Hematological Malignancy in a Hypophysectomised Acromegalic Patient Under 4-Year Therapy with Somatostatin Analogues: From a Rib Lump Underlying Bone Plasmatocytoma Features to Multiple Myeloma
Abstract
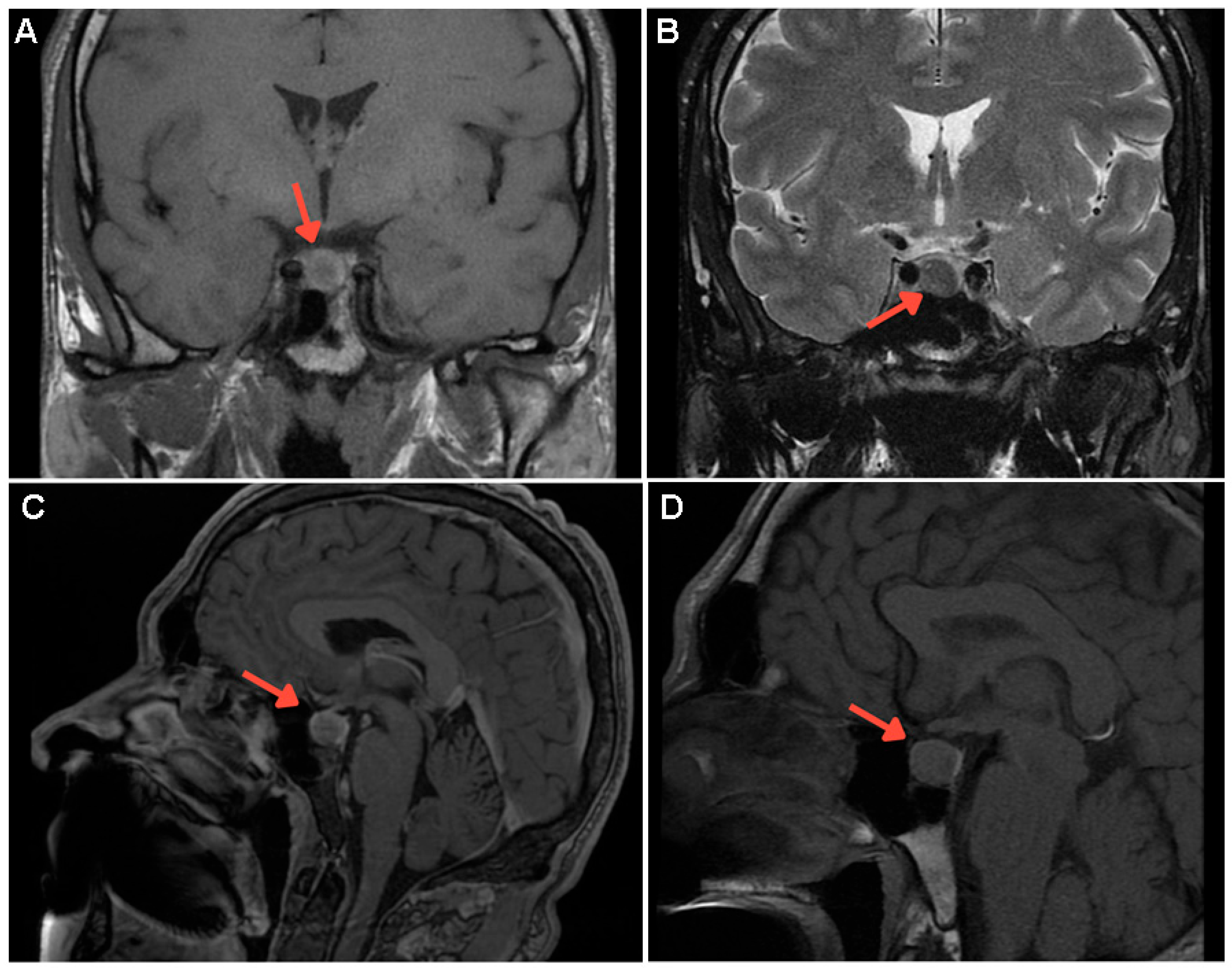
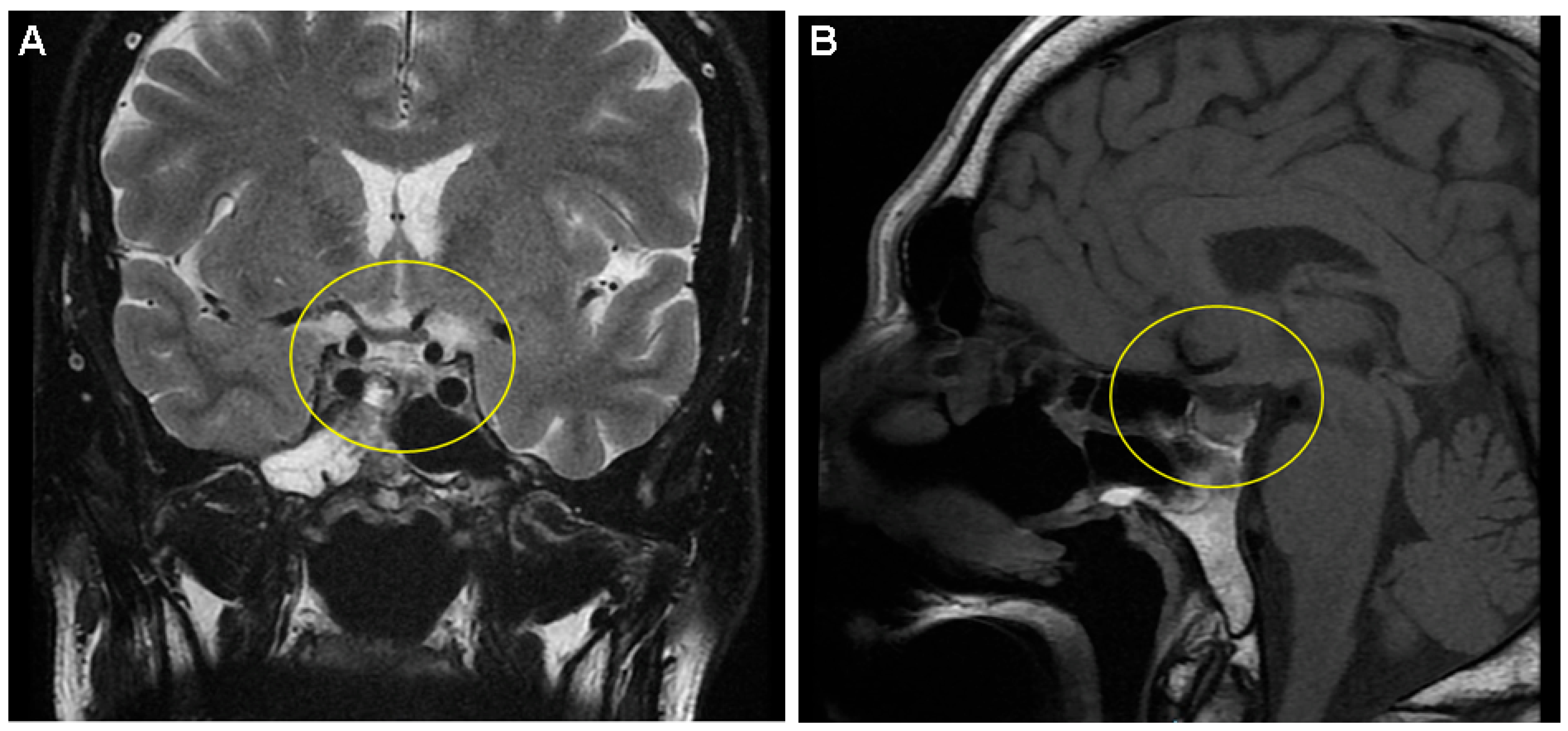
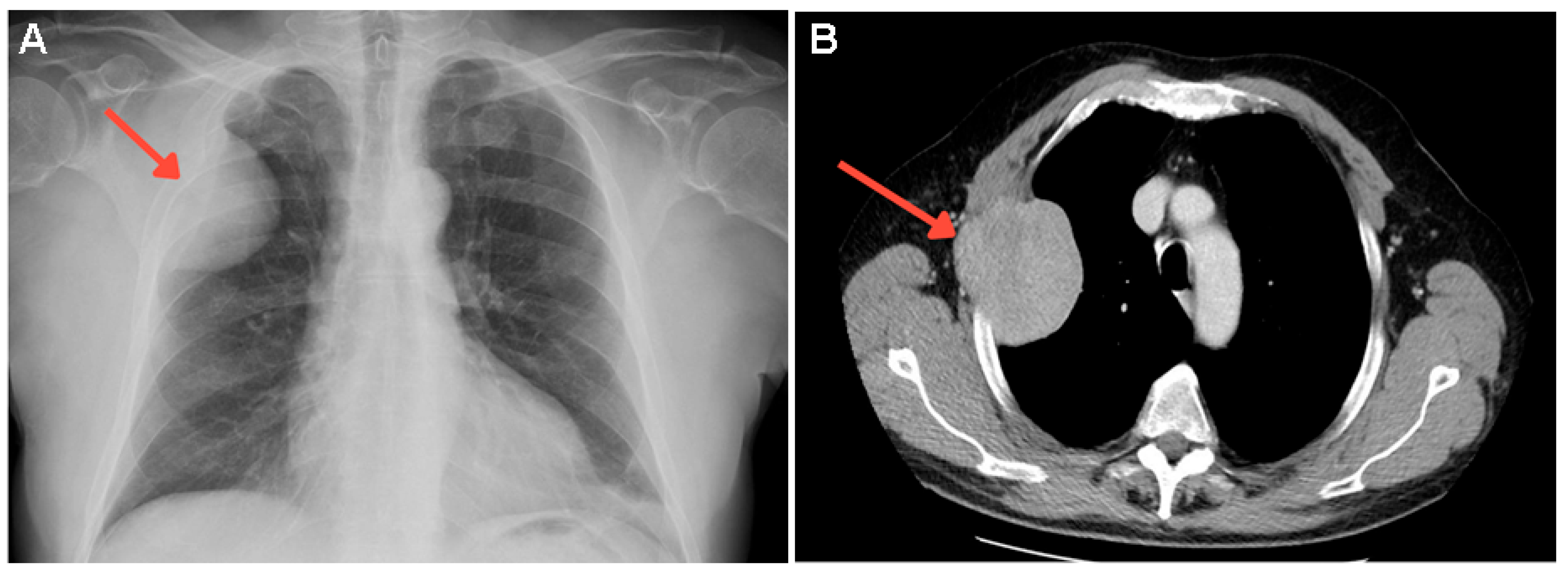
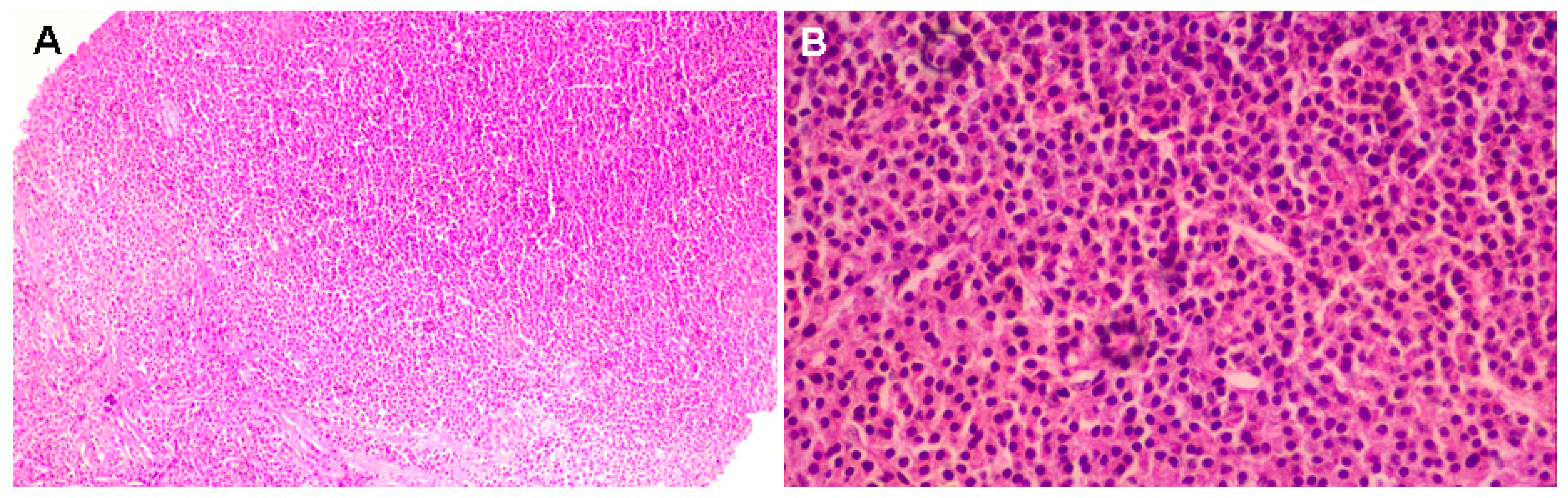


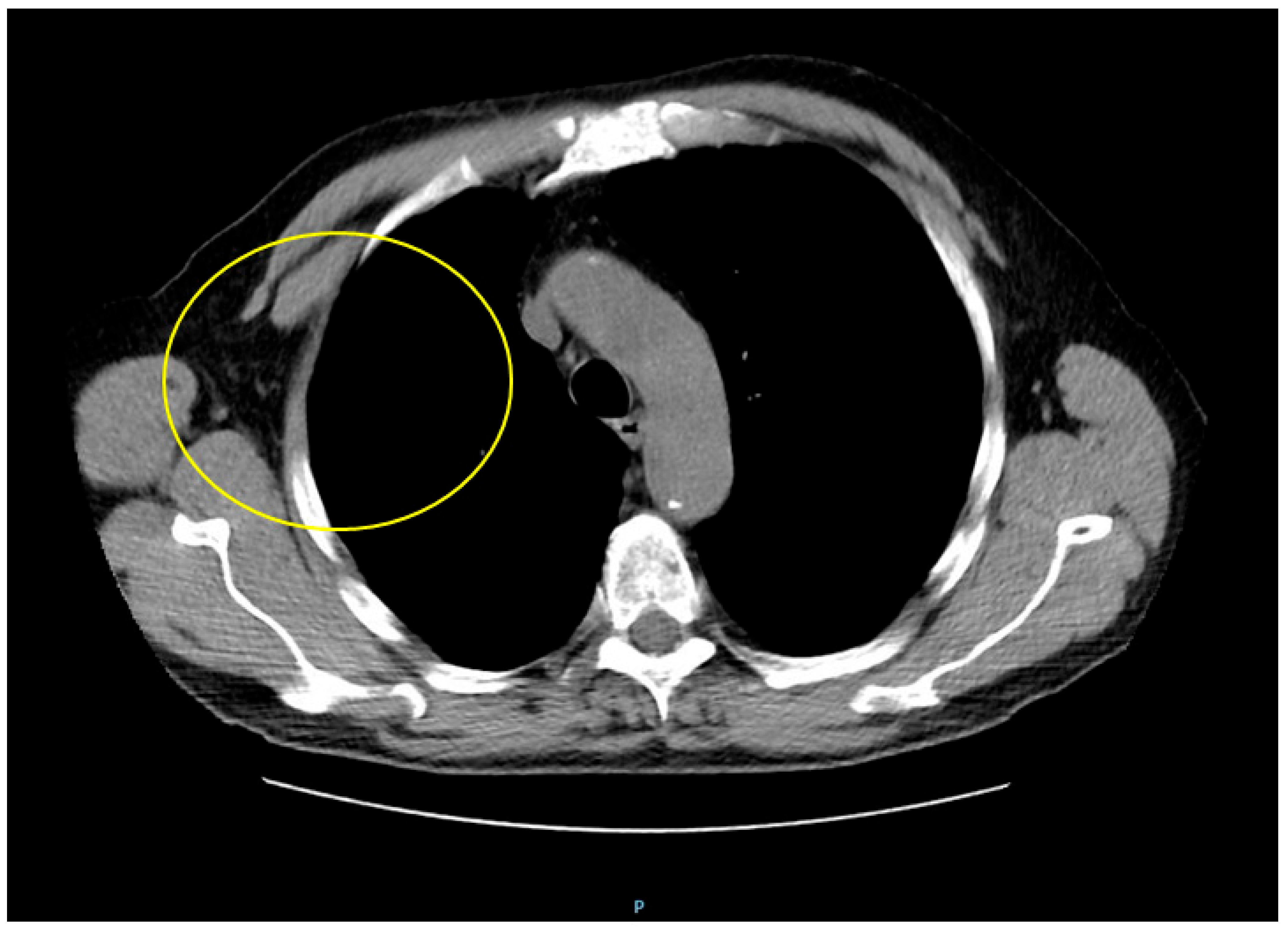
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| ASCT | autologous hematopoietic stem-cell transplantation |
| cm | centimetre |
| CECT | contrast-enhanced computed tomography |
| CRAB | hypercalcaemia, renal failure, anaemia, lytic bone lesions |
| CDKN1B | cyclin-dependent kinase inhibitor |
| DW-MRI | whole-body diffusion-weighted magnetic resonance imaging. |
| FSH | follicle-stimulating hormone |
| FDG | fluorodeoxyglucose |
| GH | growth hormone |
| GIST | gastrointestinal stromal tumour |
| IGF-1 | insulin-like growth factor |
| IGF-1R | IGF-1 receptor |
| IGFBP-3 | insulin-like growth factor binding protein type 3 |
| Ig | immunoglobulin |
| LAR | long-acting release |
| LH | luteinizing hormone |
| MRI | magnetic resonance imagining |
| MGG | May–Grunwald Giemsa |
| MEN | Multiple Endocrine Neoplasia |
| MGUS | monoclonal gammapathy of undetermined significance |
| MAPK | mitogen activated protein kinase |
| NA | not available |
| OGTT | oral glucose tolerance test |
| PTH | parathyroid hormone |
