Neutrophil Percentage/Albumin Ratio as an Independent Predictor of the No-Reflow Phenomenon in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Material and Methods
2.1. Criteria for Participant Inclusion
2.2. Data Collection and Definitions
2.3. Definition of the No-Reflow Phenomenon
2.4. Coronary Diagnostic Imaging and Percutaneous Intervention
2.5. Medication Protocols
2.6. Biochemical Testing and Cardiac Ultrasound Analysis
3. Statistical Analysis
4. Results
5. Discussion
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Poudel, I.; Tejpal, C.; Rashid, H.; Jahan, N. Major Adverse Cardiovascular Events: An Inevitable Outcome of ST-Elevation Myocardial Infarction? A Literature Review. Cureus 2019, 11, e5280. [Google Scholar] [CrossRef] [PubMed]
- Grüntzig, A.R.; Senning, A.; Siegenthaler, W.E. Nonoperative Dilatation of Coronary-Artery Stenosis: Percutaneous Transluminal Coronary Angioplasty. N. Engl. J. Med. 1979, 301, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Pelliccia, F.; Niccoli, G.; Zimarino, M.; Andò, G.; Porto, I.; Calabrò, P.; De Rosa, S.; Gragnano, F.; Piccolo, R.; Moscarella, E.; et al. Pathophysiology and Treatment of the No-Reflow Phenomenon in ST-Segment Elevation Myocardial Infarction: Focus on Low-Dose Fibrinolysis during Primary Percutaneous Intervention. Rev. Cardiovasc. Med. 2023, 24, 365. [Google Scholar] [CrossRef]
- Niccoli, G.; Burzotta, F.; Galiuto, L.; Crea, F. Myocardial No-Reflow in Humans. J. Am. Coll. Cardiol. 2009, 54, 281–292. [Google Scholar] [CrossRef]
- Gerber, B.L.; Rochitte, C.E.; Melin, J.A.; McVeigh, E.R.; Bluemke, D.A.; Wu, K.C.; Becker, L.C.; Lima, J.A. Microvascular Obstruction and Left Ventricular Remodeling Early After Acute Myocardial Infarction. Circulation 2000, 101, 2734–2741. [Google Scholar] [CrossRef]
- Annibali, G.; Scrocca, I.; Aranzulla, T.C.; Meliga, E.; Maiellaro, F.; Musumeci, G. “No-Reflow” Phenomenon: A Contemporary Review. J. Clin. Med. 2022, 11, 2233. [Google Scholar] [CrossRef]
- Engler, R.L.; Schmid-Schönbein, G.W.; Pavelec, R.S. Leukocyte Capillary Plugging in Myocardial Ischemia and Reperfusion in the Dog. Am. J. Pathol. 1983, 111, 98–111. [Google Scholar]
- Engler, R.L.; Dahlgren, M.D.; Morris, D.D.; Peterson, M.A.; Schmid-Schönbein, G.W. Role of Leukocytes in Response to Acute Myocardial Ischemia and Reflow in Dogs. Am. J. Physiol. 1986, 251, H314–H323. [Google Scholar] [CrossRef]
- Litt, M.R.; Jeremy, R.W.; Weisman, H.F.; Winkelstein, J.A.; Becker, L.C. Neutrophil Depletion Limited to Reperfusion Reduces Myocardial Infarct Size after 90 Minutes of Ischemia. Evidence for Neutrophil-Mediated Reperfusion Injury. Circulation 1989, 80, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, M.C.; Borgström, P.; Warnke, K.C.; Skalak, T.C.; Intaglietta, M.; Arfors, K.E. Mechanisms and Implications of Capillary Endothelial Swelling and Luminal Narrowing in Low-Flow Ischemias. Int. J. Microcirc. Clin. Exp. 1995, 15, 265–270. [Google Scholar] [CrossRef]
