed Tomography for Three-Dimensional Bone Imaging of Musculoskeletal Pathologies: A Review. J. Magn. Reson. Imaging 2022, 56, 11–34. [Google Scholar] [CrossRef]
- Low, X.Z.; Lim, M.C.; Nga, V.; Sundar, G.; Tan, A.P. Clinical application of “black bone” imaging in paediatric craniofacial disorders. Br. J. Radiol. 2021, 94, 20200061. [Google Scholar] [CrossRef]
- Tan, A.P. MRI Protocol for Craniosynostosis: Replacing Ionizing Radiation–Based CT. Am. J. Roentgenol. 2019, 213, 1374–1380. [Google Scholar] [CrossRef]
- Eshraghi Boroojeni, P.; Chen, Y.; Commean, P.K.; Eldeniz, C.; Skolnick, G.B.; Merrill, C.; Patel, K.B.; An, H. Deep-learning synthesized pseudo-CT for MR high-resolution pediatric cranial bone imaging (MR-HiPCB). Magn. Reson. Med. 2022, 88, 2285–2297. [Google Scholar] [CrossRef]
- Goh, C.Y.K.; Mohamed Ali, P.S.; Lee, K.H.C.; Sim, F.Y.; Chong, L.R. 3D MRI with CT-like bone contrast (3D-BONE): A pictorial review of clinical applications. Acta Radiol. 2025, 66, 228–240. [Google Scholar] [CrossRef]
- Leonhardt, Y.; Kronthaler, S.; Feuerriegel, G.; Karampinos, D.C.; Schwaiger, B.J.; Pfeiffer, D.; Makowski, M.R.; Koerte, I.K.; Liebig, T.; Woertler, K.; et al. CT-like MR-derived Images for the Assessment of Craniosynostosis and other Pathologies of the Pediatric Skull. Clin. Neuroradiol. 2022, 33, 57–64. [Google Scholar] [CrossRef]
- Eley, K.A.; Delso, G. Automated 3D MRI rendering of the craniofacial skeleton: Using ZTE to drive the segmentation of black bone and FIESTA-C images. Neuroradiology 2020, 63, 91–98. [Google Scholar] [CrossRef]
- Lethaus, B.; Gruichev, D.; Gräfe, D.; Bartella, A.K.; Hahnel, S.; Yovev, T.; Pausch, N.C.; Krause, M. “Black bone”: The new backbone in CAD/CAM-assisted craniosynostosis surgery? Acta Neurochir. 2020, 163, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Ducis, K.; Florman, J.E.; Rughani, A.I. Appraisal of the Quality of Neurosurgery Clinical Practice Guidelines. World Neurosurg. 2016, 90, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.; de Jong, G.; Rovers, M.; Westert, G.; Bartels, R. Importance and Presence of High-Quality Evidence for Clinical Decisions in Neurosurgery: International Survey of Neurosurgeons. Interact. J. Med. Res. 2018, 7, e16. [Google Scholar] [CrossRef] [PubMed]
- Kocabalkanli, C.; Fenton, R.; Gaetani, S.; Aalamifar, F.; Linguraru, M.G.; Seifabadi, R. Accuracy of Cranial Shape Measurements Using Smartphones: A Prospective Study. Cleft Palate Craniofacial J. 2024, 62, 1592–1597. [Google Scholar] [CrossRef]
- Colletti, G.; Di Bartolomeo, M.; Negrello, S.; Geronemus, R.G.; Cohen, B.; Chiarini, L.; Anesi, A.; Femino, R.; Mariotti, I.; Levitin, G.M.; et al. Multiple General Anesthesia in Children: A Systematic Review of Its Effect on Neurodevelopment. J. Pers. Med. 2023, 13, 867. [Google Scholar] [CrossRef]
- Grabowski, J.; Goldin, A.; Arthur, L.G.; Beres, A.L.; Guner, Y.S.; Hu, Y.Y.; Kawaguchi, A.L.; Kelley-Quon, L.I.; McAteer, J.P.; Miniati, D.; et al. The effects of early anesthesia on neurodevelopment: A systematic review. J. Pediatr. Surg. 2021, 56, 851–861. [Google Scholar] [CrossRef]
- Xiao, A.; Feng, Y.; Yu, S.; Xu, C.; Chen, J.; Wang, T.; Xiao, W. General anesthesia in children and long-term neurodevelopmental deficits: A systematic review. Front. Mol. Neurosci. 2022, 15, 972025. [Google Scholar] [CrossRef]