| PTHrP | parathyroid hormone-related peptide |
| PET/CT | positron emission tomography/computed tomography |
| PI-3K | phosphatidylinositol-3′-kinase |
| s.c | subcutaneous |
| sFLC | serum-free light chain |
| TSH | thyroid stimulating hormone |
| T4 | thyroxine |
| VRD | lenalidomide, low-dose dexamethasone, and bortezomib |
References
- Esposito, D.; Ragnarsson, O.; Johannsson, G.; Olsson, D.S. Incidence of Benign and Malignant Tumors in Patients With Acromegaly Is Increased: A Nationwide Population-based Study. J. Clin. Endocrinol. Metab. 2021, 106, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Langlois, F.; Lim, D.S.T.; Varlamov, E.V.; Melmed, S. Acromegaly: Pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 2022, 10, 804–826. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xiao, P.; Wang, Y.; Fang, C.; Li, Y. Risk of cancer in acromegaly patients: An updated meta-analysis and systematic review. PLoS ONE 2023, 18, e0285335. [Google Scholar] [CrossRef]
- Lazczak, K.; Niedobylski, S.; Warchoł, K.; Dobosz, M.; Pachciński, O. Prevalence, incidence, and risk of cancers in patients with acromegaly: Review. J. Educ. Health Sport 2022, 12, 11–25. [Google Scholar] [CrossRef]
- Elbaum, M.; Kałużny, M.; Jawiarczyk-Przybyłowska, A.; Wojtczak, B.; Zieliński, G.; Bolanowski, M. The Relationship between the Burden of Acromegaly, Associated Comorbidities, Complications and Disease Status. J. Clin. Med. 2023, 12, 6309. [Google Scholar] [CrossRef]