- Duilio, C.; Ambrosio, G.; Kuppusamy, P.; DiPaula, A.; Becker, L.C.; Zweier, J.L. Neutrophils Are Primary Source of O2 Radicals during Reperfusion after Prolonged Myocardial Ischemia. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2649–H2657. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhou, X.; Ji, W.-J.; Lu, R.-Y.; Zhang, Y.; Zhang, Y.-D.; Ma, Y.-Q.; Zhao, J.-H.; Li, Y.-M. Neutrophil Extracellular Traps in Ischemia-Reperfusion Injury-Induced Myocardial No-Reflow: Therapeutic Potential of DNase-Based Reperfusion Strategy. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H500–H509. [Google Scholar] [CrossRef] [PubMed]
- Golino, P.; Maroko, P.R.; Carew, T.E. Efficacy of Platelet Depletion in Counteracting the Detrimental Effect of Acute Hypercholesterolemia on Infarct Size and the No-Reflow Phenomenon in Rabbits Undergoing Coronary Artery Occlusion-Reperfusion. Circulation 1987, 76, 173–180. [Google Scholar] [CrossRef]
- Vakili, H.; Shirazi, M.; Charkhkar, M.; Khaheshi, I.; Memaryan, M.; Naderian, M. Correlation of Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio with Thrombolysis in Myocardial Infarction Frame Count in ST-Segment Elevation Myocardial Infarction. Eur. J. Clin. Investig. 2017, 47, 322–327. [Google Scholar] [CrossRef]
- Refaat, H.; Tantawy, A.; Gamal, A.S.; Radwan, H. Novel Predictors and Adverse Long-Term Outcomes of No-Reflow Phenomenon in Patients with Acute ST Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Indian Heart J. 2021, 73, 35–43. [Google Scholar] [CrossRef]
- Qu, Z.; Guan, X. Predictive Value of the Triglyceride-Glucose Index for No-Reflow Phenomenon after Percutaneous Coronary Intervention in Patients with Acute ST-Segment Elevation Myocardial Infarction Complicated by Metabolic Syndrome. Am. J. Transl. Res. 2024, 16, 5539–5551. [Google Scholar] [CrossRef]
- Esenboğa, K.; Kurtul, A.; Yamantürk, Y.Y.; Tan, T.S.; Tutar, D.E. Systemic Immune-Inflammation Index Predicts No-Reflow Phenomenon after Primary Percutaneous Coronary Intervention. Acta Cardiol. 2022, 77, 59–65. [Google Scholar] [CrossRef]
- Bayramoğlu, A.; Hidayet, Ş. Association between Pan-Immune-Inflammation Value and No-Reflow in Patients with ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Scand. J. Clin. Lab. Investig. 2023, 83, 384–389. [Google Scholar] [CrossRef]
- Soylu, K.; Gedikli, Ö.; Dagasan, G.; Aydin, E.; Aksan, G.; Nar, G.; İnci, S.; Yilmaz, Ö. Neutrophil-to-Lymphocyte Ratio Predicts Coronary Artery Lesion Complexity and Mortality after Non-ST-Segment Elevation Acute Coronary Syndrome. Rev. Port. Cardiol. 2015, 34, 465–471. [Google Scholar] [CrossRef]
