Advances in Cardiovascular Multimodality Imaging in Patients with Marfan Syndrome
Abstract
1. Introduction
2. The Use of Echocardiography in Marfan Syndrome
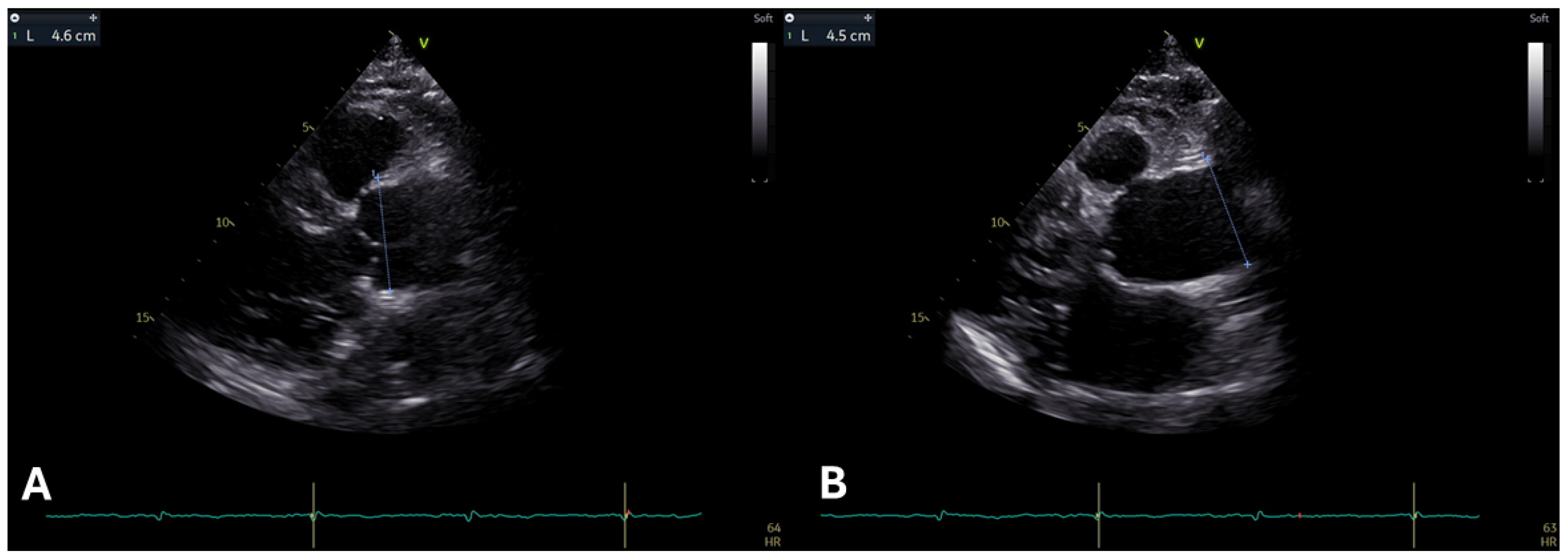
2.1. Aortic Root and Proximal Ascending Aorta Dilatation
2.2. Mitral Valve
2.3. Pulmonary Artery
2.4. Marfan Cardiomyopathy
2.5. Right Ventricle (RV) and Atria
3. The Role of Cardiovascular Magnetic Resonance in Marfan Syndrome
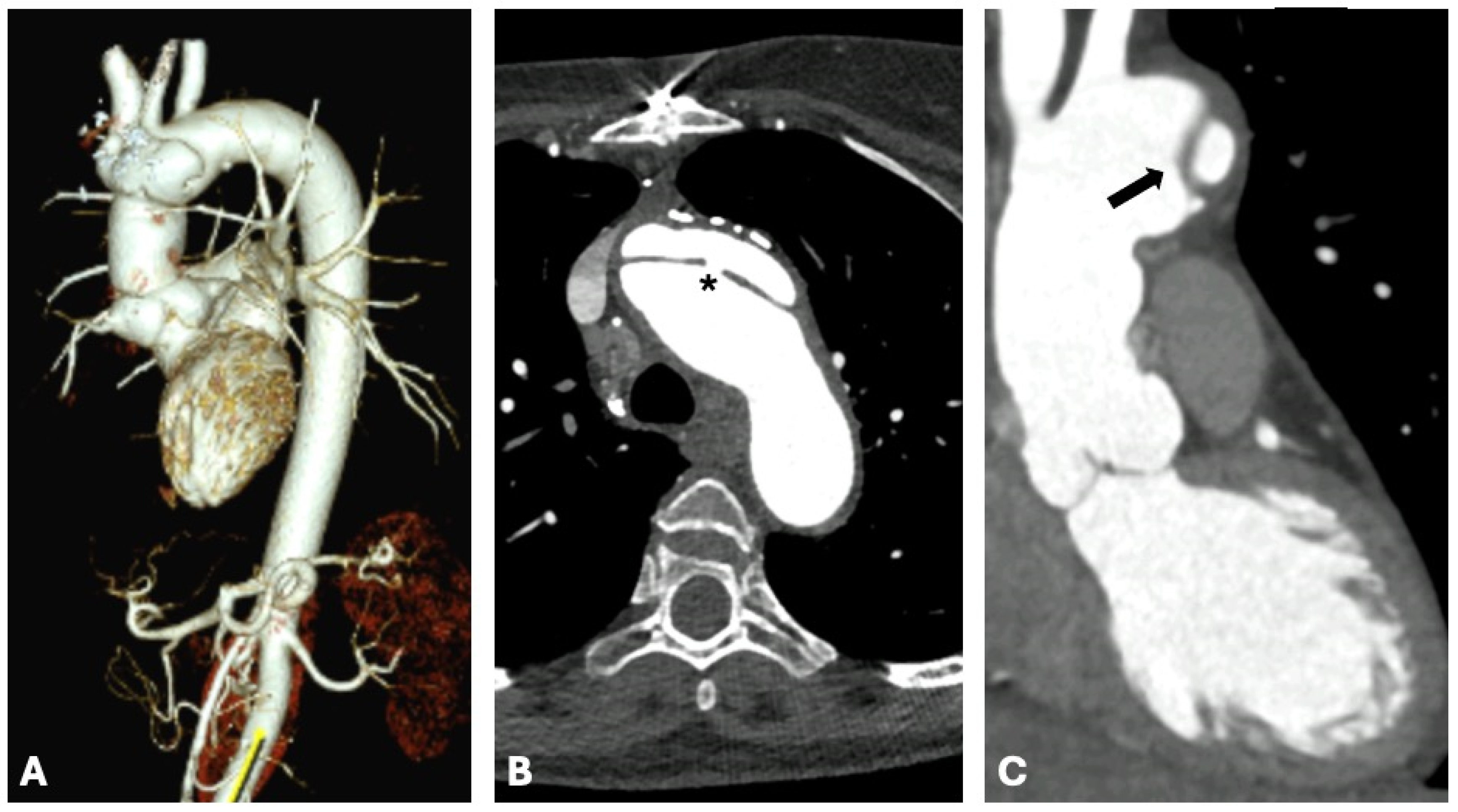
4. Computed Tomography Uses in Individuals with Marfan Syndrome
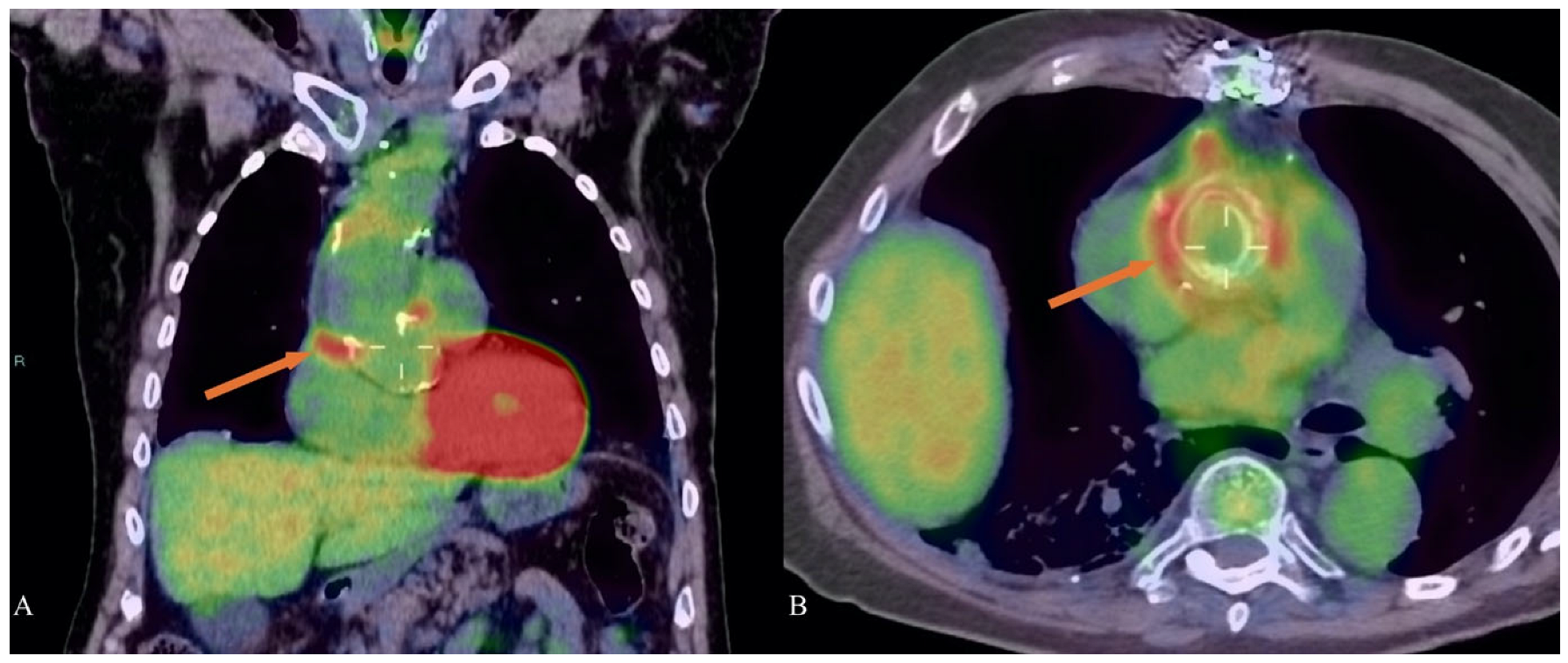
5. Nuclear Medicine and Marfan Syndrome
6. Literature Limitations in Multi-Imaging Modalities in Marfan Syndrome
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AD | Aortic Distensibility |
| BAV | Bicuspid Aortic Valve |
| b-SSFP | Balanced Steady-State Free Precession |
| BSA | Body Surface Area |
| CCT | Cardiac Computed Tomography |
| CEMRA | Contrast-Enhanced Magnetic Resonance Angiography |
| CMR | Cardiovascular Magnetic Resonance |
| EF | Ejection Fraction |
| EANM | European Association of Nuclear Medicine |
| ESC | European Society of Cardiology |
| GLS | Global Systolic Longitudinal Strain |
| HI | Haller Index |
| HU | Hounsfield Units |
| LGE | Late Gadolinium Enhancement |
| LV | Left Ventricle |
| LVEDD | Left Ventricular End-Diastolic Diameter |
| MAD | Mitral Annular Disjunction |
| MFS | Marfan Syndrome |
| MR | Mitral Regurgitation |
| MRI | Magnetic Resonance Imaging |
| MVP | Mitral Valve Prolapse |
| PC | Photon Counting |
| PET | Positron Emission Tomography |
| PSI | Pixel Signal Intensity |
| PWV | Pulse Wave Velocity |
| ROI | Region of Interest |
| RV | Right Ventricle |
| SPECT | Single Photon Emission Computed Tomography |
| SNMMI | Society of Nuclear Medicine and Molecular Imaging |
| SSFP | Steady-State Free Precession Imaging |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TDI | Tissue Doppler Imaging |
| TEE | Transesophageal Echocardiography |
| TTE | Transthoracic Echocardiography |
| VT | Ventricular Tachycardia |
| WSS | Wall Shear Stress |
| 99mTc-HMPAO | Technetium-99m hexamethylpropyleneamine oxime |
| WBC | White Blood Cell Introduction |
References
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef]
- Ramlingam, G.; Natarajasundaram, U.M. Ghent Criteria an Aid to Diagnose Latent Systemic Diseases in Marfan Syndrome. J. Clin. Diagn. Res. 2015, 9, ZJ01–ZJ02. [Google Scholar] [CrossRef]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [PubMed]
- Yu, C.C.; Su, Y.N.; Lin, J.L.; Lai, L.P. Marfan Syndrome-An Echocardiographer’s Perspective. J. Med. Ultrasound 2011, 19, 1–6. [Google Scholar] [CrossRef]
- Zeigler, S.M.; Sloan, B.; Jones, J.A. Pathophysiology and Pathogenesis of Marfan Syndrome. Adv. Exp. Med. Biol. 2021, 1348, 185–206. [Google Scholar] [PubMed]
- Selamet Tierney, E.S.; Levine, J.C.; Chen, S.; Bradley, T.J.; Pearson, G.D.; Colan, S.D.; Sleeper, L.A.; Campbell, M.J.; Cohen, M.S.; De Backer, J.; et al. Echocardiographic methods, quality review, and measurement accuracy in a randomized multicenter clinical trial of Marfan syndrome. J. Am. Soc. Echocardiogr. 2013, 26, 657–666. [Google Scholar] [CrossRef][Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Moscatelli, S.; Pergola, V.; Motta, R.; Fortuni, F.; Borrelli, N.; Sabatino, J.; Leo, I.; Avesani, M.; Montanaro, C.; Surkova, E.; et al. Multimodality Imaging Assessment of Tetralogy of Fallot: From Diagnosis to Long-Term Follow-Up. Children 2023, 10, 1747. [Google Scholar] [CrossRef]
