Clinicopathologic Disease Characteristics and Their Association with Adjuvant Chemotherapy Outcomes in Pulmonary Large-Cell Carcinoma Patients with or Without Neuroendocrine Features
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Modalities
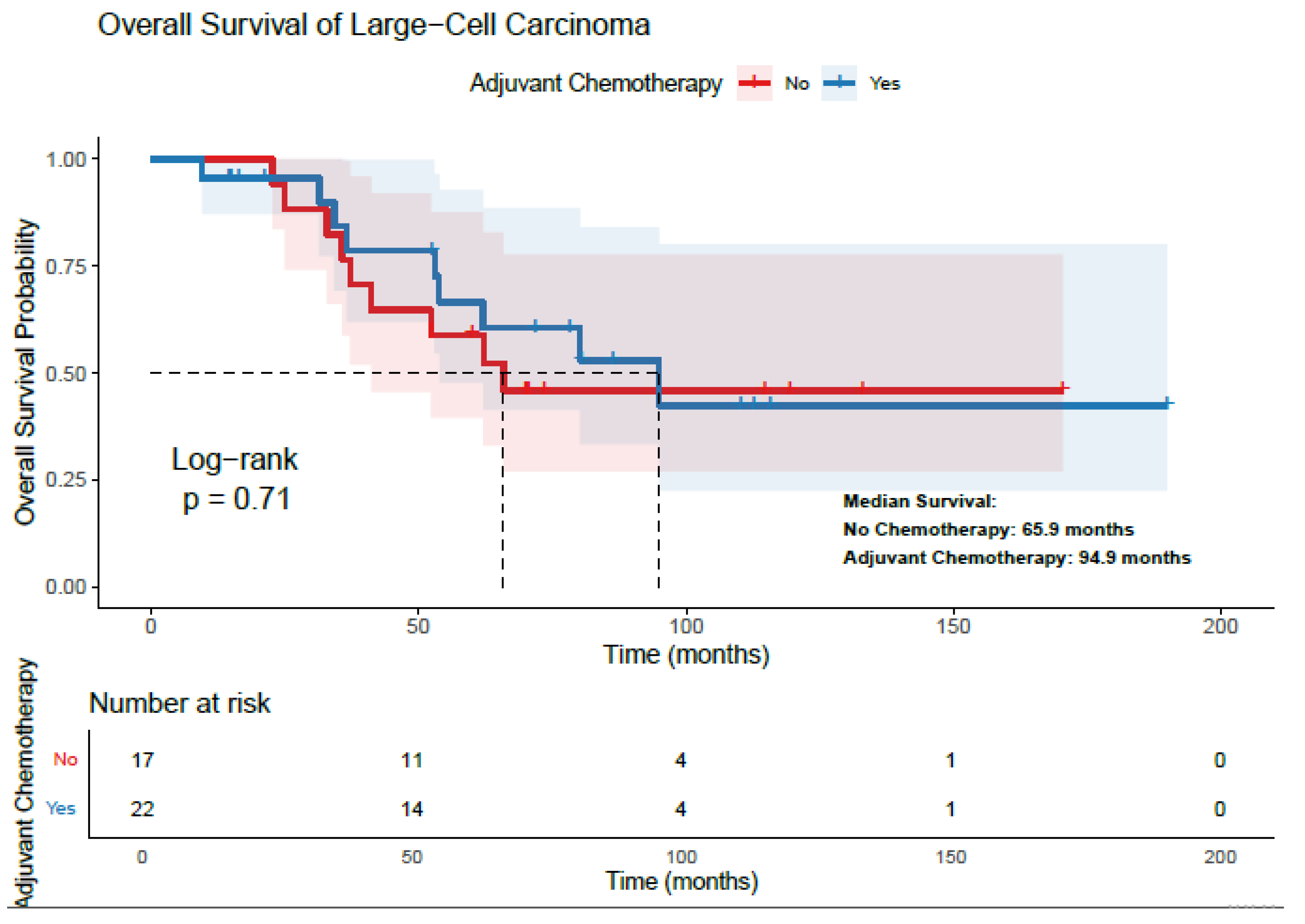
3.3. Survival Analysis and Treatment Effect—LCC Patients
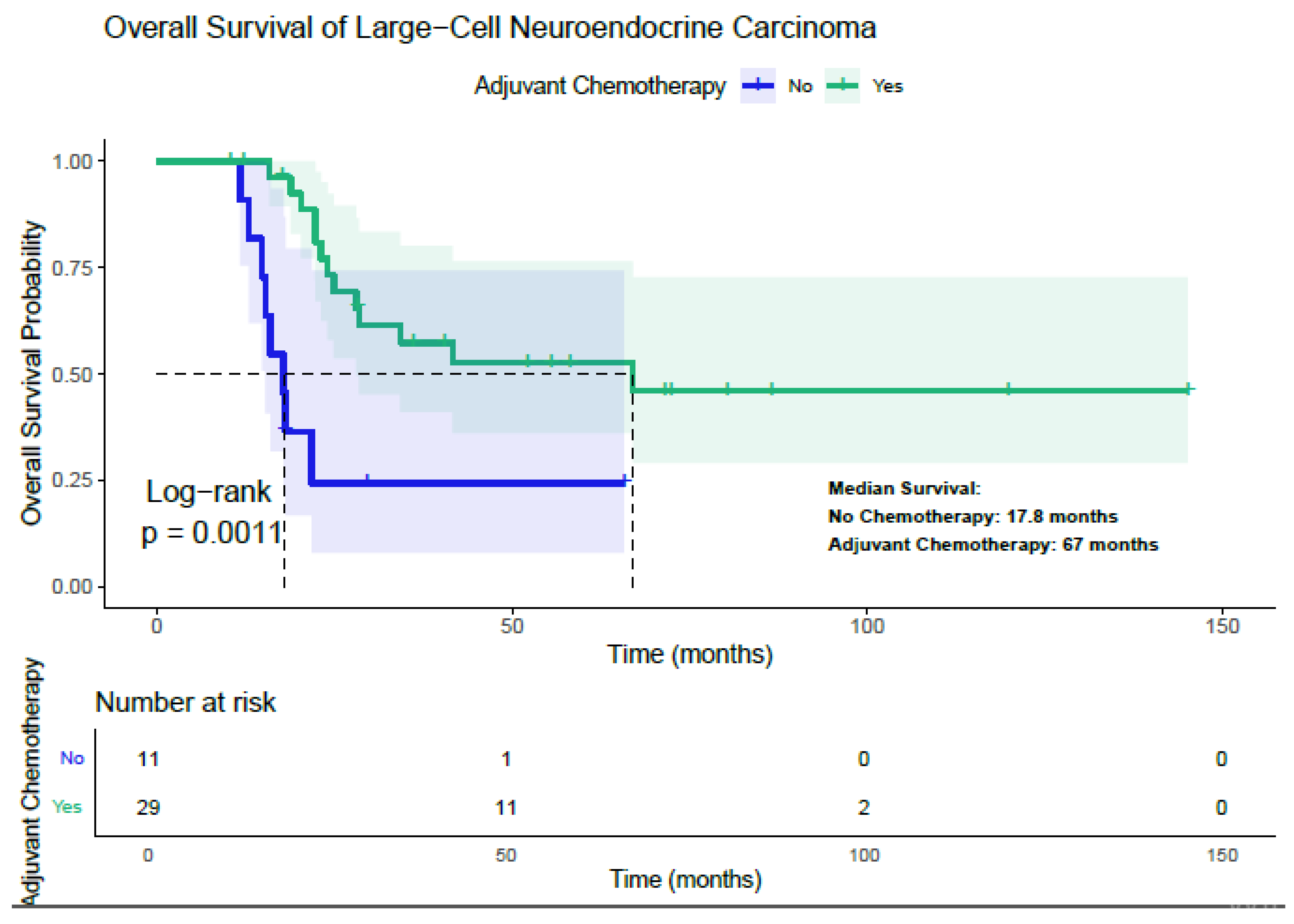
3.4. Survival Analysis and Treatment Effect—LCNEC Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Yokose, T.; Yoshida, J.; Nishiwaki, Y.; Nagai, K. Immunohistochemical neuroendocrine differentiation is an independent prognostic factor in surgically resected large cell carcinoma of the lung. Lung Cancer 2002, 38, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, W.; Hu, Q.; Zhou, P.; Zhang, Y.; Tang, Y.; Wu, Q.; Fu, Y.; Li, X.; Shao, Y.; et al. Pulmonary large cell carcinoma with neuroendocrine morphology shows genetic similarity to large cell neuroendocrine carcinoma. Diagn. Pathol. 2022, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, Y.; Lu, H. Pulmonary Large Cell Neuroendocrine Carcinoma. Pathol. Oncol. Res. 2022, 28, 1610730. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Fabbri, A.; Cossa, M.; Sonzogni, A.; Valeri, B.; Righi, L.; Papotti, M. What clinicians are asking pathologists when dealing with lung neuroendocrine neoplasms? Semin. Diagn. Pathol. 2015, 32, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Zer, A.; Ahn, M.J.; Barlesi, F.; Bubendorf, L.; De Ruysscher, D.; Garrido, P.; Gautschi, O.; Hendriks, L.E.; Jänne, P.A.; Kerr, K.M.; et al. Early and locally advanced non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 5. 2024. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (accessed on 1 May 2025).