- de Liyis, B.G.; Ciaves, A.F.; Intizam, M.H.; Jusuf, P.J.; Artha, I.M.J.R. Hematological Biomarkers of Troponin, Neutrophil-to-Lymphocyte Ratio, and Monocyte-to-Lymphocyte Ratio Serve as Effective Predictive Indicators of High-Risk Mortality in Acute Coronary Syndrome. Biomedicine 2023, 13, 32–43. [Google Scholar] [CrossRef]
- Orhan, A.L.; Şaylık, F.; Çiçek, V.; Akbulut, T.; Selçuk, M.; Çınar, T. Evaluating the Systemic Immune-Inflammation Index for in-Hospital and Long-Term Mortality in Elderly Non-ST-Elevation Myocardial Infarction Patients. Aging Clin. Exp. Res. 2022, 34, 1687–1695. [Google Scholar] [CrossRef]
- Serhatlioglu, F.; Cetinkaya, Z.; Yilmaz, Y. The Role of Glucose-Lymphocyte Ratio in Evaluating the Severity of Coronary Artery Disease. J. Clin. Med. 2024, 13, 6711. [Google Scholar] [CrossRef]
- Duran, M.; Uysal, O.K.; Günebakmaz, O.; Yılmaz, Y.; Akın, F.; Baran, O.; Inanç, M.T.; Eryol, N.K.; Ergin, A.; Oğuzhan, A.; et al. Increased Red Cell Distribution Width Level Is Associated with Absence of Coronary Collateral Vessels in Patients with Acute Coronary Syndromes. Turk. Kardiyol. Dern. Ars. 2013, 41, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ding, X.; Li, W.; Chen, H.; Li, H. The Neutrophil Percentage to Albumin Ratio as a New Predictor of In-Hospital Mortality in Patients with ST-Segment Elevation Myocardial Infarction. Med. Sci. Monit. 2019, 25, 7845–7852. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Shen, H.; Guo, Q.; Yang, J.; Zhai, G.; Zhang, J.; Zhang, B.; Ding, Y.; Cai, C.; Zhou, Y. Association between Neutrophil Percentage-to-Albumin Ratio and All-Cause Mortality in Critically Ill Patients with Coronary Artery Disease. Biomed. Res. Int. 2020, 2020, 8137576. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y.; Ling, X.; Huang, R.; Wang, S.; Min, J.; Xiao, J.; Zhang, Y.; Wang, Z. The Neutrophil Percentage-to-Albumin Ratio as a New Predictor of All-Cause Mortality in Patients with Cardiogenic Shock. Biomed. Res. Int. 2020, 2020, 7458451. [Google Scholar] [CrossRef]
- Sorci-Thomas, M.G.; Thomas, M.J. Microdomains, Inflammation, and Atherosclerosis. Circ. Res. 2016, 118, 679–691. [Google Scholar] [CrossRef]
- Milan Manani, S.; Virzì, G.M.; Clementi, A.; Brocca, A.; de Cal, M.; Tantillo, I.; Ferrando, L.; Crepaldi, C.; Ronco, C. Pro-Inflammatory Cytokines: A Possible Relationship with Dialytic Adequacy and Serum Albumin in Peritoneal Dialysis Patients. Clin. Kidney J. 2016, 9, 153–157. [Google Scholar] [CrossRef]
- Ritchie, R.F.; Palomaki, G.E.; Neveux, L.M.; Navolotskaia, O.; Ledue, T.B.; Craig, W.Y. Reference Distributions for the Negative Acute-Phase Serum Proteins, Albumin, Transferrin and Transthyretin: A Practical, Simple and Clinically Relevant Approach in a Large Cohort. J. Clin. Lab. Anal. 1999, 13, 273–279. [Google Scholar] [CrossRef]
- Mikhailidis, D.P.; Ganotakis, E.S. Plasma Albumin and Platelet Function: Relevance to Atherogenesis and Thrombosis. Platelets 1996, 7, 125–137. [Google Scholar] [CrossRef]