- Dal, J.; Leisner, M.Z.; Hermansen, K.; Farkas, D.K.; Bengtsen, M.; Kistorp, C.; Nielsen, E.H.; Andersen, M.; Feldt-Rasmussen, U.; Dekkers, O.M.; et al. Cancer Incidence in Patients With Acromegaly: A Cohort Study and Meta-Analysis of the Literature. J. Clin. Endocrinol. Metab. 2018, 103, 2182–2188. [Google Scholar] [CrossRef]
- Xiao, T.; Jiao, R.; Yang, S.; Wang, Y.; Bai, X.; Zhou, J.; Li, R.; Wang, L.; Yang, H.; Yao, Y.; et al. Incidence and risk factors of cancers in acromegaly: A Chinese single-center retrospective study. Endocrine 2023, 82, 368–378. [Google Scholar] [CrossRef]
- Demarchis, L.; Chiloiro, S.; Giampietro, A.; De Marinis, L.; Bianchi, A.; Fleseriu, M.; Pontecorvi, A. Cancer screening in patients with acromegaly: A plea for a personalized approach and international registries. Rev. Endocr. Metab. Disord. 2025, 26, 525–538. [Google Scholar] [CrossRef]
- Basu, R.; Boguszewski, C.L.; Kopchick, J.J. Growth Hormone Action as a Target in Cancer: Significance, Mechanisms, and Possible Therapies. Endocr. Rev. 2025, 46, 224–280. [Google Scholar] [CrossRef]
- Cheok, S.K.; Tavakoli-Sabour, S.; Beck, R.T.; Zwagerman, N.; Ioachimescu, A. Ends of the spectrum best practices for early detection and multidisciplinary management of acromegaly. J. Neurooncol. 2025, 171, 1–9. [Google Scholar] [CrossRef]
