1. Introduction
Anal Human Papilloma Virus (HPV) infection can cause squamous intraepithelial lesions (SIL), which are precursors of anal squamous cell carcinoma (SCC). People living with HIV (PLWH) are a population at higher risk of HPV infection and HPV-associated lesions [
1,
2,
3], and therefore deserve particular attention in terms of screening and prevention strategies.
In order to prevent the evolution of HPV infection and HPV-related lesions, it is essential to detect infected patients and to implement effective screening programs to early identify the onset of pre-cancerous lesions and their potential progression toward SCC [
4,
5]. However, relatively little is known about the long-term natural history of anal SILs, which is believed to be a dynamic process, with lesions arising and regressing over time [
6]. Nevertheless, the benefits of screening and monitoring lesions over time are supported by several reasons [
7]: (i) the high incidence of anal cancer in the populations for which screening is offered [
8]; (ii) the availability of screening methods that can effectively diagnose high-grade SILs (HSILs) [
9]; (iii) the stage at which anal cancer is detected has a substantial impact on survival rates, so early detection is crucial [
10]; (iv) the significant morbidity and mortality associated with anal cancer can be largely prevented by early screening [
10]. Therefore, it is crucial to analyze the dynamics of anal HPV infection and anal cytology over time.
The diagnostic screening algorithm for anal lesions rely on digital anorectal inspection, HPV molecular testing, cytology and high-resolution anoscopy [
8,
11,
12]. High-resolution anoscopy (gold standard) is generally reserved for those patients who first show altered results on cytology, as this technique is expensive and invasive [
13].
HPV vaccination also plays an important role for the prevention of HPV-related neoplastic lesions in PLWH and, in recent years, it is offered to all prepubescent girls and boys. In the United States, the ACIP recommends HPV vaccination for all children aged 11 to 12 years, and for everyone up to 26 years if they were not adequately vaccinated when younger. HPV vaccination may also be considered for adults aged 27 to 45 years, based on a case-by-case basis considering additional risk factors (e.g., HIV infection) [
14]. Several data demonstrated that HPV vaccination is safe in the presence of HIV [
15,
16,
17,
18,
19,
20,
21], even if some studies show a weaker and more transient immune response when compared to the general population [
19,
22,
23]. However, knowledge about this topic is still scanty and the role and persistence of vaccine protection in PLWH are still widely debated [
24,
25].
Few studies previously investigated the efficacy of HPV vaccination in formerly HPV-infected people, especially if infected by HIV [
26,
27,
28,
29]. Thus, the clinical value of vaccination is still to be determined in this setting. Consequently, it is crucial to examine the effects of HPV vaccination in PLWH already infected by HPV.
The aim of this study was to analyze the relationship between HIV infection and HPV infection over time, taking into account the incidence of:
Occurrence of new infections: in particular any new HPV infection, new Low-Risk HPV (LR-HPV) infection, new High-Risk HPV (HR-HPV) infection and the presence of new infections by HPV genotypes included in the 9-valent vaccine.
Clearance of any HPV genotype, clearance of LR-HPV and HR-HPV genotypes and clearance of HPV genotypes included in the vaccine formulation.
Occurrence of new HPV lesions (Low Grade SIL, HSIL) and their potential progression over time.
Moreover, a secondary study aim was to investigate if HPV vaccine may interact with the occurrence of new infections and/or clearance of previous infections, as well as to evaluate its role in reducing the progression of SILs and the occurrence of new HPV-related lesions.
2. Materials and Methods
This is a retrospective single-center study including PLWH in regular follow-up at the Infectious and Tropical Diseases Clinic of the Azienda Ospedaliera Universitaria Senese (AOUS) which performed longitudinal screening for anal dysplasia and HPV infection for routine clinical practice from January 2018 to March 2024. Age < 18 years was the only exclusion criterion. According to current guidelines [
30] and to our internal protocol, at our center anal HPV screening is proposed annually to all men who have sex with men (MSM) and all subjects with HPV infection or HPV-associated dysplasia at any site. The screening includes cytological analysis and molecular analysis.
Cytological analysis was performed by anal brushing (anal PAP test) and cytological abnormalities were defined according to the Bethesda classification into: no cytological abnormalities, atypical squamous cells of uncertain significance (ASC-US), low-grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesions (HSIL) and lesions that could not be evaluated (i.e., less than 2000 to 3000 nucleated squamous cells) [
31].
Molecular testing was also performed to recognize multiple HPV genotypes by multiplex PCR (Genomic DNA FFPE ONE-STEP kit), distinguishing between high risk (HR-HPV) and low risk (LR-HPV) genotypes [High risk (HR): 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68; Low risk (LR): 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, 84, 89, 90, 102].
Patients were followed from baseline (BL, time of first screening for anal HPV infection) to the last available outpatient visit. Clinical and laboratory variables were obtained from the medical record review: age, sex, risk factors for HIV infection (heterosexual, homosexual/bisexual, unspecified, other/unknown), country of origin, HPV vaccination status. Viroimmunological, therapeutical and biochemical parameters were also considered, as well as comorbidities and other sexually transmitted infections.
From these data, a database was created from which statistical analyses were then performed. Data collection was approved by the local ethic committee and informed consent was obtained from all patients before participation. The study was carried out in accordance with the ethical principles of the Declaration of Helsinki and with the Good Clinical Practice guidelines of the International Conference on Harmonization.
All patients who had at least one anal cytology screening (110 patients) were included in the study and contributed to the descriptive cross-sectional population analysis. The estimated and calculated incidence analyses were performed on subjects who had at least one post-BL follow-up (80 out of 110, longitudinal cohort).
The following outcomes were evaluated for incidence analyses: (i) any new cytological abnormality, defined as the appearance of cytological abnormalities in those with normal BL anal pap test (ii) new HSIL, defined as occurrence of new HSIL lesions in patients without previous HSIL evidence (iii) worsening cytological abnormalities, defined as worsening of cytological stage (from ASCUS to LSIL to HSIL to anal SCC) (iv) clearance of any HPV, defined as any confirmed (at least two consecutive tests) clearance of a HPV genotype during follow-up (v) clearance of LR-HPV, defined as any confirmed (at least two consecutive tests) clearance of a LR-HPV genotype during follow-up (vi) clearance of HR-HPV, defined as any confirmed (at least two consecutive tests) clearance of a HR-HPV genotype during follow-up (vii) any new HPV infection, defined as the detection of any new HPV genotype not previously detected (viii) new LR-HPV infection, defined as the detection of any new LR-HPV genotype not previously detected (ix) new HR-HPV infection, defined as the detection of any new HR-HPV genotype not previously detected. A sub-analysis evaluating new infections or clearance of any HPV genotypes included in the 9-valent HPV vaccine was also performed.