- Naruko, T.; Ueda, M.; Haze, K.; van der Wal, A.C.; van der Loos, C.M.; Itoh, A.; Komatsu, R.; Ikura, Y.; Ogami, M.; Shimada, Y.; et al. Neutrophil Infiltration of Culprit Lesions in Acute Coronary Syndromes. Circulation 2002, 106, 2894–2900. [Google Scholar] [CrossRef]
- Baetta, R.; Corsini, A. Role of Polymorphonuclear Neutrophils in Atherosclerosis: Current State and Future Perspectives. Atherosclerosis 2010, 210, 1–13. [Google Scholar] [CrossRef]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Civeira, F.; Arca, M.; Cenarro, A.; Hegele, R.A. A Mechanism-Based Operational Definition and Classification of Hypercholesterolemia. J. Clin. Lipidol. 2022, 16, 813–821. [Google Scholar] [CrossRef]
- Gibson, C.M.; de Lemos, J.A.; Murphy, S.A.; Marble, S.J.; McCabe, C.H.; Cannon, C.P.; Antman, E.M.; Braunwald, E.; TIMI Study Group. Combination Therapy with Abciximab Reduces Angiographically Evident Thrombus in Acute Myocardial Infarction: A TIMI 14 Substudy. Circulation 2001, 103, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Kurtul, A.; Yarlioglues, M.; Celik, I.E.; Duran, M.; Elcik, D.; Kilic, A.; Oksuz, F.; Murat, S.N. Association of Lymphocyte-to-Monocyte Ratio with the No-Reflow Phenomenon in Patients Who Underwent a Primary Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction. Coron. Artery Dis. 2015, 26, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, J.; Zhang, J. Meta-Analysis of the Correlation between Inflammatory Response Indices and No-Reflow after PCI in Patients with Acute STEMI. Am. J. Transl. Res. 2024, 16, 5168–5181. [Google Scholar] [CrossRef]
- Shumakov, D.V.; Shekhyan, G.G.; Zybin, D.I.; Yalymov, A.A.; Vedenikin, T.Y.; Popov, M.A. In-stent restenosis: Symptoms, hemodynamic signs, pathogenesis and treatment. Russ. Cardiol. Bull. 2021, 16, 20–27. (In Russian) [Google Scholar] [CrossRef]
- Wang, L.; Huang, S.; Zhou, Q.; Dou, L.; Lin, D. The Predictive Value of Laboratory Parameters for No-Reflow Phenomenon in Patients with ST-Elevation Myocardial Infarction Following Primary Percutaneous Coronary Intervention: A Meta-Analysis. Clin. Cardiol. 2024, 47, e24238. [Google Scholar] [CrossRef]
- Wong, D.T.L.; Puri, R.; Richardson, J.D.; Worthley, M.I.; Worthley, S.G. Myocardial ‘No-Reflow’—Diagnosis, Pathophysiology and Treatment. Int. J. Cardiol. 2013, 167, 1798–1806. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Y.; Liu, Y.; Song, X.; Zhang, M.; Xu, F.; Yuan, F.; Lyu, S. Prognostic Association of Circulating Neutrophil Count with No-Reflow in Patients with ST-Segment Elevation Myocardial Infarction Following Successful Primary Percutaneous Intervention. Dis. Markers 2017, 2017, 8458492. [Google Scholar] [CrossRef]
- Yin, R.; Zhu, W.; Chen, W.; Shen, J.; Wu, Y.; Wang, Z. The Relationship between Neutrophil Percentage-to-Albumin Ratio and Slow and Normal Coronary Flow Phenomenon. BMC Cardiovasc. Disord. 2025, 25, 64. [Google Scholar] [CrossRef]