- Wankhede, D. Evaluation of Eighth AJCC TNM Sage for Lung Cancer NSCLC: A Meta-analysis. Ann. Surg. Oncol. 2021, 28, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Iyoda, A.; Hiroshima, K.; Toyozaki, T.; Haga, Y.; Fujisawa, T.; Ohwada, H. Clinical characterization of pulmonary large cell neuroendocrine carcinoma and large cell carcinoma with neuroendocrine morphology. Cancer 2001, 91, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Liu, Y.; Li, X.; Jin, M.; Liu, X.; Yu, Q. Effect of Chemotherapy in Stage II–IV Large-Cell Lung Carcinoma and Construction of Its Predictive Nomograms: A SEER Analysis. Med. Princ. Pract. 2023, 32, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tai, Q.; Su, B.; Zhang, L. Adjuvant chemotherapy improves the prognosis of early stage resectable pulmonary large cell carcinoma: Analysis of SEER data. Ann. Palliat. Med. 2020, 9, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, F.; Dienemann, H.; Muley, T.; Warth, A.; Hoffmann, H. Predictors of survival after operation among patients with large cell neuroendocrine carcinoma of the lung. Ann. Thorac. Surg. 2015, 99, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kim, H.K.; Kim, J.; Shim, Y.M.; Ahn, M.J.; Choi, Y.L. Outcomes of Curative-Intent Surgery and Adjuvant Treatment for Pulmonary Large Cell Neuroendocrine Carcinoma. World J. Surg. 2017, 41, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G.; Morandi, U.; Alloisio, M.; Terzi, A.; Cardillo, G.; Filosso, P.; Rea, F.; Facciolo, F.; Pelosi, G.; Gandini, S.; et al. Large cell neuroendocrine carcinoma of the lung: A retrospective analysis of 144 surgical cases. Lung Cancer 2006, 53, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Filosso, P.L.; Guerrera, F.; Evangelista, A.; Galassi, C.; Welter, S.; Rendina, E.A.; Travis, W.; Lim, E.; Sarkaria, I.; Thomas, P.A. Adjuvant chemotherapy for large-cell neuroendocrine lung carcinoma: Results from the European Society for Thoracic Surgeons Lung Neuroendocrine Tumours Retrospective Database. Eur. J. Cardio-Thorac. Surg. 2017, 52, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, I.S.; Iyoda, A.; Roh, M.S.; Sica, G.; Kuk, D.; Sima, C.S.; Pietanza, M.C.; Park, B.J.; Travis, W.D.; Rusch, V.W. Neoadjuvant and adjuvant chemotherapy in resected pulmonary large cell neuroendocrine carcinomas: A single institution experience. Ann. Thorac. Surg. 2011, 92, 1180–1186; discussion 1186–1187. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Z.; Wu, Y.J.; Chen, C.S.; Yang, S.C. Impact of Smoking Status on Lung Cancer Characteristics and Mortality Rates between Screened and Non-Screened Lung Cancer Cohorts: Real-World Knowledge Translation and Education. J. Pers. Med. 2022, 12, 26. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Li, G. What’s the difference between lung adenocarcinoma and lung squamous cell carcinoma? Evidence from a retrospective analysis in a cohort of Chinese patients. Front. Endocrinol. 2022, 13, 947443. [Google Scholar] [CrossRef]
| Characteristics | Large-Cell Carcinoma n (%) | Large-Cell Neuroendocrine Carcinoma n (%) |
|---|---|---|
| Age (years) * | 61 (25–76) | 58 (43–81) |
| Gender | ||
| Male | 33 (84.6) | 35 (87.5) |
| Female | 6 (15.4) | 5 (12.5) |
| Smoking History | ||
| None | 12 (30.8) | 4 (10) |
| Ex-smoker | 21 (53.8) | 20 (50) |
| Current smoker | 6 (15.4) | 16 (40) |
| Tumor Size (cm) * | 3.6 (1.2–6.2) | 3.9 (1.2–4.9) |
| Pathological lymph node | 23 (58.9) | 21 (52.5) |
| N0 | 23 (58.9) | 21 (52.5) |
| N1 | 10 (25.7) | 11 (27.5) |
| N2 | 6 (15.4) | 8 (20) |
| Stage | ||
| I | 15 (38.5) | 16 (40) |
| II–III | 24 (61.5) | 24 (60) |
| TNM Stage | ||
| IAI | 1 (2.6) | 3 (7.5) |
| IAII | 4 (10.3) | 4 (10) |
| IAIII | 3 (7.7) | 4 (10) |
| IB | 7 (17.9) | 5 (12.5) |
| IIA | 8 (20.5) | 5 (12.5) |
| IIB | 10 (25.6) | 11 (27.5) |
| IIIA | 4 (10.3) | 8 (20) |
| IIIB | 2 (5.1) | 0 (0) |
| Tumor Localization | ||
| Central | 11 (28.2) | 18 (45) |
| Peripheral | 28 (71.8) | 22 (55) |
| TFF-1 | ||
| Positive | 7 (17.9) | 13 (32.5) |
| Negative | 32 (82.1) | 27 (67.5) |
| P40 | ||
| Positive | 12 (30.8) | 2 (5) |
| Negative | 27 (69.2) | 38 (95) |
| Synaptophysin | ||
| Positive | 10 (25.6) | 26 (65) |