- Mariucci, E.; Bonori, L.; Lovato, L.; Graziano, C.; Ciuca, C.; Pacini, D.; Di Marco, L.; Angeli, E.; Careddu, L.; Gargiulo, G.; et al. Coronary Artery Aneurysms in Patients With Marfan Syndrome: Frequent, Progressive, and Relevant. Can. J. Cardiol. 2021, 37, 1225–1231. [Google Scholar] [CrossRef]
- Colan, S.D. Normal Echocardiographic Values for Cardiovascular Structures. In Echocardiography in Pediatric and Congenital Heart Disease; Wiley: Hoboken, NJ, USA, 2016; pp. 883–901. [Google Scholar] [CrossRef]
- Chivulescu, M.; Krohg-Sørensen, K.; Scheirlynck, E.; Lindberg, B.R.; Dejgaard, L.A.; Lie, Ø.H.; Helle-Valle, T.; Skjølsvik, E.T.; Estensen, M.E.; Edvardsen, T.; et al. Mitral annulus disjunction is associated with adverse outcome in Marfan and Loeys-Dietz syndromes. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1035–1044. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B.; Kramer-Fox, R.; O’Loughlin, J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am. J. Cardiol. 1989, 64, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Lino Cardenas, C.L.; Lindsay, M.E. Hereditary Influence in Thoracic Aortic Aneurysm and Dissection. Circulation 2016, 133, 2516–2528. [Google Scholar] [CrossRef] [PubMed]
- Jondeau, G.; Detaint, D.; Tubach, F.; Arnoult, F.; Milleron, O.; Raoux, F.; Delorme, G.; Mimoun, L.; Krapf, L.; Hamroun, D.; et al. Aortic event rate in the Marfan population: A cohort study. Circulation 2012, 125, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Aburawi, E.H.; O’Sullivan, J. Relation of aortic root dilatation and age in Marfan’s syndrome. Eur. Heart J. 2007, 28, 376–379. [Google Scholar] [CrossRef]
- Taub, C.C.; Stoler, J.M.; Perez-Sanz, T.; Chu, J.; Isselbacher, E.M.; Picard, M.H.; Weyman, A.E. Mitral valve prolapse in Marfan syndrome: An old topic revisited. Echocardiography 2009, 26, 357–364. [Google Scholar] [CrossRef]
- Kunkala, M.R.; Schaff, H.V.; Li, Z.; Volguina, I.; Dietz, H.C.; LeMaire, S.A.; Coselli, J.S.; Connolly, H. Mitral valve disease in patients with Marfan syndrome undergoing aortic root replacement. Circulation 2013, 128, S243–S247. [Google Scholar] [CrossRef]
- Hayek, E.; Gring, C.N.; Griffin, B.P. Mitral valve prolapse. Lancet 2005, 365, 507–518. [Google Scholar] [CrossRef]
- Malagoli, A.; Albini, A.; Benfari, G.; Ilardi, F.; Lisi, M.; Mandoli, G.E.; Pastore, M.C.; Sperlongano, S.; Cameli, M.; D’Andrea, A. Arrhythmic mitral valve prolapse: A practical approach for asymptomatic patients. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 293–301. [Google Scholar] [CrossRef]
- Détaint, D.; Faivre, L.; Collod-Beroud, G.; Child, A.H.; Loeys, B.L.; Binquet, C.; Gautier, E.; Arbustini, E.; Mayer, K.; Arslan-Kirchner, M.; et al. Cardiovascular manifestations in men and women carrying a FBN1 mutation. Eur. Heart J. 2010, 31, 2223–2229. [Google Scholar] [CrossRef]
- Demolder, A.; Timmermans, F.; Duytschaever, M.; Muiño-Mosquera, L.; De Backer, J. Association of Mitral Annular Disjunction With Cardiovascular Outcomes Among Patients With Marfan Syndrome. JAMA Cardiol. 2021, 6, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef] [PubMed]
- Carmo, P.; Andrade, M.J.; Aguiar, C.; Rodrigues, R.; Gouveia, R.; Silva, J.A. Mitral annular disjunction in myxomatous mitral valve disease: A relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc. Ultrasound 2010, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.P.; Jin, C.N.; Fan, Y.; Wong, R.H.L.; Underwood, M.J.; Wan, S. Functional Implication of Mitral Annular Disjunction in Mitral Valve Prolapse: A Quantitative Dynamic 3D Echocardiographic Study. JACC Cardiovasc. Imaging 2017, 10, 1424–1433. [Google Scholar] [CrossRef]
- Nollen, G.J.; Mulder, B.J. What is new in the Marfan syndrome? Int. J. Cardiol. 2004, 97 (Suppl. S1), 103–108. [Google Scholar] [CrossRef]