- Gupta, P.; Dutta, P. Co-Occurrence of Acromegaly and Hematological Disorders: A Myth or Common Pathogenic Mechanism. Integr. Med. Int. 2017, 4, 94–100. [Google Scholar] [CrossRef]
- Hägg, E.; Asplund, K.; Holm, J. Acromegaly and multiple myeloma. Ann. Intern. Med. 1988, 109, 437–438. [Google Scholar] [CrossRef]
- Atmaca, M.; Yildiz, S.; Kalan, I.; Özbay, M.F.; Seven, I.; Öztürk, M. Association of acromegaly and multiple myeloma: A case report. Turk. Jem. 2013, 17, 75–78. [Google Scholar] [CrossRef]
- Kang, Y.M.; Choi, J.H.; Lee, M.J.; Ahn, A.; Park, C.J.; Chang, K.; Seo, S.; Hong, S.I.; Kim, M.S. Multiple myeloma in a patient with acromegaly. Endocrinol. Metab. 2015, 30, 110–115. [Google Scholar] [CrossRef]
- Peng, Y.; Li, F.; Zhang, P.; Wang, X.; Shen, Y.; Feng, Y.; Jia, Y.; Zhang, R.; Hu, J.; He, A. IGF-1 promotes multiple myeloma progression through PI3K/Akt mediated epithelial-mesenchymal transition. Life Sci. 2020, 249, 117503. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.R.; Vieira Neto, L.; Lima, G.A.; Wildemberg, L.E.; Portugal, R.; Gadelha, M.R. Hematologic neoplasias and acromegaly. Pituitary 2011, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Yamaguchi, T.; Yamane, Y.; Murakami, N.; Kato, Y.; Sugimoto, T. Acromegaly associated with monoclonal gammopathy of undetermined significance (MGUS). Endocr. J. 2006, 53, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Tucci, A.; Bonadonna, S.; Cattaneo, C.; Ungari, M.; Giustina, A.; Guiseppe, R. Transformation