| Negative | 29 (74.4) | 15 (35) |
| Chromogranin A | ||
| Positive | 4 (10.3) | 19 (47.5) |
| Negative | 35 (89.7) | 21 (52.5) |
| CD56 | ||
| Positive | 12 (30.8) | 28 (70) |
| Negative | 27 (69.2) | 12 (30) |
| Large-Cell Carcinoma n % | Large-Cell Neuroendocrine Carcinoma n % | |
|---|---|---|
| Curative Surgery | 39 (100) | 40 (100) |
| Lobectomy | 28 (71.8) | 34 (85) |
| Pneumonectomy | 11 (28.2) | 6 (15) |
| Adjuvant Chemotherapy | 22 (56.4) | 29 (72.5) |
| Cisplatin-Vinorelbine | 19 (86.4) | 1 (3.4) |
| Carboplatin-Paclitaxel | 2 (9.1) | 0 |
| Carboplatin-Vinorelbine | 1 (4.5) | 0 |
| Cisplatin-Etoposide | 0 | 17 (77.2) |
| Carboplatin-Etoposide | 0 | 10 |
| Carboplatin- Gemcitabine | 0 | 1 (3.4) |
| Adjuvant Radiotherapy | 4 (10.3) | 9 (22.5) |
| Relapse After Adjuvant Treatments | 19 (48.7) | 24 (60) |
| Local Recurrence | 4 | 4 |
| Distant Organ Metastasis | 15 | 20 |
| Post-Relapse Treatment | ||
| 1st Line Chemotherapy | 8 | 19 |
| Radiotherapy | 5 | 2 |
| Surgery | 4 | 2 |
| Supportive Care | 2 | 1 |
| 1st Line Chemotherapy | ||
| Carboplatin-Pemetrexed | 4 | 0 |
| Carboplatin-Paclitaxel | 2 | 0 |
| Docetaxel | 2 | 0 |
| Platin-Etoposide | 0 | 14 |
| Cisplatin-Gemcitabine | 0 | 1 |
| Irinotecan | 0 | 1 |
| Topotecan | 0 | 2 |
| CAVI | 0 | 1 |
| Large-Cell Carcinoma | |||||||
|---|---|---|---|---|---|---|---|
| mOS (Months) | Univariate (p Value) | Multivariate p Value (HR) | mDFS (Months) | Univariate (p Value) | Multivariate p Value (HR) | ||
| Gender | Male | 80.1 | 0.4 | 64.1 | 0.32 | ||
| Female | NR | NR | |||||
| Age | <61 | NR | 0.020 | 0.047 (HR: 0.279) | NR | 0.065 | |
| ≥61 | 62.029 | 53.06 | |||||
| Smoking | Yes | 80.1 | 0.56 | 77.1 | 0.53 | ||
| No | NR | NR | |||||
| Stage | I | NR | 0.028 | 0.016 (HR: 0.198) | NR | 0.02 | 0.022 (HR: 0.26) |
| II–III | 62.02 | 47.2 | |||||
| Tumor Localization | Central | 65.8 | 0.49 | 66.1 | 0.74 | ||
| Peripheral | 80 | 77.14 | |||||
| TTF-1 | Positive | NR | 0.3 | NR | 0.26 | ||