- Nollen, G.J.; van Schijndel, K.E.; Timmermans, J.; Groenink, M.; Barentsz, J.O.; van der Wall, E.E.; Stoker, J.; Mulder, B.J. Pulmonary artery root dilatation in Marfan syndrome: Quantitative assessment of an unknown criterion. Heart 2002, 87, 470–471. [Google Scholar] [CrossRef]
- De Backer, J.; Loeys, B.; Devos, D.; Dietz, H.; De Sutter, J.; De Paepe, A. A critical analysis of minor cardiovascular criteria in the diagnostic evaluation of patients with Marfan syndrome. Genet. Med. 2006, 8, 401–408. [Google Scholar] [CrossRef]
- Hetzer, R.; Siegel, G.; Delmo Walter, E.M. Cardiomyopathy in Marfan syndrome. Eur. J. Cardiothorac. Surg. 2016, 49, 561–567. [Google Scholar] [CrossRef]
- Meijboom, L.J.; Timmermans, J.; van Tintelen, J.P.; Nollen, G.J.; De Backer, J.; van den Berg, M.P.; Boers, G.H.; Mulder, B.J. Evaluation of left ventricular dimensions and function in Marfan’s syndrome without significant valvular regurgitation. Am. J. Cardiol. 2005, 95, 795–797. [Google Scholar] [CrossRef]
- Yetman, A.T.; Bornemeier, R.A.; McCrindle, B.W. Long-term outcome in patients with Marfan syndrome: Is aortic dissection the only cause of sudden death? J. Am. Coll. Cardiol. 2003, 41, 329–332. [Google Scholar] [CrossRef]
- Rybczynski, M.; Koschyk, D.H.; Aydin, M.A.; Robinson, P.N.; Brinken, T.; Franzen, O.; Berger, J.; Hofmann, T.; Meinertz, T.; von Kodolitsch, Y. Tissue Doppler imaging identifies myocardial dysfunction in adults with Marfan syndrome. Clin. Cardiol. 2007, 30, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Taylor, A.L.; Yetman, A.T. Left ventricular diastolic dysfunction in children and young adults with Marfan syndrome. Pediatr. Cardiol. 2006, 27, 256–258. [Google Scholar] [CrossRef] [PubMed]
- De Backer, J.F.; Devos, D.; Segers, P.; Matthys, D.; François, K.; Gillebert, T.C.; De Paepe, A.M.; De Sutter, J. Primary impairment of left ventricular function in Marfan syndrome. Int. J. Cardiol. 2006, 112, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Demolder, A.; von Kodolitsch, Y.; Muiño-Mosquera, L.; De Backer, J. Myocardial Function, Heart Failure and Arrhythmia in Marfan Syndrome: A Systematic Literature Review. Diagnostics 2020, 10, 751. [Google Scholar] [CrossRef]
- Perrone, M.A.; Zaninotto, M.; Masotti, S.; Musetti, V.; Padoan, A.; Prontera, C.; Plebani, M.; Passino, C.; Romeo, F.; Bernardini, S.; et al. The combined measurement of high-sensitivity cardiac troponins and natriuretic peptides: A useful tool for clinicians? J. Cardiovasc. Med. 2020, 21, 953–963. [Google Scholar] [CrossRef]
- Kiotsekoglou, A.; Bajpai, A.; Bijnens, B.H.; Kapetanakis, V.; Athanassopoulos, G.; Moggridge, J.C.; Mullen, M.J.; Nassiri, D.K.; Camm, J.; Sutherland, G.R.; et al. Early impairment of left ventricular long-axis systolic function demonstrated by reduced atrioventricular plane displacement in patients with Marfan syndrome. Eur. J. Echocardiogr. 2008, 9, 605–613. [Google Scholar] [CrossRef][Green Version]
- Abd El Rahman, M.; Haase, D.; Rentzsch, A.; Olchvary, J.; Schäfers, H.J.; Henn, W.; Wagenpfeil, S.; Abdul-Khaliq, H. Left ventricular systolic dysfunction in asymptomatic Marfan syndrome patients is related to the severity of gene mutation: Insights from the novel three dimensional speckle tracking echocardiography. PLoS ONE 2015, 10, e0124112. [Google Scholar] [CrossRef]
- Kiotsekoglou, A.; Saha, S.; Moggridge, J.C.; Kapetanakis, V.; Govindan, M.; Alpendurada, F.; Mullen, M.J.; Nassiri, D.K.; Camm, J.; Sutherland, G.R.; et al. Impaired biventricular deformation in Marfan syndrome: A strain and strain rate study in adult unoperated patients. Echocardiography 2011, 28, 416–430. [Google Scholar] [CrossRef]