Descriptive statistics (number, proportion, median, interquartile range, 95% confidence interval) were used to describe the baseline characteristics of the patients. Categorical variables were compared between groups using the χ2 test or Fisher’s exact test, as appropriate. Continuous variables were compared using the non-parametric Mann-Whitney U-test. Only values of p < 0.05 were considered significant. To evaluate the occurrence of the different outcomes over time, their incidence was calculated as 100-person year of follow-up (PYFU) with a 95% confidence interval (CI). Kaplan–Meier curves were then used to calculate the estimated incidence at different time points, using log rank test to compare subgroups. We also explored predictors of a new HPV infection or clearance of any HPV genotype by Cox regression analysis; variables associated with the outcome at univariate analysis were then evaluated in a multivariate model to confirm independent associations.
All analyses were performed using SPSS software v.25.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Population Characteristics
A total of 110 PLWH aged between 43 and 56 years (median age 49) were included, of whom 85.5% were males. Population characteristics are reported in
Table 1. The population had the following risk factors for HIV infection: 14.5% were heterosexual patients, while over 54.5% were men who have sex with men (MSM), 0.9% injecting drug users (IDU) and 30% had unknown/other risk factors.
Among this population, 34 patients (30.9%) were vaccinated for HPV, of which only 10 received the vaccine before BL (9.1%). Six of the 34 vaccinated subjects (17.6%) did not complete the full vaccination schedule (3 and 3 patients performed only 1 and 2 doses, respectively).
On average, the median number of years since HIV diagnosis was 8.5. Twenty (18.2%) patients had a history of AIDS events [overall 29 events, the most frequent were: 6 (20.7%) Pneumocystis jirovecii pneumonia, 6 (20.7%) Kaposi Sarcoma, 4 (13.8%) oesophageal candidiasis, 3 (10.3%) non Hodgkin lymphomas, 3 (10.3%) wasting syndromes]. At BL, 3 patients (2.7%) were positive for HbsAg, 36 (32.7%) were positive for HbcAb, 5 (4.5%) had a previous HCV infection and 32 (29.1%) had a history of syphilis. Regarding HIV infection, 84.5% of patients had HIV-RNA < 50 copies/mL and a median CD4 lymphocyte count of 643 cells/mmc, with a median CD4/CD8 ratio of 0.90.
3.2. HPV Infection and Cytological Abnormalities
The presence of HPV infection and cytological abnormalities at baseline and their evolution over follow-up are detailed in
Table 2. At first screening, anal HPV infection was observed in 95 (86.4%) patients, including 26 LR-HPV genotype infections (23.6%) and 69 HR-HPV genotype infections (62.7%). The presence of abnormal cytology was demonstrated in 43 subjects (39.1%), of whom ASCUS
n = 7, 6.4%; LSIL
n = 34, 30.9%; HSIL
n = 2, 1.8%. A total of 33 (30%) patients had lesions that could not be evaluated according to Bethesda classification; of these, only 7 (21.2%) subjects did not show HPV infection, while 7 (21.2%) and 19 (57.6%) individuals demonstrated LR-HPV and HR-HPV, respectively.
A total of 80 (72.7%) PLWH repeated screening during follow-up (longitudinal cohort). The characteristics of patients in the longitudinal cohort were similar to those who did not perform repeated HPV testing, with the exception of a longer history of HIV infection (median 14 versus 8 years, p = 0.011) and a longer exposure to ART (median 10 versus 5 years, p = 0.021) in the former group. In the longitudinal cohort, 28 (35%) received HPV vaccination.
During a median follow-up of 35.3 months (interquartile range, IQR 20.4–49.0) with a median of 4 (IQR 2.3–5) screenings for each patient, no cases of SCC were observed. The presence of any new cytological atypia during follow-up was found in 57 patients (71.3%), the presence of HSIL in 6 patients (7.5%), the progression of cytological lesions in 6 patients (7.5%). Evaluating HPV infection during the follow-up period, 58 cases (72.5%) of new HPV infection occurred and clearance of any HPV genotype in 48 subjects (60.0%) was recorded.
3.3. HPV Vaccination
Characteristics of HPV-vaccinated (
n = 34) or unvaccinated (
n = 76) patients are reported in
Table 3. The median age of the vaccinated population was significantly lower (47 years) than that of the unvaccinated (50 years) (
p = 0.014). Moreover, vaccinated subjects had a shorter time from HIV diagnosis [median 3.0 years (IQR 2.0–13.5) versus 11 years (IQR 5.0–17.0),
p = 0.001] and from antiretroviral therapy initiation [median 3.0 years (IQR 1.0–10.0) versus 8.0 years (IQR 4.0–14.0),
p = 0.001] than unvaccinated individuals. Vaccinated and unvaccinated patients did not show significant differences in other main characteristics (e.g., risk factor, coinfections, viral load, CD4 count, antiretroviral regimen).
The presence of HPV infection and cytological abnormalities at baseline and their evolution over follow-up in vaccinated and unvaccinated patients are detailed in
Table 4.
At BL, the proportion of HPV infection in vaccinated patients was 82.4% (of which 26.5% with LR-HPV infection and 55.9% with HR-HPV infection) compared to 88.2% (of which 22.4% present LR-HPV infection and 65.8% present HR-HPV infection) in unvaccinated (p = 0.604).
At first screening, no cytological abnormalities were seen in 32.4% of vaccinated and 30.3% of unvaccinated patients, respectively (p = 0.348). Cytological abnormalities in vaccinated versus unvaccinated subjects were: ASC-US 0% versus 9.2%, LSILs 35.3% versus 28.9%, HSILs 0% versus 2.6%; moreover, there were some lesions that could not be evaluated (32.4% in vaccinated and 28.9% in unvaccinated patients).