of a MGUS to overt multiple myeloma: The possible role of a pituitary macroadenoma secreting high levels of insulin-like growth factor 1 (IGF-1). Leuk. Lymphoma 2003, 44, 543–545. [Google Scholar] [CrossRef]
- Jawiarczyk-Przybyłowska, A.; Wojtczak, B.; Whitworth, J.; Sutkowski, K.; Bidlingmaier, M.; Korbonits, M.; Bolanowski, M. Acromegaly associated with GIST, non-small cell lung carcinoma, clear cell renal carcinoma, multiple myeloma, medulla oblongata tumour, adrenal adenoma, and follicular thyroid nodules. Endokrynol. Pol. 2019, 70, 213–217. [Google Scholar] [CrossRef]
- Knuppel, A.; Fensom, G.K.; Watts, E.L.; Gunter, M.J.; Murphy, N.; Papier, K.; Perez-Cornago, A.; Schmidt, J.A.; Smith Byrne, K.; Travis, R.C.; et al. Circulating Insulin-like Growth Factor-I Concentrations and Risk of 30 Cancers: Prospective Analyses in UK Biobank. Cancer Res. 2020, 80, 4014–4022. [Google Scholar] [CrossRef]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef]
- Caers, J.; Paiva, B.; Zamagni, E.; Leleu, X.; Bladé, J.; Kristinsson, S.Y.; Touzou, C.; Abildgaard, N.; Terpos, E.; Heusschen, R.; et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: Updated recommendations from a European Expert Panel. J. Hematol. Oncol. 