- Angtuaco, M.J.; Vyas, H.V.; Malik, S.; Holleman, B.N.; Gossett, J.M.; Sachdeva, R. Early detection of cardiac dysfunction by strain and strain rate imaging in children and young adults with marfan syndrome. J. Ultrasound Med. 2012, 31, 1609–1616. [Google Scholar] [CrossRef]
- Winther, S.; Williams, L.K.; Keir, M.; Connelly, K.A.; Bradley, T.J.; Rakowski, H.; Crean, A.M. Cardiovascular Magnetic Resonance Provides Evidence of Abnormal Myocardial Strain and Primary Cardiomyopathy in Marfan syndrome. J. Comput. Assist. Tomogr. 2019, 43, 410–415. [Google Scholar] [CrossRef]
- Kiotsekoglou, A.; Sutherland, G.R.; Moggridge, J.C.; Kapetanakis, V.; Bajpai, A.; Bunce, N.; Mullen, M.J.; Louridas, G.; Nassiri, D.K.; Camm, J.; et al. Impaired right ventricular systolic function demonstrated by reduced atrioventricular plane displacement in adults with Marfan syndrome. Eur. J. Echocardiogr. 2009, 10, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kiotsekoglou, A.; Moggridge, J.C.; Bijnens, B.H.; Kapetanakis, V.; Alpendurada, F.; Mullen, M.J.; Saha, S.; Nassiri, D.K.; Camm, J.; Sutherland, G.R.; et al. Biventricular and atrial diastolic function assessment using conventional echocardiography and tissue-Doppler imaging in adults with Marfan syndrome. Eur. J. Echocardiogr. 2009, 10, 947–955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Witte, P.; Aalberts, J.J.; Radonic, T.; Timmermans, J.; Scholte, A.J.; Zwinderman, A.H.; Mulder, B.J.; Groenink, M.; van den Berg, M.P. Intrinsic biventricular dysfunction in Marfan syndrome. Heart 2011, 97, 2063–2068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alpendurada, F.; Wong, J.; Kiotsekoglou, A.; Banya, W.; Child, A.; Prasad, S.K.; Pennell, D.J.; Mohiaddin, R.H. Evidence for Marfan cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 1085–1091. [Google Scholar] [CrossRef]
- Hoffmann, B.A.; Rybczynski, M.; Rostock, T.; Servatius, H.; Drewitz, I.; Steven, D.; Aydin, A.; Sheikhzadeh, S.; Darko, V.; von Kodolitsch, Y.; et al. Prospective risk stratification of sudden cardiac death in Marfan’s syndrome. Int. J. Cardiol. 2013, 167, 2539–2545. [Google Scholar] [CrossRef]
- Demolder, A.; Bianco, L.; Caruana, M.; Cervi, E.; Evangelista, A.; Jondeau, G.; Buttigieg, L.L.; López-Sainz, Á.; Delmás, E.M.; Pini, A.; et al. Arrhythmia and impaired myocardial function in heritable thoracic aortic disease: An international retrospective cohort study. Eur. J. Med. Genet. 2022, 65, 104503. [Google Scholar] [CrossRef]
- Shiga, T.; Wajima, Z.; Apfel, C.C.; Inoue, T.; Ohe, Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection. Arch. Intern. Med. 2006, 166, 1350–1356. [Google Scholar] [CrossRef]
- Wright, F.; Warncke, M.; Sinn, M.; Ristow, I.; Lenz, A.; Riedel, C.; Schoennagel, B.P.; Zhang, S.; Kaul, M.G.; Adam, G.; et al. Assessment of aortic diameter in Marfan patients: Intraindividual comparison of 3D-Dixon and 2D-SSFP magnetic resonance imaging. Eur. Radiol. 2023, 33, 1687–1697. [Google Scholar] [CrossRef]
- Evangelista, A.; Sitges, M.; Jondeau, G.; Nijveldt, R.; Pepi, M.; Cuellar, H.; Pontone, G.; Bossone, E.; Groenink, M.; Dweck, M.R.; et al. Multimodality imaging in thoracic aortic diseases: A clinical consensus statement from the European Association of Cardiovascular Imaging and the European Society of Cardiology working group on aorta and peripheral vascular diseases. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e65–e85. [Google Scholar] [CrossRef]
- Whitlock, M.C.; Hundley, W.G. Noninvasive Imaging of Flow and Vascular Function in Disease of the Aorta. JACC Cardiovasc. Imaging 2015, 8, 1094–1106. [Google Scholar] [CrossRef]