- Moltoni, G.; Lucignani, G.; Sgrò, S.; Guarnera, A.; Rossi Espagnet, M.C.; Dellepiane, F.; Carducci, C.; Liberi, S.; Iacoella, E.; Evangelisti, G.; et al. MRI scan with the “feed and wrap” technique and with an optimized anesthesia protocol: A retrospective analysis of a single-center experience. Front. Pediatr. 2024, 12, 1415603. [Google Scholar] [CrossRef]
- Liu, J.; Elkhill, C.; LeBeau, S.; French, B.; Lepore, N.; Linguraru, M.G.; Porras, A.R. Data-driven Normative Reference of Pediatric Cranial Bone Development. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4457. [Google Scholar] [CrossRef]
- Robertson, E.; Boulanger, P.; Kwan, P.; Louie, G.; Aalto, D. Improving Cranial Vault Remodeling for Unilateral Coronal Craniosynostosis—Introducing Automated Surgical Planning. Craniomaxillofacial Trauma. Reconstr. 2024, 17, 203–213. [Google Scholar] [CrossRef]
- Sharma, D.; Holden, A.M.; Nezamivand-Chegini, S. Augmented Reality Integration in Surgery for Craniosynostoses: Advancing Precision in the Management of Craniofacial Deformities. J. Clin. Med. 2025, 14, 4359. [Google Scholar] [CrossRef]
- Soldozy, S.; Yağmurlu, K.; Akyeampong, D.K.; Burke, R.; Morgenstern, P.F.; Keating, R.F.; Black, J.S.; Jane, J.A.; Syed, H.R. Three-dimensional printing and craniosynostosis surgery. Child’s Nerv. Syst. 2021, 37, 2487–2495. [Google Scholar] [CrossRef]
- Yoshida, M.M.; Freitas, A.L.P.d.; Carvalho Júnior, J.d.C.; Souza, J.S.d.; Baptista, V.S.; Ferreira, L.M. Holographic model of craniosynostosis for HoloLens. Acta Cirúrgica Bras. 2025, 40, e404825. [Google Scholar] [CrossRef]
| Outcome | Modality | Certainty of Evidence | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Comments |
|---|---|---|---|---|---|---|---|---|
| Suture Visualization | 3DCT | High | Low | Low | Low | Low | Low | Highly accurate, widely used as gold standard |
| MRI | Moderate | Low | Moderate | Low | Moderate | Low | Advanced sequences show promising accuracy; availability varies | |
| US | High | Moderate | Moderate | Low | Moderate | Moderate | High sensitivity in infants | |
| 3DSPG | Low | High | High | Low | High | Moderate | Only assesses external morphology; cannot visualize sutures | |
| Bone Thickness and Density | 3DCT | High | Low | Low | Low | Low | Low | High-resolution assessment of cortical bone with quantitative tools |
| MRI | Moderate | Moderate | Moderate | Low | Moderate | Moderate | Emerging sequences effective, but less validated | |
| US | Low | High | High | High | High | High | Cannot quantify bone density | |
| 3DSPG | Low | High | High | High | High | High | Shape surface imaging only | |
| Intracranial Anatomy Visualization | 3DCT | Moderate | Moderate | Moderate | Low | Low | Low | Limited soft tissue visualization |
| MRI | High | Low | Low | Low | Low | Low | Excellent detailed visualization of brain, CSF, venous structures | |
| US | Moderate | High | High | Low | High | Moderate | Limited brain visualization, only while the fontanel is opened; not suitable for deep structures, close to the skull base | |
| 3DSPG | Low | High | High | High | High | High | No intracranial data available | |
