Abstract
Objectives: We aimed to analyze potential predictors for the development of metachronous fractures (MFs) after osteoporotic vertebral fractures (OVFs), with particular focus on radiological variables obtained at initial X-rays and computed tomography (CT) examinations, treatment applied (conservative management [CM] versus percutaneous vertebroplasty [PV]), and fractures located at the thoracolumbar junction (T11-L2). Methods: We conducted a two-center, observational retrospective study, including patients with single-level OVFs treated with CM or VP. We collected socio-demographic, radiological and treatment-related variables. We performed descriptive and contrastive bivariate analyses based on the presence of MFs and univariate and multivariate logistic regression analyses to obtain adjusted and crude odds ratios (aOR and cOR, respectively) for predicting MFs. Finally, we performed receiver-operating characteristic (ROC) curve analyses to determine the discriminative power of the models obtained. Results: Of the 90 patients included, 20 (22.2%) developed one or more MFs (15 in CM and 5 in PV groups, respectively; p = 0.037). The treatment group (aOR for PV, 0.087; 95%CI, 0.015–0.379), presence of intravertebral cleft (aOR, 5.62; 95%CI, 1.84–19.2) and difference in posterior height loss between X-rays and CT (aOR, 0.926; 95%CI, 0.856–0.992) were identified as significant predictors for MFs, while Genant’s numerical classification showed a trend toward significance (aOR, 1.97; 95%CI, 0.983–4.19; p = 0.064). A multivariate model combining these four variables showed optimal fitting and correctly discriminated over 80% of cases (AUC, 0.828; 95%CI, 0.725–0.930). Factors associated with MFs in thoracolumbar junction OVFs were intravertebral cleft, CM, posterior height loss in CT, and DGOU OF3 fractures. Conclusions: The presence of intravertebral cleft, a difference in posterior height loss between X-rays and CT equal to or lower than 2.4%, higher grades of Genant’s numerical classification, and application of CM instead of PV are predictors of MFs. These findings improve our understanding of the factors involved in the development of MFs, but they need to be validated prospectively.
1. Introduction
Osteoporotic vertebral fractures (OVFs) represent a health problem of high prevalence and increasing incidence due to the aging of the population [1,2]. These fractures may be associated with scarce or even absent symptoms, and some studies have suggested that only one-third of these fractures are clinically detected [3]. However, they are a major cause of morbidity, mortality and healthcare costs [1,4]. Up to 20% of OVFs may occur without previous trauma [2] and their presence increases the risk of metachronous vertebral fractures due to the highly deteriorated bone fragility in these patients [5,6].
There are different treatment options available for OVFs [7] and no universal agreement on their optimal management has been achieved to date. An increasing body of evidence emphasizes the need for adjusting the treatment strategy to the patient profile [8]. It is widely accepted that the first approach should be based on analgesic management and treating osteoporosis itself with hygienic–dietary and pharmacological measures [9,10]. However, there is no current consensus on the optimal therapeutic strategy for fractures. In most cases, conservative management (CM) is the first approach, although this decision may be contingent upon the fracture type, stability or presence of neurological deficits [11].
In general, CM consists of the use of rigid, semi-rigid and soft orthoses, which aim to minimize the shear forces on the fractured vertebrae. However, the scientific evidence supporting the usefulness of orthoses is limited [12]. In addition, CM is not adequately standardized, and up to one-third of patients with osteoporosis do not respond to it [13]. In cases refractory to CM, it is possible to perform vertebral augmentation techniques. Finally, more aggressive (i.e., surgical) techniques such as decompression of the central canal or vertebral fixation can be necessary, although the risks of the latter are considerable [9].
Percutaneous vertebroplasty (PV) was the first vertebral augmentation technique developed. It was performed in France in 1984 for the treatment of an aggressive hemangioma at C2 [14] and, by the end of the 1990s, it was widely adopted in clinical practice for the treatment of different types of vertebral fractures [15,16,17]. Briefly, PV is an imaging-guided percutaneous procedure by which a biological cement (usually polymethylmethacrylate) is injected into the fractured vertebral body, with the aim of relieving pain and increasing stability at the fracture site [9].
The efficacy of PV has been the subject of an ongoing debate since the publication of two randomized clinical trials in which no benefit was found in comparison with placebo in terms of pain control, improved functionality nor quality of life [18,19]. Likewise, some authors argue that PV may be responsible for the development of metachronous vertebral fractures (MFs) in adjacent vertebrae [20,21]. A relatively recent meta-analysis by Buchbinder et al. [22] concluded that there is no scientific evidence to recommend its use due to concerns related to both its efficacy and safety.
However, several clinical trials support the safety and efficacy of PV, as is the case with VAPOUR (2016), in which the time from fracture onset to PV was short [23], or the recent VERTOS V trial on chronic OVFs [24], which showed positive results favoring PV. Recent meta-analyses concluded that PV is effective, with proven benefits compared to CM, although the importance of using adequate selection criteria for patients has been stressed [25]. In this context, elderly people with recent, painful OVFs seem to be the most suitable patient profile that can benefit from PV [25,26].