- Kröner, E.S.; Scholte, A.J.; de Koning, P.J.; Boogaard, P.J.v.D.; Kroft, L.J.; van der Geest, R.J.; Hilhorst-Hofstee, Y.; Lamb, H.J.; Siebelink, H.-M.J.; Mulder, B.J.; et al. MRI-assessed regional pulse wave velocity for predicting absence of regional aorta luminal growth in marfan syndrome. Int. J. Cardiol. 2013, 167, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Guala, A.; Rodriguez-Palomares, J.; Dux-Santoy, L.; Teixido-Tura, G.; Maldonado, G.; Galian, L.; Huguet, M.; Valente, F.; Gutiérrez, L.; González-Alujas, T.; et al. Influence of Aortic Dilation on the Regional Aortic Stiffness of Bicuspid Aortic Valve Assessed by 4-Dimensional Flow Cardiac Magnetic Resonance: Comparison With Marfan Syndrome and Degenerative Aortic Aneurysm. JACC Cardiovasc. Imaging 2019, 12, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Fujiseki, Y.; Okuno, K.; Tanaka, M.; Shimada, M.; Takahashi, M.; Kawanishi, K. Myocardial involvement in the Marfan syndrome. Jpn. Heart J. 1985, 26, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Pieri, M.; Marchei, M.; Sergi, D.; Bernardini, S.; Romeo, F. Serum free light chains in patients with ST elevation myocardial infarction (STEMI): A possible correlation with left ventricle dysfunction. Int. J. Cardiol. 2019, 292, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Leo, I.; Bianco, F.; Borrelli, N.; Beltrami, M.; Garofalo, M.; Milano, E.G.; Bisaccia, G.; Iellamo, F.; Bassareo, P.P.; et al. The Role of Multimodality Imaging in Pediatric Cardiomyopathies. J. Clin. Med. 2023, 12, 4866. [Google Scholar] [CrossRef]
- Loeper, F.; Oosterhof, J.; Dorpel, M.v.D.; van der Linde, D.; Lu, Y.; Robertson, E.; Hambly, B.; Jeremy, R. Ventricular-Vascular Coupling in Marfan and Non-Marfan Aortopathies. J. Am. Heart Assoc. 2016, 5, e003705. [Google Scholar] [CrossRef]
- Connor, B.S.; Algaze, C.A.; Narkevičiūtė, A.; Anguiano, B.; Pariani, M.; Zarate, Y.A.; Collins, R.T. Prevalence and Outcomes of Primary Left Ventricular Dysfunction in Marfan Syndrome. Am. J. Cardiol. 2022, 175, 119–126. [Google Scholar] [CrossRef]
- Muiño-Mosquera, L.; De Wilde, H.; Devos, D.; Babin, D.; Jordaens, L.; Demolder, A.; De Groote, K.; De Wolf, D.; De Backer, J. Myocardial disease and ventricular arrhythmia in Marfan syndrome: A prospective study. Orphanet J. Rare Dis. 2020, 15, 300. [Google Scholar] [CrossRef]
- Van Lam, H.; Groth, M.; Mir, T.; Bannas, P.; Lund, G.K.; Jahnke, C.M.; Warncke, M.; Maas, K.-J.; Adam, G.; Herrmann, J.; et al. Impact of chest wall deformity on cardiac function by CMR and feature-tracking strain analysis in paediatric patients with Marfan syndrome. Eur. Radiol. 2021, 31, 3973–3982. [Google Scholar] [CrossRef]
- Groth, M.; Henes, F.; Müllerleile, K.; Bannas, P.; Adam, G.; Regier, M. Accuracy of thoracic aortic measurements assessed by contrast enhanced and unenhanced magnetic resonance imaging. Eur. J. Radiol. 2012, 81, 762–766. [Google Scholar] [CrossRef]
- Leonardi, B.; D’Avenio, G.; Vitanovski, D.; Grigioni, M.; Perrone, M.A.; Romeo, F.; Secinaro, A.; Everett, A.D.; Pongiglione, G. Patient-specific three-dimensional aortic arch modeling for automatic measurements: Clinical validation in aortic coarctation. J. Cardiovasc. Med. 2020, 21, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, B.; Gentili, F.; Perrone, M.A.; Sollazzo, F.; Cocomello, L.; Kikina, S.S.; Wald, R.M.; Palmieri, V.; Secinaro, A.; Gagliardi, M.G.; et al. Cardiopulmonary Exercise Testing in Repaired Tetralogy of Fallot: Multiparametric Overview and Correlation with Cardiac Magnetic Resonance and Physical Activity Level. J. Cardiovasc. Dev. Dis. 2022, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Adsay, B.A.; Sheikhzadeh, S.; Keyser, B.; Rybczynski, M.; Sondermann, C.; Detter, C.; Steven, D.; Robinson, P.N.; Berger, J.; et al. Observational cohort study of ventricular arrhythmia in adults with marfan syndrome caused by FBN1 mutations. PLoS ONE 2013, 8, e81281. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Ortiz-Pérez, J.T.; Fernández-Armenta, J.; Guiu, E.; Acosta, J.; Prat-González, S.; De Caralt, T.M.; Perea, R.J.; Garrido, C.; Mont, L.; et al. 3D delayed-enhanced magnetic resonance sequences improve conducting channel delineation prior to ventricular tachycardia ablation. Europace 2015, 17, 938–945. [Google Scholar] [CrossRef]
- Writing Committee Members; Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., III; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; et al. ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar]
- Leonardi, B.; Secinaro, A.; Calvieri, C.; Perrone, M.A.; Gimigliano, F.; Muscogiuri, G.; Carotti, A.; Drago, F. The role of 3D imaging in the follow-up of patients with repaired tetralogy of Fallot. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1698–1709. [Google Scholar] [CrossRef]
- Braverman, A.C.; Mittauer, E.; Harris, K.M.; Evangelista, A.; Pyeritz, R.E.; Brinster, D.; Conklin, L.; Suzuki, T.; Fanola, C.; Ouzounian, M.; et al. Clinical Features and Outcomes of Pregnancy-Related Acute Aortic Dissection. JAMA Cardiol. 2021, 6, 58–66. [Google Scholar] [CrossRef]
- Saremi, F. Cardiac CT and MR for Adult Congenital Heart Disease; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Steinbrecher, K.L.; Marquis, K.M.; Braverman, A.C.; Ohman, J.W.; Bhalla, S.; Lin, C.-Y.; Naeem, M.; Raptis, C.A. Imaging of Genetic Thoracic Aortopathy. RadioGraphics 2022, 42, 1283–1302. [Google Scholar] [CrossRef]
- Festa, P.; Lovato, L.; Bianco, F.; Alaimo, A.; Angeli, E.; Baccano, G.; Barbi, E.; Bennati, E.; Bonhoeffer, P.; Bucciarelli, V.; et al. Recommendations for cardiovascular magnetic resonance and computed tomography in congenital heart disease: A consensus paper from the CMR/CCT Working Group of the Italian Society of Pediatric Cardiology and the Italian College of Cardiac Radiology endorsed by the Italian Society of Medical and Interventional Radiology (Part II). J. Cardiovasc. Med. 2024, 25, 473–487. [Google Scholar] [CrossRef]
- Moscatelli, S.; Bianco, F.; Cimini, A.; Panebianco, M.; Leo, I.; Bucciarelli-Ducci, C.; Perrone, M.A. The Use of Stress Cardiovascular Imaging in Pediatric Population. Children 2023, 10, 218. [Google Scholar] [CrossRef]
- Beetz, N.L.; Trippel, T.D.; Philipp, K.; Maier, C.; Walter-Rittel, T.; Shnayien, S.; Gehle, P. Discrepancy of echocardiography and computed tomography in initial assessment and 2-year follow-up for monitoring Marfan syndrome and related disorders. Sci. Rep. 2022, 12, 15333. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.C.; Yeh, S.H. Marfan syndrome with myocarditis demonstrated by 99Tcm-HMPAO-labelled WBC and 201Tl scintig-raphy: Report of three cases in a Chinese family. Nucl. Med. Commun. 1993, 14, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Leo, I.; Bianco, F.; Surkova, E.; Pezel, T.; Donald, N.A.; Triumbari, E.K.A.; Bassareo, P.P.; Pradhan, A.; Cimini, A.; et al. The Role of Multimodality Imaging in Patients with Congenital Heart Disease and Infective Endocarditis. Diagnostics 2023, 13, 3638. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Wanjari, A. Cardiac Complications in Marfan Syndrome: A Review. Cureus 2022, 14, e29800. [Google Scholar] [CrossRef]