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.W.; Campbell, B.A.; Goda, J.S.; Kelsey, C.R.; Kirova, Y.M.; Parikh, R.R.; Ng, A.K.; Ricardi, U.; Suh, C.O.; Mauch, P.M.; et al. Radiation Therapy for Solitary Plasmacytoma and Multiple Myeloma: Guidelines From the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.; Ciuche, A.; Constantinescu, I. Emergency surgical tracheal decompression in a huge retrosternal goiter. Acta Endocrinol. 2017, 13, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, L.; Johnsen, H.E.; Maes, K.; Menu, E.; Van Valckenborgh, E.; Overgaard, M.T.; Nyegaard, M.; Conover, C.A.; Vanderkerken, K.; De Bruyne, E. The insulin-like growth factor system in multiple myeloma: Diagnostic and therapeutic potential. Oncotarget 2016, 7, 48732–48752. [Google Scholar] [CrossRef]
- Georgii-Hemming, P.; Wiklund, H.J.; Ljunggren, O.; Nilsson, K. Insulin-like growth factor I is a growth and survival factor in human multiple myeloma cell lines. Blood 1996, 88, 2250–2258. [Google Scholar] [CrossRef]
- Maiza, J.C.; Revel, C. Evolution of (18)FDG pituitary uptake after medical control of acromegaly. Pituitary 2014, 17, 296–297. [Google Scholar] [CrossRef]
- Maiza, J.C.; Zunic, P.; Revel, C.; Schneebeli, S. Acromegaly revealed by 18FDG-PET/CT in a plasmocytoma patient. Pituitary 2012, 15, 614–615. [Google Scholar] [CrossRef]
- Nistor, C.E.; Bugala, N.M.; Daguci, C.; Daguci, L.; Diaconu, O.A.; Rica, A.M. Multiple endocrine neoplasia type 2 syndrome and osteoporosis. Aging Clin. Exp. Res. 2023, 35, S387. [Google Scholar]