| Negative | 65.873 | 64.1 | |||||
| P40 | Positive | 62.0 | 0.73 | 50.1 | 0.41 | ||
| Negative | 80.1 | 77.1 | |||||
| Synaptophysin | Positive | NR | 0.81 | 45.8 | 0.42 | ||
| Negative | 79.6 | 91.1 | |||||
| Chromogranin A | Positive | 52.3 | 0.017 | 0.011 (HR: 0.088) | 43.1 | 0.056 | |
| Negative | 94.9 | 122.3 | |||||
| CD56 | Positive | 65.3 | 0.15 | 32.7 | 0.043 | 0.034 (HR: 0.35) | |
| Negative | 83.8 | 91.1 | |||||
| Surgical Procedure | Lobectomy | 92.8 | 0.35 | 91.1 | 0.64 | ||
| Pneumonectomy | 62.0 | 53.0 | |||||
| Adjuvant Chemotherapy | Yes | 94.9 | 0.70 | 77.1 | 0.73 | ||
| No | 65.8 | 64.1 | |||||
| Adjuvant Radiotherapy | Yes | 36.5 | 0.96 | 34.3 | 0.86 | ||
| No | 80.1 | 77.1 | |||||
| Adjuvant Chemotherapy Yes | Adjuvant Chemotherapy No | |||||
|---|---|---|---|---|---|---|
| n | mOS | n | mOS | p | ||
| Gender | Female | 2 | NR | 4 | NR | 0.85 |
| Male | 20 | 94.9 | 13 | 62.0 | 0.46 | |
| Age | <61 | 8 | NR | 6 | NR | 0.39 |
| ≥61 | 14 | 66.8 | 11 | 70.2 | 0.97 | |
| Stage | I | 3 | NR | 12 | NR | 0.37 |
| II–III | 19 | 80.1 | 5 | 32.7 | 0.009 | |
| Smoking | Yes | 18 | 80.1 | 9 | NR | 0.669 |
| No | 4 | NR | 8 | NR | 0.107 | |
| Tumor Localization | Central | 7 | 94.7 | 4 | 62.0 | 0.206 |
| Peripheral | 15 | 80.1 | 13 | NR | 0.87 | |
| TTF-1 | Positive | 3 | NR | 4 | NR | 0.18 |
| Negative | 19 | 80.1 | 13 | 65.8 | 0.95 | |
| P40 | Positive | 9 | 94.9 | 3 | 62.0 | 0.88 |
| Negative | 13 | 80.1 | 14 | 65.8 | 0.66 | |
| Synaptophysin | Positive | 8 | NR | 2 | 52.3 | 0.810 |
| Negative | 14 | 94.9 | 15 | 65.8 | 0.609 | |
| Chromogranin A | Positive | 3 | 52.3 | 1 | 41.6 | 0.56 |
| Negative | 19 | 65.8 | 16 | 94.9 | 0.67 | |
| CD56 | Positive | 7 | 41.0 | 5 | 53.8 | 0.38 |
| Negative | 15 | 94.9 | 12 | NR | 0.926 | |
| Surgical Procedure | Lobectomy | 14 | 62.0 | 14 | 52.3 | 0.86 |
| Pneumonectomy | 8 | 94.9 | 3 | 65.8 | 0.181 | |
| Large-Cell Neuroendocrine Carcinoma | |||||||
|---|---|---|---|---|---|---|---|