The percentage of patients with an available follow-up after the first screening was 82.4% in the vaccinated population and 68.4% in the non-vaccinated population (p = 0.199). The duration of follow-up was significantly different in the two groups (p = 0.036), with an average of 36.9 months in the vaccinated and 20 months in the unvaccinated.
The vaccinated population who was followed over the time showed an occurrence of any new cytological abnormalities in 58.8% of cases versus 59.2% in unvaccinated. Of these new lesions, the occurrence of HSIL was 5.9% in the vaccinated and 5.3% in the unvaccinated groups. The rate of worsening of cytological lesions was 8.8% in the vaccinated and 3.9% in the unvaccinated (p = 0.371).
Regarding HPV infection during follow-up, there was a significantly higher rate of any new HPV infections (70.6%) in the vaccinated group of patients compared to the unvaccinated (44.7%) (p = 0.021). Of all acquired infections, those from genotypes included in the 9-valent vaccine were 13/21 (61.9%) in vaccinated and 26/74 (35.1%) in unvaccinated patients (p = 0.051).
During follow-up, the clearance of infection occurred in 52.9% and 39.5% in the vaccinated and unvaccinated population, respectively. In particular, the clearance of genotypes contained in the 9-valent vaccine was 61.9% and 35.1% in the vaccinated and non-vaccinated population, respectively (p = 0.051).
3.4. Incidence of HPV Infection/Clearance and Evolution of Cytological Abnormalities over Time
We further analyzed the incidence of HPV infection/clearance and the evolution of cytological abnormalities over time in patients with an available follow-up. The overall number of patients in regular follow-up was 80, of which 28 vaccinated subjects and 52 unvaccinated subjects. Of the 43 subjects with abnormal cytology at baseline, 35 (81.4%) were included in the longitudinal cohort. The median follow-up was 35.3 months (95% CI 24.8–45.8).
During follow-up, the incidence of the different outcomes was as follow: (i) any new cytological abnormality 20 per 100 PYFU (95% CI 8.3–31.7), (ii) new HSIL 2.4 per 100 PYFU (95% CI −0.4–5.2), (iii) worsening cytological abnormalities 2.3 per 100 PYFU (95% CI −0.5–5.1), (iv) clearance of any HPV 24.9 per 100 PYFU (95% CI 16.8–33.0), (v) clearance of LR-HPV 15.1 per 100 PYFU (95% CI 8.4–21.8), (vi) clearance of HR-HPV 13.3 per 100 PYFU (95% CI 7.0–19.6), (vii) any new HPV infection 38.2 per 100 PYFU (95% CI 29.1–47.2), (viii) new LR-HPV infection 9.6 per 100 PYFU (95% CI 4.1–15.1), (ix) new HR-HPV infection 28.6 per 100 PYFU (95% CI 20.2–37.0).