- Karasu, M.; Karaca, Y.; Yıldırım, E.; Kobat, M.A.; Er, F. Neutrophil-to-Albumin Ratio: A Promising Tool for CAD Assessment in Non-ST Elevation AMI. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 11832–11839. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Huang, X.; Zhang, Y.; Wang, Z.; Zhang, S.; Peng, J. Predictive Value of the Neutrophil Percentage-to-Albumin Ratio for Coronary Atherosclerosis Severity in Patients with CKD. BMC Cardiovasc. Disord. 2024, 24, 277. [Google Scholar] [CrossRef]
- Serhatlioglu, F.; Yilmaz, Y.; Baran, O.; Yilmaz, H.; Kelesoglu, S. Inflammatory Markers and Postoperative New-Onset Atrial Fibrillation: Prognostic Predictions of Neutrophil Percent to Albumin Ratio in Patients with CABG. Diagnostics 2025, 15, 741. [Google Scholar] [CrossRef]
- Karaca, M.; Gumusdag, A. Prognostic Role of Neutrophil Percentage-to-Albumin Ratio in Patients with Non-ST-Elevation Myocardial Infarction. Medicina 2024, 60, 2101. [Google Scholar] [CrossRef]
- Pinheiro Machado, G.; Araujo, G.N.; Carpes, C.K.; Lech, M.C.; Mariani, S.; Valle, F.H.; Bergoli, L.C.C.; Wainstein, R.V.; Wainstein, M.V. Elevated Neutrophil-to-Lymphocyte Ratio Can Predict Procedural Adverse Events in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Coron. Artery Dis. 2019, 30, 20–25. [Google Scholar] [CrossRef]
- Matteucci, A.; Bonanni, M.; Versaci, F.; Frati, G.; Peruzzi, M.; Sangiorgi, G.; Biondi-Zoccai, G.; Massaro, G. Cardiovascular Medicine: A Year in Review. Minerva Cardiol. Angiol. 2022, 70, 40–55. [Google Scholar] [CrossRef]
- Arques, S. Human Serum Albumin in Cardiovascular Diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef] [PubMed]
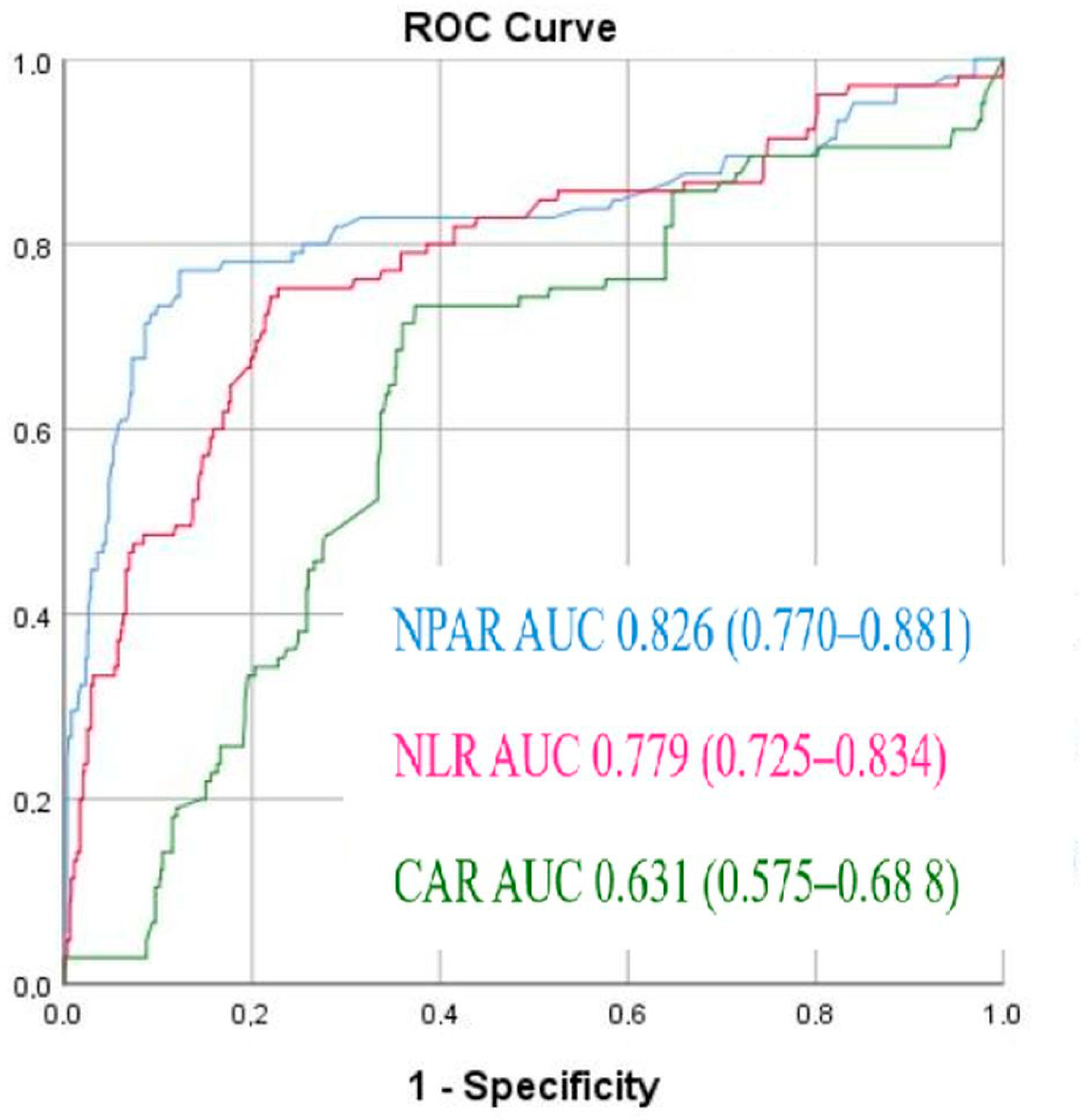
| Normal-Reflow n = 653 | No-Reflow n = 105 | p Value | |
|---|---|---|---|
| Age (year) | 54 (45–62) | 60 (53–67) | <0.001 |
| Male (n, %) | 461 (71%) | 79 (75.2%) | 0.33 |
| Hypertension (n, %) | 362 (55.4) | 64 (60.1%) | 0.089 |
| DM (n, %) | 258 (39.5%) | 42 (40%) | 0.815 |
| Dyslipidemia (n, %) | 87 (13.3%) | 18 (17.1%) | 0.318 |
| CAD (n, %) | 124 (19%) | 26 (24.7%) | 0.655 |
| Smokers (n, %) | 315 (48.2%) | 49 (46.6%) | 0.354 |
| LVEF (%) | 39.2 ± 6.9 | 31.8 ± 5.1 | <0.001 |
| Killip III–IV (n, %) | 29 (4.4%) | 9 (8.2%) | 0.062 |
| BMI (kg/m2) | 26.5 (23.6–30.8) | 28.4 (26–31) | <0.001 |
| Glomerular filtration rate (mL/min/1.73 m2) | 85.29 ± 39.4 | 81.9 ± 37.2 | 0.17 |
| Serum Glucose (mg/dL) | 109.29 ± 68.6 | 129.5 ± 34.5 | 0.144 |
| Total cholesterol (mg/dL) | 156.3 (132–179) | 175 (141–210) | 0.291 |
| High-density lipoprotein cholesterol (mg/dL) | 39 (29–47) | 37 (31–45) | 0.713 |
| Low-density lipoprotein cholesterol (mg/dL) | 101 (79–135) | 106 (82–176) | 0.645 |
| Triglyceride (mg/dL) | 129 (83–211) | 144 (91–233) | 0.536 |