| Radiation Safety | 3DCT | Low | Low | Low | Low | Low | Low | Highest ionizing radiation burden; concern in pediatrics |
| MRI | High | Low | Low | Low | Low | Low | No radiation | |
| US | High | Low | Low | Low | Low | Low | No radiation; easy repeated use | |
| 3DSPG | High | Low | Low | Low | Low | Low | No radiation; rapid acquisition | |
| Surgical Planning Capability | 3DCT | High | Low | Low | Low | Low | Low | Detailed bone 3D modeling |
| MRI | Moderate | Moderate | Moderate | Low | Moderate | Low | MRI-based VSP emerging | |
| Ultrasound | Low | High | High | Low | Moderate | Moderate | Very limited surgical usefulness | |
| 3DSPG | Low | High | High | Low | Moderate | Moderate | Useful for pre/post-op morphology tracking only |
| 3DCT Feature | Normal Finding | Abnormal in CS | Clinical Utility | Acquisition/Interpretation Method |
|---|---|---|---|---|
| Suture Visualization | Patent cranial sutures, clear suture lines | Suture fusion, absent suture, bony ridge formation | Direct identification of fused sutures | Multiplanar/3D volume rendering |
| Skull Morphology | Smooth cranial contours and adequate volume | Regional flattening, bossing, abnormal cranial shape | Objective measurement of cranial deformity | High-resolution 3D surface model reconstruction |
| Bone Thickness and Density | Normal cortical thickness and uniform bone density | Areas of thickening, abnormal density, suture overlap | Evaluation of structural changes associated with craniosynostosis | Hounsfield unit analysis, bone window adjustment |
| Surgical Planning Capability | Not applicable | Detailed mapping for osteotomies, bone defect visualization | Preoperative planning, intraoperative navigation | 3DCT models with/without surgical simulation software |
| Intracranial Visualization | Mainly osseous anatomy, basic brain anatomy | Indirect findings, basic brain anatomy | Limited visualization of brain morphology and dynamics | Not primary modality for soft-tissue/brain analysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micovic, M.; Zivkovic, B.; Vukasinovic, I.; Jelovac, D.; Stojicic, M.; Bascarevic, V. Imaging Modalities in Craniosynostosis: A Systematic Review and Proposal of the ARCANA Protocol for Multimodal Radiation-Free Assessment. Diagnostics 2025, 15, 2632. https://doi.org/10.3390/diagnostics15202632
Micovic M, Zivkovic B, Vukasinovic I, Jelovac D, Stojicic M, Bascarevic V. Imaging Modalities in Craniosynostosis: A Systematic Review and Proposal of the ARCANA Protocol for Multimodal Radiation-Free Assessment. Diagnostics. 2025; 15(20):2632. https://doi.org/10.3390/diagnostics15202632
Chicago/Turabian StyleMicovic, Mirko, Bojana Zivkovic, Ivan Vukasinovic, Drago Jelovac, Milan Stojicic, and Vladimir Bascarevic. 2025. "Imaging Modalities in Craniosynostosis: A Systematic Review and Proposal of the ARCANA Protocol for Multimodal Radiation-Free Assessment" Diagnostics 15, no. 20: 2632. https://doi.org/10.3390/diagnostics15202632
APA StyleMicovic, M., Zivkovic, B., Vukasinovic, I., Jelovac, D., Stojicic, M., & Bascarevic, V. (2025). Imaging Modalities in Craniosynostosis: A Systematic Review and Proposal of the ARCANA Protocol for Multimodal Radiation-Free Assessment. Diagnostics, 15(20), 2632. https://doi.org/10.3390/diagnostics15202632