- Goltzman, D. Nonparathyroid Hypercalcemia. Front. Horm. Res. 2019, 51, 77–90. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yun, J.S.; Kim, H.; Jeun, S.S.; Kim, B.; Lee, S.W.; Lee, J.E.; Kim, K.; Ko, S.H.; Ahn, Y.B.; et al. Acromegaly and the risk of cancer: A nationwide population-based cohort study in Korea. Eur. J. Endocrinol. 2025, 192, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bhatty, S.A.; Siddiqui, A.S.; Talib, A.; Mahmood, K.; Naqvi, I.; Khan, A.N.; Nizam, M.; Saiyed, A. Amyloidosis with Multiple Myeloma Presenting with Acromegalic Features. J. Coll. Physicians Surg Pak. 2015, 25 (Suppl. 2), S112–S114. [Google Scholar] [CrossRef]
- Peixe, C.; Sánchez-García, M.; Grossman, A.B.; Korbonits, M.; Marques, P. Biochemical discrepancies in the evaluation of the somatotroph axis: Elevated GH or IGF-1 levels do not always diagnose acromegaly. Growth Horm. IGF Res. 2022, 64, 101467. [Google Scholar] [CrossRef]
- Georgii-Hemming, P.; Strömberg, T.; Janson, E.T.; Stridsberg, M.; Wiklund, H.J.; Nilsson, K. The somatostatin analog octreotide inhibits growth of interleukin-6 (IL-6)-dependent and IL-6-independent human multiple myeloma cell lines. Blood 1999, 93, 1724–1731. [Google Scholar] [CrossRef]
- Kumar, U. Somatostatin and Somatostatin Receptors in Tumour Biology. Int. J. Mol. Sci. 2023, 25, 436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciu, M.; Cătană, A.; Ristea, R.P.; Tanasescu, D.; Carsote, M.; Popa, F.L.; Lebădă, I.