| Albumin (mg/dL) | 4.2 (3.5–4.4) | 3.9 (3.5–4.1) | 0.021 |
| Hemoglobin (g/L) | 14.2 (13.3–15.2) | 14.3 (12.9–15.1) | 0.883 |
| Platelet (103/μL) | 220 (188–263) | 227 (187–257) | 0.716 |
| WBC (103/μL) | 7.4 (5.8–8.9) | 12.3 (9.6–16.9) | <0.001 |
| Neutrophil (103/μL) | 4.1 (3.3–6.9) | 7 (4.5–13.5) | <0.001 |
| Neutrophil percentage (%) | 67.2 (45.2–75.3) | 81.9 (66.9–89.1) | <0.001 |
| Lymphocyte (103/μL) | 1.8 (1.3–2.5) | 1.7 (1.3–2.3) | 0.168 |
| CRP (mg/dL) | 3.2 (2.4–6) | 5 (3.6–8) | <0.001 |
| NLR | 2.1 (1.5–3) | 4.6 (2.9–9.6) | <0.001 |
| NPAR | 16.32 ± 4.14 | 21.01 ± 3.65 | <0.001 |
| CAR | 0.75 (0.59–1.7) | 1.1 (0.74–2.1) | <0.001 |
| Infarct Related Artery | |||
|---|---|---|---|
| LAD | 76 (49%) | 113 (45%) | 0.322 |
| RCA | 42 (27%) | 71 (28%) | 0.843 |
| CX | 35 (23%) | 67 (26%) | 0.334 |
| Coronary artery involvement | |||
| Stent length (mm) | 21.1 ± 5.4 | 20.6 ± 6.7 | 0.139 |
| Stent diameter (mm) | 2.54 ± 0.85 | 2.61 ± 0.73 | 0.380 |
| Predilation | 103 (67%) | 148 (60%) | 0.113 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | Unadjusted OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
| Age | 1.057 | 1.036–1.079 | <0.001 | 1.108 | 1.072–1.144 | <0.001 |
| BMI | 1.063 | 1.018–1.111 | 0.006 | 1.016 | 1.011–1.148 | 0.022 |
| LVEF | 0.592 | 0.345–0.878 | 0.026 | |||
| Albumin | 0.488 | 0.303–0.786 | 0.003 | |||
| WBC | 1.403 | 1.312–1.501 | <0.001 | |||
| Neutrophil | 1.501 | 1.386–1.625 | <0.001 | |||
| Neutrophil percentage | 3.625 | 2.982–5.193 | <0.001 | |||
| CRP | 1.068 | 1.020–1.119 | 0.005 | |||
| NLR * | 1.324 | 1.245–1.408 | <0.001 | 1.158 | 1.074–1.249 | <0.001 |
| NPAR * | 4.622 | 3.452–6.190 | <0.001 | 5.482 | 3.254–9.234 | <0.001 |
| CAR | 1.369 | 1.137–1.647 | 0.001 | 1.344 | 1.024–1.763 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yavcin, O.; Yilmaz, Y. Neutrophil Percentage/Albumin Ratio as an Independent Predictor of the No-Reflow Phenomenon in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Diagnostics 2025, 15, 2609. https://doi.org/10.3390/diagnostics15202609
Yavcin O, Yilmaz Y. Neutrophil Percentage/Albumin Ratio as an Independent Predictor of the No-Reflow Phenomenon in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Diagnostics. 2025; 15(20):2609. https://doi.org/10.3390/diagnostics15202609
Chicago/Turabian StyleYavcin, Ozkan, and Yucel Yilmaz. 2025. "Neutrophil Percentage/Albumin Ratio as an Independent Predictor of the No-Reflow Phenomenon in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention" Diagnostics 15, no. 20: 2609. https://doi.org/10.3390/diagnostics15202609
APA StyleYavcin, O., & Yilmaz, Y. (2025). Neutrophil Percentage/Albumin Ratio as an Independent Predictor of the No-Reflow Phenomenon in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Diagnostics, 15(20), 2609. https://doi.org/10.3390/diagnostics15202609