Despite the increasing knowledge on the pathophysiology of OVFs, the question on whether PV increases the risk of MFs compared to CM remains unsolved. In addition, the identification of predictive factors involved in the development of metachronous OVFs is still limited. Some conditions (e.g., diabetes mellitus, chronic obstructive pulmonary disease), medications (e.g., corticosteroids), bone health indicators (e.g., bone mineral density) and demographic factors (e.g., age, sex, body mass index) have been linked to a higher risk of MFs [27,28]. However, radiological variables on X-rays and computed tomography (CT) images, which are the most frequently used imaging examinations when acute OVF is suspected due to their greater availability as compared to magnetic resonance imaging (MRI) [29,30], have been insufficiently explored in this setting. Of note, OVFs at the thoracolumbar junction deserve particular attention, as they have been reported to be a specific risk factor for higher kyphotic deformity and subsequent loss of sagittal balance, increasing the odds of developing MFs [31].
We previously reported that some radiological variables such as differences in posterior vertebral height loss between standing X-rays and CT or the fractured vs. non-fractured vertebral density ratio were associated with the development of vertebral collapse in OVFs treated with CM [32]. On the basis of these findings, we hypothesized that radiological variables from initial imaging examinations could be helpful for predicting the development of MFs and that the application of PV could be an important factor to be considered regarding this complication.
The main objective of this study was to identify potential predictors for the development of MFs after OVFs, with particular focus on radiological variables obtained at initial X-rays and CT examinations as well as the treatment applied (CM vs. PV). A secondary objective was to identify such predictors in the subgroup of patients with fractures located in the thoracolumbar junction.
2. Materials and Methods
2.1. Study Design and Patient Selection
A retrospective observational study was designed from a consecutive series of patients with acute OVF of the thoracolumbar spine diagnosed by imaging examinations at the Hospital Universitario Virgen de las Nieves and the Hospital Universitario San Cecilio between 1 January 2019 and 31 December 2023. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed in the design of this study [33]. This study was approved by the Provincial Ethics Committee of Granada (code TFG-FX-2019).
The following inclusion criteria were established:
- Patients diagnosed with acute OVF by X-rays and CT.
- Patients with radiological follow-up of their fracture at least 12 months after diagnosis.
- Type A fractures of the AO Spine Classification [34,35].
- Management with CM or PV (in the latter case, during the first 6 months after diagnosis).
The exclusion criteria were:
- Lack of follow-up X-rays and/or CT at least 12 months after initial diagnosis.
- Presence of two or more simultaneous OVFs.
- Patients treated by spinal surgery prior to the diagnosis of OVF or during follow-up.
- Patients with non-diagnostic-quality imaging examinations.
To ensure a balanced distribution of patients treated conservatively and with PV, patient selection was performed as follows: search filters were applied in the hospital radiology information system, which is common to both institutions involved, using the keywords “vertebral fracture” or “spinal fracture” (filter: all words). Single vertebral fracture cases were checked one by one and recorded in a spreadsheet. Next, the treatment applied was examined and classified according to whether it was fully conservative or with PV (in the first 6 months after diagnosis). Finally, the inclusion and exclusion criteria were applied consecutively by date of imaging examination and the first 45 patients treated conservatively, and the first 45 patients treated with PV were selected. Therefore, a total of 90 patients with OVF were analyzed in this study.
2.2. Study Variables
The dependent variable was the development of MFs on follow-up. The independent qualitative variables were sex, fracture location, cause (spontaneous, stress, fall), type of treatment applied (PV or CM), and presence of intravertebral cleft. In addition, we considered the type of fracture at initial diagnosis based on the following classification systems:
- Morphological (wedge-shaped, biconcave, crush) and quantitative (grade 0, 1, 2, 3) classifications by Genant (34).
- Classification of the German Society of Orthopedics and Traumatology (DGOU) (OF2-OF5) (35).
- Classification of the AO Spine (A1 to A4) (32,33).
- Sugita classification (swelled-front, bow-shaped, projecting, concave, dented) (36).
Independent quantitative variables were patient age, vertebral height loss in anterior, middle and posterior walls at diagnosis, difference in anterior, middle and posterior height loss between standing X-rays and supine CT images at diagnosis, density of the fractured and non-fractured vertebral bodies as well as fractured/non-fractured vertebrae and fractured vertebra/aorta density ratios.
To calculate vertebral height loss, the fractured vertebral body measurements were divided by the mean of the measurements made on the two adjacent vertebrae. To compare the variability between X-rays and CT measurements, the differences in height loss of each wall (i.e., anterior, middle and posterior) between both imaging techniques were calculated. Figure 1 shows illustrative examples of the measurements made.