- Emrich, F.C.; Okamura, H.; Dalal, A.R.; Penov, K.; Merk, D.R.; Raaz, U.; Hennigs, J.K.; Chin, J.T.; Miller, M.O.; Pedroza, A.J.; et al. Enhanced caspase activity contributes to aortic wall remodeling and early aneurysm development in a murine model of marfan syndrome. Arter. Thromb. Vasc. Biol. 2015, 35, 146–154. [Google Scholar] [CrossRef]
- Jamar, F.; Buscombe, J.; Chiti, A.; Christian, P.E.; Delbeke, D.; Donohoe, K.J.; Israel, O.; Martin-Comin, J.; Signore, A. EANM/SNMMI guideline for18F-FDG use in inflammation and infection. J. Nucl. Med. 2013, 54, 647–658. [Google Scholar] [CrossRef]
- Perrone, M.A.; Cimini, A.; Ricci, M.; Pizzoferro, M.; Garganese, M.C.; Raponi, M.; Schillaci, O. Myocardial Functional Imaging in Pediatric Nuclear Cardiology. J. Cardiovasc. Dev. Dis. 2023, 10, 361. [Google Scholar] [CrossRef]
- Brili, S.V.; Gkotzamanis, V.; Kafouris, P.; Mystakidi, V.-C.; Sakalidis, A.; Antonopoulos, A.S.; Oikonomou, E.; Anagnostopoulos, C.; Tsioufis, K. The use of 18-Fluorodeoxyglucose positron emission and computed tomography in the evaluation of Marfan syndrome patients. JACC 2023, 81, 2017. [Google Scholar] [CrossRef]
- Lindsay, D.; Ismajli, M.; Bucknall, R.; Lipscomb, G.; Boyle, J.J.; Mason, J.C.; Wig, S. Simultaneous presentation of IgG4-related chronic pe-ri-aortitis and coeliac disease in a patient with Marfan’s Syndrome. Rheumatology 2016, 55, 1141–1143. [Google Scholar] [CrossRef]
- Johnston, P.W.; Kennedy, P.T.; Blair, P.H. Periaortitis complicating chronic aortic dissection. Eur. Heart J. 2007, 28, 442. [Google Scholar] [CrossRef]
- Yamanaka, K.; Matsueda, T.; Miyahara, S.; Nomura, Y.; Sakamoto, T.; Morimoto, N.; Inoue, T.; Matsumori, M.; Okada, K.; Okita, Y. Surgical strategy for aortic prosthetic graft infection with (18)F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 549–551. [Google Scholar] [CrossRef]
- Shim, H.; Sung, K.; Kim, W.S.; Lee, Y.T.; Park, P.W.; Jeong, D.S. Diagnosis of Graft Infection Using FDG PET-CT. Korean J. Thorac. Cardiovasc. Surg. 2012, 45, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Chiocchi, M.; Spiritigliozzi, L.; Di Tosto, F.; Benelli, L.; D’Errico, F.; Presicce, M.; Pugliese, L.; Ricci, F.; De Stasio, V.; Di Donna, C.; et al. Ascending aorta pseudoaneurysm simulating mediastinal lymphoma in computed tomography, a possible diagnostic error: A case report. J. Med. Case Rep. 2020, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Calabria, F.F.; Guadagnino, G.; Cimini, A.; Leporace, M. PET/CT Imaging of Infectious Diseases: Overview of Novel Radi-opharmaceuticals. Diagnostics 2024, 14, 1043. [Google Scholar] [CrossRef]
- Perrone, M.A.; Intorcia, A.; Morgagni, R.; Marchei, M.; Sergi, D.; Pugliese, L.; Ferrante, P.; Chiocchi, M.; Borzi, M.; Romeo, F. Primary cardiac lymphoma: The role of multimodality imaging. J. Cardiovasc. Med. 2018, 19, 455–458. [Google Scholar] [CrossRef]
- Cimini, A.; Ricci, M.; Chiaravalloti, A.; Filippi, L.; Schillaci, O. Theragnostic Aspects and Radioimmunotherapy in Pediatric Tumors. Int. J. Mol. Sci. 2020, 21, 3849. [Google Scholar] [CrossRef]
- Bobbio, E.; Dudás, A.; Bergström, A.; Esposito, D.; Angerås, O.; Taha, A.; van Essen, M.; Björkenstam, M.; Karason, K.; Bollano, E.; et al. Incidental cardiac findings on somatostatin receptor PET/CT: What do they indicate and are they of clinical relevance? J. Nucl. Cardiol. 2022, 29, 1159–1165. [Google Scholar] [CrossRef]
- Jaleel, J.; Patel, C.D.; Chandra, K.B.; Ramakrishnan, S.; Seth, S. Imaging Acute Myocarditis with 68Ga-DOTANOC PET/CT. Indian J. Nucl. Med. 2023, 38, 69–70. [Google Scholar] [CrossRef]
- Available online: https://www.clinicaltrials.gov/study/NCT04206163?cond=Myocardial%20Inflammation&term=PET%2FCT&rank=6 (accessed on 24 November 2024).