Kaplan–Meier analysis was used to estimate incidence of the different outcomes at various time points (see
Supplementary Figure S1). At 24 months, incidence was as follow in the overall population: (i) any new cytological abnormalities 33.1% (95% CI 18–48.2), (ii) new HSIL 3.1% (95% CI −0.4–6.6), (iii) worsening cytological abnormalities 2.7% (95% CI −0.8–6.2), (iv) clearance of any HPV 39.5% (95% CI 28.1–50.9), (v) clearance of LR-HPV 23.9% (95% CI 13.5–34.3), (vi) clearance of HR-HPV 20.9% (95% CI 11.5–30.3), (vii) any new HPV infections 54.7% (95% CI 42.7–66.7), (viii) new LR-HPV infection 20.6% (95% CI 10.8–30.4), (ix) new HR-HPV infection 38.3 (95% CI 26.5/50.1).
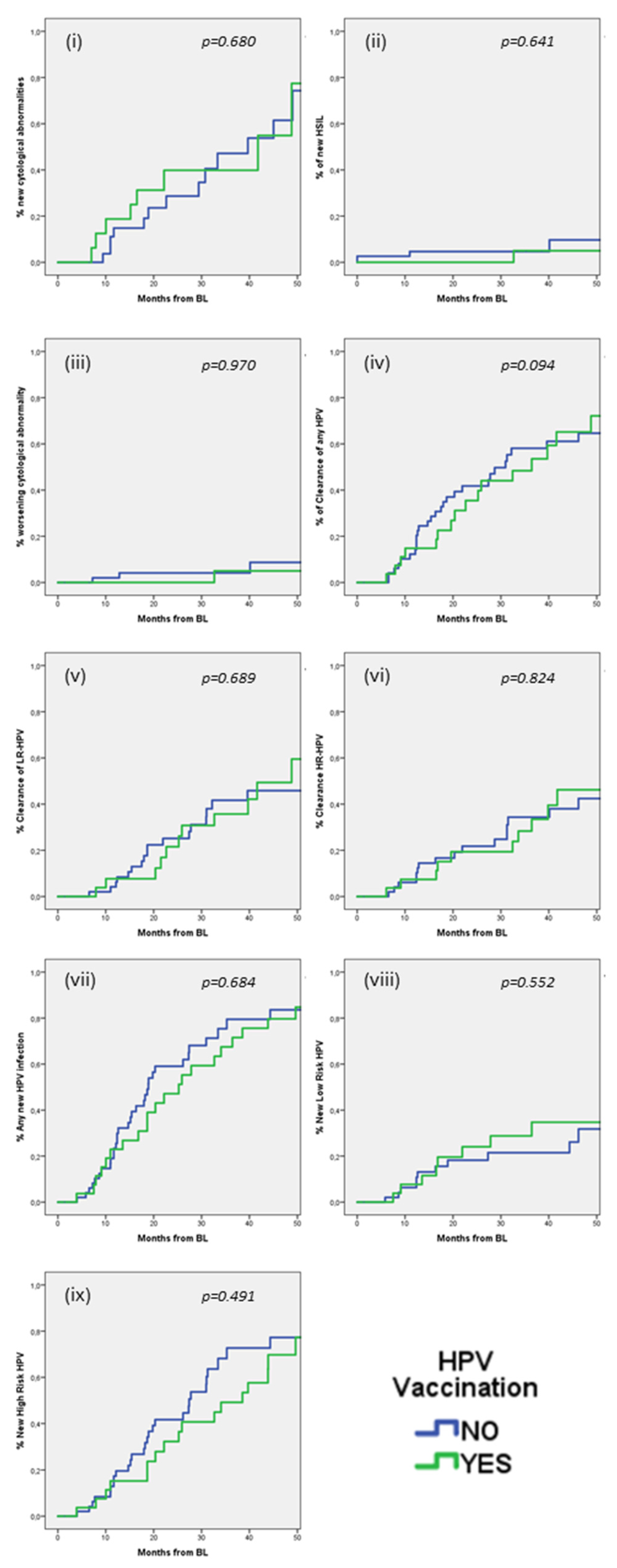
We also explored if HPV vaccination could influence these outcomes (
Figure 1). No significant differences were observed in any of the outcomes (i–ix) between the group of HPV vaccinated and the group of HPV unvaccinated PLWH. At 24 months, the estimated incidences in vaccinated versus unvaccinated patients were: (i) any new cytological abnormalities 39.8% (95% CI 14.5–65.1) versus 28.7% (95% CI 10.5–46.9) (
p = 0.680), (ii) new HSIL 0% versus 4.7% (95% CI −0.6–10.0) (
p = 0.641), (iii) worsening cytological abnormalities 0% versus 4.1% (95% CI −1.6–9.8) (
p = 0.970), (iv) clearance of any HPV 35.5% (95% CI 16.7–54.3) versus 41.8% (95% CI 27.7–55.9) (
p = 0.094), (v) clearance of LR-HPV 21.5% (95% CI 4.6–38.4) versus 25.1% (95% CI 12.2–38.0) (
p = 0.689), (vi) clearance of HR-HPV 19.4% (95% CI 4.1–34.7) versus 21.8% (95% CI 9.6–34.0) (
p = 0.824), (vii) any new HPV infections 47.1% (95% CI 27.7–66.5) versus 59.1% (95% CI 44.2–74.0) (
p = 0.684), (viii) new LR-HPV infection 24.0% (95% CI 7.1–40.9) versus 18.2% (95% CI 6.6–29.8) (
p = 0.552), (ix) new HR-HPV infection 32.2% (95% CI 13.8–50.6) versus 41.7% (95% CI 26.8–56.6) (
p = 0.491).
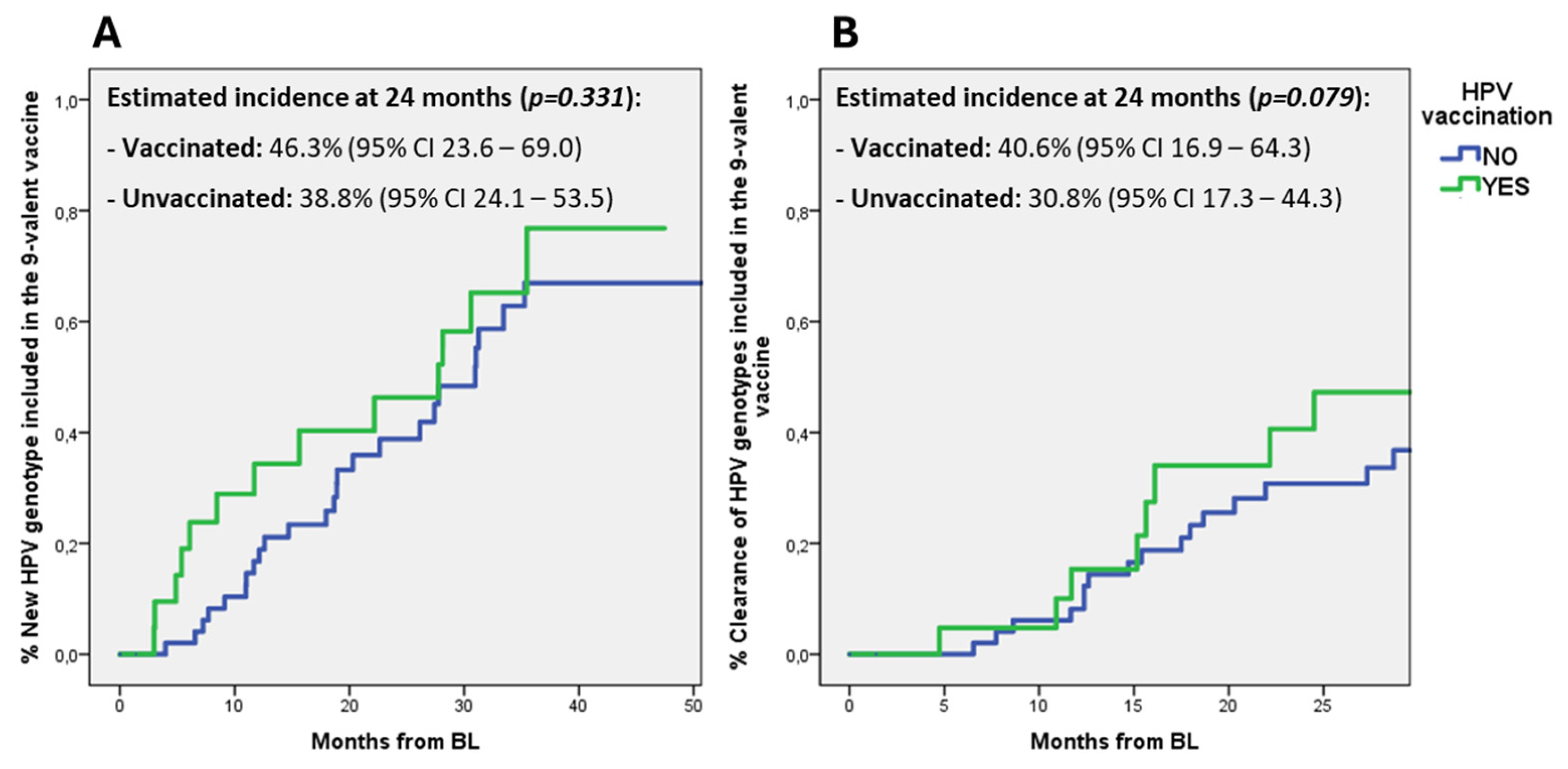
Furthermore, we also explored if HPV vaccination could influence the incidence of new infections or clearance of HPV genotypes included in the 9-valent HPV vaccine. While no differences were observed between vaccinated and unvaccinated patients in the incidence of new infections by HPV genotypes included in the 9-valent vaccine, vaccinated subjects showed a trend toward an increased clearance of vaccinal HPV genotypes when compared to unvaccinated: at 24 months the estimated incidence was 40.6% (95% CI 16.9–64.3) versus 30.8% (95% CI 17.3–44.3), respectively (
p = 0.079) (
Figure 2).