-C. Hematological Malignancy in a Hypophysectomised Acromegalic Patient Under 4-Year Therapy with Somatostatin Analogues: From a Rib Lump Underlying Bone Plasmatocytoma Features to Multiple Myeloma. Diagnostics 2025, 15, 2623. https://doi.org/10.3390/diagnostics15202623
Stanciu M, Cătană A, Ristea RP, Tanasescu D, Carsote M, Popa FL, Lebădă I-C. Hematological Malignancy in a Hypophysectomised Acromegalic Patient Under 4-Year Therapy with Somatostatin Analogues: From a Rib Lump Underlying Bone Plasmatocytoma Features to Multiple Myeloma. Diagnostics. 2025; 15(20):2623. https://doi.org/10.3390/diagnostics15202623
Chicago/Turabian StyleStanciu, Mihaela, Alina Cătană, Ruxandra Paula Ristea, Denisa Tanasescu, Mara Carsote, Florina Ligia Popa, and Ioana-Codruța Lebădă. 2025. "Hematological Malignancy in a Hypophysectomised Acromegalic Patient Under 4-Year Therapy with Somatostatin Analogues: From a Rib Lump Underlying Bone Plasmatocytoma Features to Multiple Myeloma" Diagnostics 15, no. 20: 2623. https://doi.org/10.3390/diagnostics15202623
APA StyleStanciu, M., Cătană, A., Ristea, R. P., Tanasescu, D., Carsote, M., Popa, F. L., & Lebădă, I.-C. (2025). Hematological Malignancy in a Hypophysectomised Acromegalic Patient Under 4-Year Therapy with Somatostatin Analogues: From a Rib Lump Underlying Bone Plasmatocytoma Features to Multiple Myeloma. Diagnostics, 15(20), 2623. https://doi.org/10.3390/diagnostics15202623