- Singh, P.; Almarzooq, Z.; Salata, B.; Devereux, R.B. Role of molecular imaging with positron emission tomographic in aortic aneurysms. J. Thorac. Dis. 2017, 9, S333–S342. [Google Scholar] [CrossRef]
- Rutten, D.W.; Aarts-Janssen, I.J.; Kempers, M.J.; Reimer, A.G.; Cate, F.E.U.T.; Loeys, B.L.; Slieker, M.G. Comparability of different Z-score equations for aortic root dimensions in children with Marfan syndrome. Cardiol. Young 2021, 31, 1962–1968. [Google Scholar] [CrossRef] [PubMed]



| Criteria | Diagnosis of Marfan Syndrome | Additional Considerations |
|---|---|---|
| In the Absence of Family History | ||
| Aortic Root Dilatation (Z-score ≥ 2) AND Ectopia Lentis | Marfan Syndrome | The presence of aortic root enlargement (Z-score ≥ 2, standardized by age and body size) or dissection with ectopia lentis confirms Marfan Syndrome. Differential diagnoses such as Shprintzen-Goldberg syndrome, Loeys-Dietz syndrome (TGFBR1/2, SMAD3, TGFB2, TGFB3), or vascular Ehlers-Danlos syndrome (COL3A1) must be excluded in the case of the presence of suggestive features. |
| Aortic Root Dilatation Z score ≥ 2 AND FBN1 Mutation | Marfan Syndrome | Diagnosis is confirmed with aortic root enlargement (Z ≥ 2) or dissection alongside a verified FBN1 mutation, even in the absence of ectopia lentis. |
| Aortic Root Dilatation Z score ≥ 2 AND Systemic Score ≥ 7 points | Marfan Syndrome | A diagnosis is supported when systemic findings (≥7 points) accompany aortic root enlargement (Z ≥ 2) or dissection. Features indicative of other syndromes (e.g., Shprintzen-Goldberg syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syndrome) require genetic testing of TGFBR1/2, SMAD3, TGFB2, TGFB3, or COL3A1. |
| Ectopia Lentis AND FBN1 Mutation associated with Aortic Root Dilatation | Marfan Syndrome | If ectopia lentis is present without aortic enlargement or dissection, an FBN1 mutation linked to aortic disease is required for diagnosis. |
| In the Presence of Family History | ||
| Ectopia lentis AND Family History of Marfan syndrome (as defined above) | Marfan Syndrome | Diagnosis is established if ectopia lentis is present with a family history meeting the criteria for Marfan Syndrome (as defined in 1–4 above). |
| A systemic score ≥ 7 points AND Family History of Marfan syndrome (as defined above) | Marfan Syndrome | A systemic score of ≥7 points combined with a family history confirms the diagnosis. Alternative syndromes, such as Loeys-Dietz (TGFBR1/2, SMAD3, TGFB2, TGFB3) or vascular Ehlers-Danlos syndrome (COL3A1), must be excluded via genetic testing in the case of the presence of suggestive features. |
| Aortic Root Dilatation Z score ≥ 2 above 20 yrs. old, ≥3 below 20 yrs. old + Family History of Marfan syndrome (as defined above) | Marfan Syndrome | For individuals aged >20 years (Z ≥ 2) or ≤20 years (Z ≥ 3) with a relevant family history, the diagnosis is confirmed. Differential diagnoses, including Loeys-Dietz (TGFBR1/2, SMAD3, TGFB2, TGFB3) and vascular Ehlers-Danlos syndrome (COL3A1), must be excluded through appropriate genetic testing in the case of the presence of suggestive features. |
| Modality | Specific Recommendations |
|---|---|
| Echocardiography | Regularly evaluate aortic root and ascending aorta dimensions using standardized techniques (e.g., leading-edge-to-leading-edge in adults). Index measurements to body surface area and calculate Z-scores (≥2 indicates concern). Screen for mitral valve prolapse (MVP) and mitral annular disjunction (MAD) and monitor for complications like regurgitation. Include left and right ventricular function assessment, integrating strain imaging for early myocardial dysfunction detection. Monitor pulmonary artery dimensions, especially the root, for potential dilation. |
| Cardiovascular Magnetic Resonance (CMR) | Use CMR for comprehensive evaluation of the entire aorta, including aneurysms, dissections, and hemodynamic properties (4D flow). Incorporate CMR into routine monitoring to detect disease progression, particularly in younger patients. Report the measurement techniques to ensure consistency in measurements. Use feature-tracking techniques to detect early myocardial dysfunction and evaluate the impact of skeletal deformities on cardiac function, including right ventricular impairment. Use contrast-enhanced imaging to identify arrhythmogenic regions, particularly in the case of MAD. |
| Computed Tomography (CT) | Use CT for detailed evaluation of the entire aorta, particularly in emergencies like dissections. Perform assessments in multiple phases (non-contrast, arterial, and late) for accurate characterization of calcifications, intramural hematomas, and contrast leaks. Apply dose-reduction techniques to minimize radiation exposure, particularly in pediatric populations. Administer contrast tailored to patient size and condition (e.g., 2 mL/kg in children, 1.5 mL/kg in adults) with appropriate infusion rates for optimal imaging quality. Standardize aortic measurements in diastole, reporting dimensions consistently (e.g., outer-to-outer for thickened walls, inner-to-inner for routine measurements). Employ 3D reconstruction tools for surgical planning and follow-up monitoring. Integrate CT into longitudinal follow-up of aortic enlargement, particularly in cases where echocardiography alone provides inconsistent results. |
| Nuclear Medicine | Use 18F-FDG PET/CT to detect aortic wall inflammation and complications such as chronic peri-aortitis or infections in prosthetic grafts. Utilize myocardial SPECT with 201Tl or 99mTc-based tracers for evaluating perfusion, viability, and cardiac pump function in Marfan cardiomyopathy, particularly when echocardiographic or CMR data are inconclusive. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, M.A.; Moscatelli, S.; Guglielmi, G.; Bianco, F.; Cappelletti, D.; Pellizzon, A.; Baggiano, A.; Diviggiano, E.E.; Ricci, M.; Bassareo, P.P.; et al. Advances in Cardiovascular Multimodality Imaging in Patients with Marfan Syndrome. Diagnostics 2025, 15, 172. https://doi.org/10.3390/diagnostics15020172
Perrone MA, Moscatelli S, Guglielmi G, Bianco F, Cappelletti D, Pellizzon A, Baggiano A, Diviggiano EE, Ricci M, Bassareo PP, et al. Advances in Cardiovascular Multimodality Imaging in Patients with Marfan Syndrome. Diagnostics. 2025; 15(2):172. https://doi.org/10.3390/diagnostics15020172
Chicago/Turabian StylePerrone, Marco Alfonso, Sara Moscatelli, Giulia Guglielmi, Francesco Bianco, Deborah Cappelletti, Amedeo Pellizzon, Andrea Baggiano, Enrico Emilio Diviggiano, Maria Ricci, Pier Paolo Bassareo, and et al. 2025. "Advances in Cardiovascular Multimodality Imaging in Patients with Marfan Syndrome" Diagnostics 15, no. 2: 172. https://doi.org/10.3390/diagnostics15020172
APA StylePerrone, M. A., Moscatelli, S., Guglielmi, G., Bianco, F., Cappelletti, D., Pellizzon, A., Baggiano, A., Diviggiano, E. E., Ricci, M., Bassareo, P. P., Pradhan, A., Mandoli, G. E., Cimini, A., & Caminiti, G. (2025). Advances in Cardiovascular Multimodality Imaging in Patients with Marfan Syndrome. Diagnostics, 15(2), 172. https://doi.org/10.3390/diagnostics15020172