3.5. Predictors of HPV Infection and Clearance over Time
We also explored whether demographic, HPV-related, or HIV-related variables could be associated with the occurrence of a new HPV infection or clearance of any HPV genotype, using Cox regression analysis (
Table 5).
In univariate analysis, prior AIDS-related events were associated with a lower probability of new HPV infections (hazard ratio, HR 0.40, 95% CI 0.17–0.95, p = 0.037), while a CD4 count > 500 cells/mm3 was associated with a higher probability (HR 1.89, 95% CI 1.03–3.47, p = 0.041). However, statistical significance was not confirmed in multivariate analysis [AIDS: adjusted HR (aHR) 0.48, 95% CI 0.20–1.16, p = 0.103; CD4 > 500 cells/mmc: aHR 1.58, 95% CI 0.84–2.97, p = 0.155).
Exploring predictors of HPV clearance, in univariate analysis subjects with HR-HPV showed a lower probability (HR 0.50, 95% CI 0.28–0.91, p = 0.023), while those with CD4 count > 500 cells/mmc had a higher probability (HR 2.62, 95% CI 1.24–5.54, p = 0.011) of any HPV genotype clearance. However, in the multivariate analysis only CD4 cells > 500/mmc remained an independent predictor of HPV clearance (aHR 2.44, 95% CI 1.15–5.18, p = 0.021), while infections with HR-HPV genotypes showed only a nearly significant trend towards a reduced probability of clearance (aHR 0.56, 95% CI 0.31–1.01, p = 0.054).
4. Discussion
In our study, we evaluated the prevalence and the evolution over time of anal HPV infection and HPV-associated cytological abnormalities in a population of PLWH. At the first screening, we observed a high prevalence (86.4%) of anal HPV infection. This high prevalence is similar to that reported in other cohorts of PLWH [
12,
32,
33,
34], which are a population at increased risk of HPV infection and its associated pre-cancerous/cancerous lesions [
35]. Moreover, our cohort was predominantly constituted by MSM, which are a group at increased risk of anal HPV infection, and this could have contributed to the high prevalence of HPV infection [
36].
Since most HPV infections were sustained by HR-HPV genotypes (62.7%), this could translate in a higher risk of developing cytological abnormalities. Indeed, at the first screening, cytological abnormalities were quite common in our cohort, being recognized in 39.1% of patients. Most of them were classified as LSIL (30.9%), with only 1.8% of HSIL and no cases of SCC. Since the evolution of SILs toward overt cancerous lesions is increased in PLWH, the high prevalence of SIL in our cohort highlights the importance of a longitudinal screening program in PLWH to early diagnose anal HPV infection and pre-cancerous lesions to prevent anal SCC [
4,
11].
Despite repeated screenings being proposed to all subjects in our cohort, only 72.7% consented to re-testing and had an available follow-up. This highlights the importance of strengthening counseling to all PLWH at risk for HPV infection, to improve adherence to screening procedures. Moreover, several structural and organizational adjustments should be considered at a local level to link screening procedures for HPV to the routine clinical care of PLWH, thus making screening compliance easier.
During follow-up, no cases of anal SCC were observed in our cohort. However, in the longitudinal cohort, the proportion of patients with cytological abnormalities increased to 71.3%, with 7.5% of patients showing a progression of cytological abnormalities over time and 7.5% developing HSIL. The incidence of any new cytological abnormalities was 20 per 100 PYFU, with an incidence of HSIL and of worsening cytological abnormalities of 1.6 and 2.3 per 100 PYFU, respectively. These data confirm that anal lesions can evolve over time, thus further enhancing the relevance of implementing longitudinal screening for SCC prevention. It will be relevant to evaluate if a further progression of cytological abnormalities and eventually the appearance of cases of anal SCC will be observed by prolonging follow-up.
In our longitudinal cohort, we also explored the dynamics of HPV infection over time by evaluating the appearance/clearance of HPV genotypes and we estimated incidence of various outcomes. In general, a dynamic process was observed with appearance and clearance over time of different genotypes also in the same patient. Overall, 60% of patients showed the clearance of at least one HPV genotype, with an incidence of 24.9 per 100 PYFU. The proportion of clearance was similar for HR-HPV and LR-HPV (38.8% and 37.5%, respectively). On the other side, appearance of new HPV genotypes was also observed in a substantial proportion of patients, since during follow-up 72.5% showed new genotypes not detected at baseline, with an incidence of 38.2 per 100 PYFU. This was more common for HR-HPV than for LR-HPV (61.3% versus 21.3% of subjects, respectively). These data can have several explanations. HPV persistence in tissues is a dynamic process that is influenced by a balance between HPV replication and immune system activity [
37,
38]. Indeed, clearance of HPV genotypes can be the result of an adequate immune pression on the virus. However, also inadequate sampling could have played a role. On the other side, the appearance of new HPV genotypes could be the result of new HPV infections or of HPV reactivation due to an impaired immune system.
The acquisition of HPV and its dynamics over time can be influenced by HPV vaccination, the effectiveness of which is widely documented also in PLWH for primary prevention [
20]. In our population, only 9.1% of patients had received HPV vaccine before baseline. The low proportion of patients previously vaccinated against HPV can partly be related to the median age (49 years) of our population, mainly constituted by subjects born when universal HPV vaccination in prepubertal children had not yet been introduced. Indeed, median age of vaccinated patients was lower than that of unvaccinated (47 versus 50 years). The proportion of vaccinated subjects increased to 30.9% after first screening, despite the efficacy of HPV vaccine for preventing anal SCC in adult PLWH who already acquired HPV is controversial [
39].