| mOS (Months) | Univariate p | Multivariate p (HR) | mDFS (Months) | Univariate p | Multivariate p (HR) | ||
| Gender | Male | 28.02 | 0.27 | 24.1 | 0.49 | ||
| Female | NR | 61.9 | |||||
| Age | <58 | 28.3 | 0.78 | 26.7 | 0.93 | ||
| ≥58 | 41.2 | 31.3 | |||||
| Smoking | Yes | 28.3 | 0.48 | 26.7 | 0.37 | ||
| No | 41.5 | 36.0 | |||||
| Stage | I | NR | 0.001 | 0.017 (HR: 0.20) | NR | 0.001 | 0.005 (HR: 0.20) |
| II–III | 24.0 | 15.0 | |||||
| Tumor Localization | Central | 28.0 | 0.32 | 15.4 | 0.064 | ||
| Peripheral | 41.5 | 35.8 | |||||
| TTF-1 | Positive | 41.5 | 0.55 | 29.8 | 0.46 | ||
| Negative | 24.8 | 17.6 | |||||
| Synaptophysin | Positive | 24.8 | 0.014 | 0.037 (HR: 0.30) | 17.6 | 0.057 | |
| Negative | 39.4 | 61.9 | |||||
| Chromogranin A | Positive | 21.7 | 0.028 | 0.14 | 14.8 | 0.077 | |
| Negative | 66.9 | 40.0 | |||||
| CD56 | Positive | 23.0 | 0.056 | 35.2 | 0.087 | ||
| Negative | NR | 14.4 | |||||
| Surgical Procedure | Lobectomy | 66.9 | 0.001 | 0.048 (HR: 0.31) | 36.0 | 0.002 | 0.57 |
| Pneumonectomy | 17.8 | 13.5 | |||||
| Adjuvant Chemotherapy | Yes | 67.0 | 0.0011 | 0.030 (HR: 0.264) | 61.9 | 0.001 | 0.02 (HR: 0.211) |
| No | 17.8 | 12.9 | |||||
| Adjuvant Radiotherapy | Yes | 36.8 | 0.95 | 26.7 | 0.47 | ||
| No | 28.3 | 31.3 | |||||
| Adjuvant Chemotherapy Yes | Adjuvant Chemotherapy No | |||||
|---|---|---|---|---|---|---|
| n | mOS | n | mOS | p | ||
| Gender | Female | 4 | 64.6 | 1 | 21.7 | 0.046 |
| Male | 25 | 34.2 | 10 | 15.9 | 0.007 | |
| Age | <58 | 15 | NR | 6 | 15.2 | 0.006 |
| ≥58 | 14 | 41.5 | 5 | 21.7 | 0.055 | |
| Stage | I | 12 | NR | 4 | 21.7 | 0.038 |
| II–III | 17 | 28.0 | 7 | 15.2 | 0.004 | |
| Smoking | Yes | 25 | 66.9 | 11 | 17.8 | 0.002 |
| No | 4 | 41.5 | 0 | - | - | |
| Tumor Localization | Central | 12 | 28.3 | 6 | 14.7 | 0.33 |
| Peripheral | 17 | NR | 5 | 17.08 | 0.001 | |
| TTF-1 | Positive | 11 | 41.5 | 2 | 17.8 | 0.016 |
| Negative | 21 | 66.9 | 9 | 15.9 | 0.008 | |
| Synaptophysin | Positive | 17 | 28.3 | 9 | 17.8 | 0.051 |
| Negative | 12 | NR | 2 | 12.9 | 0.33 | |
| Chromogranin A | Positive | 10 | 21.7 | 9 | 15.9 | 0.05 |
| Negative | 19 | 28.0 | 2 | 16.8 | 0.42 | |
| CD56 | Positive | 18 | 41.5 | 10 | 15.9 | 0.01 |
| Negative | 10 | 23.5 | 1 | - | 0.46 | |
| Surgical Procedure | Lobectomy | 26 | 66.9 | 8 | 18.0 | 0.018 |
| Pneumonectomy | 3 | 22.3 | 3 | 15.2 | 0.55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayram, D.; Bal, O.; Erdat, E.C.; Sekmek, S.; Yılmaz, S.; Perkin, P.; Güneş, S.G.; Algin, E.; Yenigün, B.M. Clinicopathologic Disease Characteristics and Their Association with Adjuvant Chemotherapy Outcomes in Pulmonary Large-Cell Carcinoma Patients with or Without Neuroendocrine Features. Diagnostics 2025, 15, 2582. https://doi.org/10.3390/diagnostics15202582
Bayram D, Bal O, Erdat EC, Sekmek S, Yılmaz S, Perkin P, Güneş SG, Algin E, Yenigün BM. Clinicopathologic Disease Characteristics and Their Association with Adjuvant Chemotherapy Outcomes in Pulmonary Large-Cell Carcinoma Patients with or Without Neuroendocrine Features. Diagnostics. 2025; 15(20):2582. https://doi.org/10.3390/diagnostics15202582
Chicago/Turabian StyleBayram, Doğan, Oznur Bal, Efe Cem Erdat, Serhat Sekmek, Saliha Yılmaz, Perihan Perkin, Süleyman Gökalp Güneş, Efnan Algin, and Bülent Mustafa Yenigün. 2025. "Clinicopathologic Disease Characteristics and Their Association with Adjuvant Chemotherapy Outcomes in Pulmonary Large-Cell Carcinoma Patients with or Without Neuroendocrine Features" Diagnostics 15, no. 20: 2582. https://doi.org/10.3390/diagnostics15202582
APA StyleBayram, D., Bal, O., Erdat, E. C., Sekmek, S., Yılmaz, S., Perkin, P., Güneş, S. G., Algin, E., & Yenigün, B. M. (2025). Clinicopathologic Disease Characteristics and Their Association with Adjuvant Chemotherapy Outcomes in Pulmonary Large-Cell Carcinoma Patients with or Without Neuroendocrine Features. Diagnostics, 15(20), 2582. https://doi.org/10.3390/diagnostics15202582