We also explored if HPV vaccination could influence HPV acquisition/clearance and the evolution of cytological abnormalities. No significant differences were observed between the group of HPV vaccinated and the group of HPV unvaccinated patients in the incidence of any new cytological abnormality, clearance or acquisition of any HPV. However, 9-valent vaccinated subjects showed a trend toward an increased clearance of HPV vaccinal genotypes (40.6% versus 30.8% when compared to unvaccinated, p = 0.079). Although not reaching statistical significance partly due to the small sample size, this observation raises questions whether HPV vaccination may contribute to enhance the immunological response against genotypes it contains, potentially leading to HPV clearance in patients who had previously acquired the infection.
In our population, we could not demonstrate any independent predictors of infections with new HPV genotypes. However, a CD4 count > 500 cells/mmc was independently associated with a higher likelihood of HPV clearance, suggesting that a compromised immune system may play a relevant role in HPV persistence. We also observed that HR-HPV genotypes were less likely to be cleared over time than LR-HPV, thus highlighting the importance of strengthening prevention measures (e.g., vaccination) to prevent HR-HPV infections, which are associated with an increased risk of cytological abnormalities and progression to SCC. Some limitations should be recognized when interpreting the results of our study. Its retrospective and observational design could have introduced potential biases, since patients undergoing screening procedures could have been selected based on unaccounted variables. However, our protocol for PLWH recommends HPV screening in all MSM and other groups at high risk for HPV infection and its related cytological abnormalities, therefore, screening should have been proposed to all eligible patients. Not all patients were adherent to longitudinal screening, the duration of follow-up was limited (median nearly 3 years) and few subjects performed the suggested HPV vaccination, thus reducing sample size and consequently statistical power of the analyses. Based on this, it is not possible to draw definitive conclusions on whether the vaccine is effective or not in preventing new infections and/or increasing the resolution of previous infections. Moreover, at baseline some patients had lesions that could not be evaluated according to Bethesda classification, and this can influence incidence estimates of cytological abnormalities. However, high rates of unsatisfactory anal cytology have been previously described in other cohorts, mainly attributed to inadequate sample collection [
40]. Therefore, more standardization in sampling techniques is advisable to avoid misclassification.
5. Conclusions
A high prevalence of anal HPV infection and squamous intraepithelial lesions (SILs) was observed in our cohort of PLWH; furthermore, incidence estimates suggest a continuing increase over time in new infections and associated lesions.
This highlights the importance of strengthening immunization programs and continuous screening for anal HPV infection as well as for the presence of anal dysplasia in this population.
This study could not demonstrate the efficacy of the 9-valent HPV vaccine in preventing either the acquisition of HPV genotypes nor the appearance/evolution of pre-neoplastic lesions in PLWH (especially in patients already infected with another HPV genotype). However, a trend toward greater clearance of genotypes included in 9-valent HPV vaccine was observed in vaccinated patients, raising the question of whether HPV vaccine may be at least partially efficacious in stimulating the immune system of these patients. Given the small sample size and the still short duration of follow-up, considering the normal evolution of HPV infection, further prospective studies are needed to verify these results.
Author Contributions
Conceptualization, F.M. (Francesca Montagnani)., M.T. and M.F.; methodology, F.M. (Francesca Montagnani) and M.F.; formal analysis, M.F.; investigation, M.S., A.B., F.M. (Francesca Montagnani), M.G., N.C., V.M., F.M. (Federico Mariani), M.T., S.L., F.R. and M.F.; data curation, M.S., A.B., A.D. and M.F.; writing—original draft preparation, M.S. and M.F.; writing—review and editing, F.M. (Francesca Montagnani) and M.F.; supervision, F.M. (Francesca Montagnani), M.T. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to guidelines of the Declaration of Helsinki, and approved by Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana, CEAVSE (protocol code 1237, date of approval 21 July 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical reasons.
Acknowledgments
We acknowledge all patients enrolled in the study and nurse staff for their support. We thank Riccardo Rimondi for reviewing the English language.
Conflicts of Interest
M.F. received speakers’ honoraria, support for travel to meetings, and/or fees for attending advisory boards from Bristol Myers Squibb (BMS), Gilead, Janssen-Cilag, Merck Sharp and Dohme (MSD), and ViiV Healthcare. The authors declare no conflicts of interest.
References
- Khandwala, P.; Singhal, S.; Desai, D.; Parsi, M.; Potdar, R. HIV-Associated Anal Cancer. Cureus 2021, 13, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Marra, E.; Lin, C.; Clifford, G.M. Type-Specific Anal Human Papillomavirus Prevalence among Men, According to Sexual Preference and HIV Status: A Systematic Literature Review and Meta-Analysis. J. Infect. Dis. 2019, 219, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Revollo, B.; Videla, S.; Sirera, G.; Garcia-Cuyas, F.; Pares, D.; Corral, J.; Clotet, B.; Llibre, J. Natural History of Anal Squamous Intraepithelial Lesions in HIV-Positive Men with Normal Baseline Cytology. AIDS Patient Care STDS 2019, 33, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Poljak, M.; Šterbenc, A.; Lunar, M.M. Prevention of Human Papillomavirus (HPV)-Related Tumors in People Living with Human Immunodeficiency Virus (HIV). Expert Rev. Anti. Infect. Ther. 2017, 15, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Roelandt, P.; De Looze, D.; De Schepper, H.; Ledouble, V.; Surmont, M.; Cuming, T. Diagnosis and Screening for Anal Intraepithelial Neoplasia in Belgium: Position Statement. Acta Gastroenterol. Belg. 2022, 85, 625–631. [Google Scholar] [CrossRef]
- Barroso, L.F. The Natural History of Anal Dysplasia: Unwrapping the Riddle to Find an Enigma. J. Infect. Dis. 2020, 222, 7–8. [Google Scholar] [CrossRef]
- Chiao, E.Y.; Giordano, T.P.; Palefsky, J.M.; Tyring, S.; Serag, H. El Screening HIV-Infected Individuals for Anal Cancer Precursor Lesions: A Systematic Review. Clin. Infect. Dis. 2006, 43, 223–233. [Google Scholar] [CrossRef]
- Stier, E.A.; Clarke, M.A.; Deshmukh, A.A.; Wentzensen, N.; Liu, Y.; Poynten, I.M.; Cavallari, E.N.; Fink, V.; Barroso, L.F.; Clifford, G.M.; et al. International Anal Neoplasia Society’s Consensus Guidelines for Anal Cancer Screening. Int. J. Cancer 2024, 154, 1694–1702. [Google Scholar] [CrossRef]
- Albuquerque, A. Cytology in Anal Cancer Screening: Practical Review for Clinicians. Acta Cytol. 2020, 64, 281–287. [Google Scholar] [CrossRef]
- Palefsky, J.M.; Lee, J.Y.; Jay, N.; Goldstone, S.E.; Darragh, T.M.; Dunlevy, H.A.; Rosa-Cunha, I.; Arons, A.; Pugliese, J.C.; Vena, D.; et al. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N. Engl. J. Med. 2022, 386, 2273–2282. [Google Scholar] [CrossRef]
- Park, I.U.; Palefsky, J.M. Evaluation and Management of Anal Intraepithelial Neoplasia in HIV-Negative and HIV-Positive Men Who Have Sex with Men. Curr. Infect. Dis. Rep. 2010, 12, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Rodríguez-Rivero, S.; Fernández-Veiga, P.; Flores, E.; Poveda, E.; González-Carreró, J.; Pérez-Castro, S.; Labajo-Leal, L.; Miralles, C.; Ocampo, A. Anal Dysplasia Screening in People Living with HIV: Long-Term Follow-Up in a Large Cohort from Northwest Spain. AIDS Patient Care STDS 2024, 38, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.M.; Palefsky, J.M.; Jay, N.; Cheng, S.C.; Darragh, T.M.; Chin-Hong, P.V. Performance Characteristics of Anal Cytology and Human Papillomavirus Testing in Patients with High-Resolution Anoscopy-Guided Biopsy of High-Grade Anal Intraepithelial Neoplasia. Dis. Colon Rectum 2009, 52, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Meites, E.; Szilagyi, P.G.; Harrell, C.W.; Chesson, W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Morbidity and Mortality Weekly Report Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. US Dep. Health Hum. Serv. 2019, 68, 698–702. [Google Scholar]
- Kahn, J.A.; Xu, J.; Kapogiannis, B.G.; Rudy, B.; Gonin, R.; Liu, N.; Wilson, C.M.; Worrell, C.; Squires, K.E. Immunogenicity and Safety of the Human Papillomavirus 6, 11, 16, 18 Vaccine in HIV-Infected Young Women. Clin. Infect. Dis. 2013, 57, 735–744. [Google Scholar] [CrossRef][Green Version]
- Kojic, E.M.; Kang, M.; Cespedes, M.S.; Umbleja, T.; Godfrey, C.; Allen, R.T.; Firnhaber, C.; Grinsztejn, B.; Palefsky, J.M.; Webster-Cyriaque, J.Y.; et al. Immunogenicity and Safety of the Quadrivalent Human Papillomavirus Vaccine in HIV-1-Infected Women. Clin. Infect. Dis. 2014, 59, 127–135. [Google Scholar] [CrossRef]
- Wilkin, T.; Lee, J.Y.; Lensing, S.Y.; Stier, E.A.; Goldstone, S.E.; Berry, J.M.; Jay, N.; Aboulafia, D.; Cohn, D.L.; Einstein, M.H.; et al. Safety and Immunogenicity of the Quadrivalent Human Papillomavirus Vaccine in HIV-1-Infected Men. J. Infect. Dis. 2010, 202, 1246–1253. [Google Scholar] [CrossRef]
- Denny, L.; Hendricks, B.; Gordon, C.; Thomas, F.; Hezareh, M.; Dobbelaere, K.; Durand, C.; Hervé, C.; Descamps, D. Safety and Immunogenicity of the HPV-16/18 AS04-Adjuvanted Vaccine in HIV-Positive Women in South Africa: A Partially-Blind Randomised Placebo-Controlled Study. Vaccine 2013, 31, 5745–5753. [Google Scholar] [CrossRef]
- Bergman, H.; Buckley, B.S.; Villanueva, G.; Petkovic, J.; Garritty, C.; Lutje, V.; Riveros-Balta, A.X.; Low, N.; Henschke, N. Comparison of Different Human Papillomavirus (HPV) Vaccine Types and Dose Schedules for Prevention of HPV-Related Disease in Females and Males. Cochrane Database Syst. Rev. 2019, 2019, CD013479. [Google Scholar] [CrossRef]
- Zizza, A.; Banchelli, F.; Guido, M.; Marotta, C.; Di Gennaro, F.; Mazzucco, W.; Pistotti, V.; D’Amico, R. Efficacy and Safety of Human Papillomavirus Vaccination in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 4954. [Google Scholar] [CrossRef]
- Tortellini, E.; Fosso Ngangue, Y.C.; Dominelli, F.; Guardiani, M.; Falvino, C.; Mengoni, F.; Carraro, A.; Marocco, R.; Pasculli, P.; Mastroianni, C.M.; et al. Immunogenicity and Efficacy of Vaccination in People Living with Human Immunodeficiency Virus. Viruses 2023, 15, 1844. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.; Bitnun, A.; Alimenti, A.; Lapointe, N.; Samson, L.; Read, S.; Karatzios, C.; Dobson, S.; Moses, E.; Blitz, S.; et al. Immunogenicity and Safety of the Quadrivalent Human Papillomavirus Vaccine in Girls Living with HIV. Pediatr. Infect. Dis. J. 2018, 37, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Huang, S.; Moscicki, A.B.; Song, L.Y.; Read, J.S.; Meyer, W.A.; Saah, A.J.; Richardson, K.; Weinberg, A. Four-Year Persistence of Type-Specific Immunity after Quadrivalent Human Papillomavirus Vaccination in HIV-Infected Children: Effect of a Fourth Dose of Vaccine. Vaccine 2017, 35, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Lacey, C.J. HPV Vaccination in HIV Infection. Papillomavirus Res. 2019, 8, 100174. [Google Scholar] [CrossRef]
- Losada, C.; Samaha, H.; Scherer, E.M.; Kazzi, B.; Khalil, L.; Ofotokun, I.; Rouphael, N. Efficacy and Durability of Immune Response after Receipt of HPV Vaccines in People Living with HIV. Vaccines 2023, 11, 1067. [Google Scholar] [CrossRef]
- Szarewski, A.; Poppe, W.A.J.; Skinner, S.R.; Wheeler, C.M.; Paavonen, J.; Naud, P.; Salmeron, J.; Chow, S.N.; Apter, D.; Kitchener, H.; et al. Efficacy of the Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted Vaccine in Women Aged 15-25 Years with and without Serological Evidence of Previous Exposure to HPV-16/18. Int. J. Cancer 2012, 131, 106–116. [Google Scholar] [CrossRef]
- Mensah, F.A.; Mehta, M.R.; Lewis, J.S.; Lockhart, A.C. The Human Papillomavirus Vaccine: Current Perspective and Future Role in Prevention and Treatment of Anal Intraepithelial Neoplasia and Anal Cancer. Oncologist 2016, 21, 453–460. [Google Scholar] [CrossRef]
- Beachler, D.C.; Kreimer, A.R.; Schiffman, M.; Herrero, R.; Wacholder, S.; Rodriguez, A.C.; Lowy, D.R.; Porras, C.; Schiller, J.T.; Quint, W.; et al. Multisite HPV16/18 Vaccine Efficacy Against Cervical, Anal, and Oral HPV Infection. J. Natl. Cancer Inst. 2016, 108, djv302. [Google Scholar] [CrossRef]
- López-Codony, V.; De Andrés-Pablo, Á.; Ferrando-Díez, A.; Fernández-Montolí, M.E.; Lopez-Querol, M.; Tous, S.; Ortega-Expósito, C.; Torrejón-Becerra, J.C.; Pérez, Y.; Ferrer-Artola, A.; et al. Assessing the Reduction of Viral Infectivity in HPV16/18-Positive Women after One, Two, and Three Doses of Gardasil-9 (RIFT): Study Protocol. PLoS ONE 2024, 19, e0304080. [Google Scholar] [CrossRef]
- EACS Secretariat EACS Guidelines Version 12.0. Available online: https://www.eacsociety.org/media/guidelines-12.0.pdf (accessed on 22 September 2022).
- Solomon, D.; Davey, D.; Kurman, R.; Moriarty, A.; Connor, D.O.; Raab, S.; Sherman, M.; Wilbur, D.; Wright, T.; Young, N. Human Papillomavirus, Indigenous Paraguayan Women, Genital Infections, Cervical Inflammation. J. Am. Med. Assoc. 2002, 287, 2114–2119. [Google Scholar] [CrossRef]
- Phanuphak, N.; Teeraananchai, S.; Hansudewechakul, R.; Gatechompol, S.; Chokephaibulkit, K.; Dang, H.L.D.; Tran, D.N.H.; Achalapong, J.; Teeratakulpisarn, N.; Chalermchockcharoenkit, A.; et al. Incidence and Persistence of High-Risk Anogenital Human Papillomavirus Infection among Female Youth with and Without Perinatally Acquired Human Immunodefiency Virus Infection: A 3-Year Observational Cohort Study. Clin. Infect. Dis. 2020, 71, E270–E280. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.; Walker, S.; Grulich, A.; Hoy, J.; Read, T.R.H.; Bradshaw, C.; Chen, M.; Garland, S.M.; Cornall, A.; Hillman, R.; et al. Incidence, Clearance, and Persistence of Anal Human Papillomavirus in Men Who Have Sex with Men Living with Human Immunodeficiency Virus: Implications for Human Papillomavirus Vaccination. Sex. Transm. Dis. 2019, 46, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.A.; Belzer, M.; Chi, X.; Lee, J.; Gaur, A.H.; Mayer, K.; Martinez, J.; Futterman, D.C.; Stier, E.A.; Paul, M.E.; et al. Pre-Vaccination Prevalence of Anogenital and Oral Human Papillomavirus in Young HIV-Infected Men Who Have Sex with Men. Papillomavirus Res. 2019, 7, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.J.; Lau, B.; Justice, A.C.; Engels, E.; Gill, M.J.; Goedert, J.J.; Kirk, G.D.; D’Souza, G.; Bosch, R.J.; Brooks, J.T.; et al. Risk of Anal Cancer in HIV-Infected and HIV-Uninfected Individuals in North America. Clin. Infect. Dis. 2012, 54, 1026–1034. [Google Scholar] [CrossRef]
- Machalek, D.A.; Poynten, M.; Jin, F.; Fairley, C.K.; Farnsworth, A.; Garland, S.M.; Hillman, R.J.; Petoumenos, K.; Roberts, J.; Tabrizi, S.N.; et al. Anal Human Papillomavirus Infection and Associated Neoplastic Lesions in Men Who Have Sex with Men: A Systematic Review and Meta-Analysis. Lancet Oncol. 2012, 13, 487–500. [Google Scholar] [CrossRef]
- Hibma, M.H. The Immune Response to Papillomavirus During Infection Persistence and Regression. Open Virol. J. 2013, 6, 241–248. [Google Scholar] [CrossRef]
- Castro-Muñoz, L.J.; Rocha-Zavaleta, L.; Lizano, M.; Ramírez-Alcántara, K.M.; Madrid-Marina, V.; Manzo-Merino, J. Alteration of the IFN-Pathway by Human Papillomavirus Proteins: Antiviral Immune Response Evasion Mechanism. Biomedicines 2022, 10, 2965. [Google Scholar] [CrossRef]
- Wilkin, T.J.; Chen, H.; Cespedes, M.S.; Leon-Cruz, J.T.; Godfrey, C.; Chiao, E.Y.; Bastow, B.; Webster-Cyriaque, J.; Feng, Q.; Dragavon, J.; et al. A Randomized, Placebo-Controlled Trial of the Quadrivalent Human Papillomavirus Vaccine in Human Immunodeficiency Virus-Infected Adults Aged 27 Years or Older: AIDS Clinical Trials Group Protocol A5298. Clin. Infect. Dis. 2018, 67, 1339–1346. [Google Scholar] [CrossRef]
- Khattab, R.; McMeekin, E.; Taege, A.J.; Hekman, J.M.; Brainard, J.A.; Underwood, D.; Procop, G.W.; Sturgis, C.D. Unsatisfactory Exfoliative Anal Cytology Samples, 15-Year Experience with Histologic, Cytologic, and Molecular Follow-Up. Diagn. Cytopathol. 2018, 46, 117–121. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).