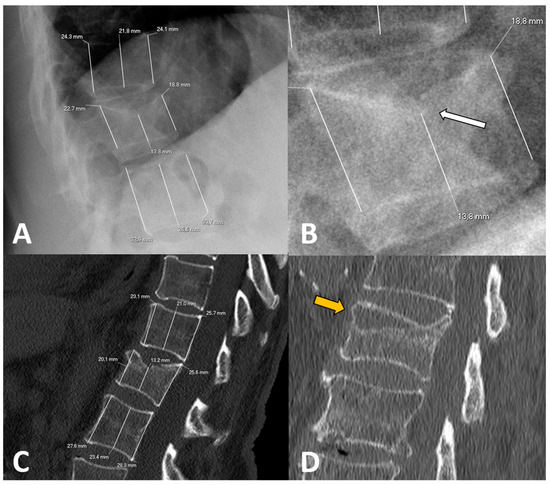
Figure 1.
Examples of radiological variables assessed in our sample, from a 58-year-old woman (A–C) and an 88-year-old man (D). Patient 1. X-rays ((A), magnified in (B)) and CT at diagnosis (C). The height of each wall (anterior, middle and posterior) was measured through lines parallel to the anterior vertebral walls. Relative measurements were obtained for each fractured vertebral body by dividing them between the mean of the respective measurements in the cephalad and caudal vertebral bodies. Note that some cases involved subtle findings such as the asymmetric, focal endplate depression depicted with a white arrow in (B). Patient 2. CT at diagnosis (D) showing an obvious subchondral defect (orange arrow) consistent with a large intravertebral cleft.
As in our previous study [32], the values of CT density in Hounsfield Units (HU) [36] were measured by applying 1.5–2 cm2 oval region of interest (ROI) areas at two different levels of the trabecular bone of the fractured vertebra, in the two adjacent healthy vertebrae and in the aortic lumen. The mean value of these measurements was used as the final density value for the fractured vertebra, the normal vertebra and the aorta, respectively (Figure 2). For measurements in the fractured vertebra, cystic cavities or sclerotic lines of impaction were avoided. The aorta was chosen as the internal reference standard.
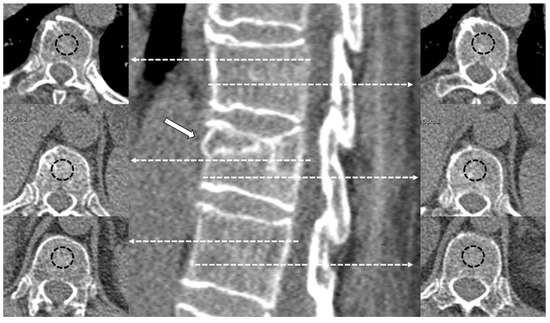
Figure 2.
Examples of CT density measurements in a fractured vertebral body (arrow) and adjacent healthy vertebrae. The dashed lines with arrows indicate the level of different measurements in the axial plane. The dashed circles represent the region of interest (ROI) measured in the central part of the vertebral body.
All CT scans were performed using 64-detector-row (Lightspeed VCT, General Electric®, Boston, MA, USA) and 128-detector-row (Ingenuity, Philips®, Amsterdam, The Netherlands) multidetector machines. CT images with a thickness of 0.63–1.25 mm and reconstructions with the same range were obtained. Two radiologists (ANONYMIZED and ANONYMIZED) with 1 and 7 years of experience, respectively, performed the measurements independently using the Carestream Vue system (Phillips®). The mean values of both measurements were used as final values. Fracture classification was also performed by both radiologists independently. In case of disagreement, the study was reviewed by a senior radiologist (ANONYMIZED) who decided the final classification for the fracture.
2.3. Statistical Analysis
A descriptive analysis was performed expressing qualitative variables as absolute and relative frequencies and quantitative variables as means and standard deviations. To analyze potential confounders that could be involved in the treatment applied, we performed both a univariate descriptive analysis for the whole sample and a bivariate descriptive analysis, including separated descriptive data for each of the treatment groups. This provided information on variables that could differ between patients managed with CM and PV.
Bivariate analyses were then performed to compare the group of patients who developed MFs and the group of patients who did not develop them. For these bivariate analyses, quantitative and qualitative variables were compared using Student’s t-tests and chi-squared tests (or Mann–Whitney/Fisher’s exact tests when the conditions to apply the respective parametric tests were not met).
Then, to analyze how the independent variables which showed statistically significant differences in the bivariate analyses comparing the categories of the treatment applied were able to predict the development of MFs, we performed univariate and multivariate binary logistic regression analyses. Crude and adjusted odds ratios (cOR and aOR, respectively) with their corresponding 95% confidence intervals (95%CIs) were obtained. For aORs related to the treatment applied, we adjusted the models for the variables that had resulted as significantly different in the corresponding bivariate descriptive analysis. To compare the consistency and fit of the multivariate models, we utilized the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC). Finally, receiver-operating characteristic (ROC) curves were used for discriminant analysis, and the Youden index was used to select the cut-off points that maximize the sensitivity and specificity of the variables of the models obtained.
As for the secondary objective of this study, all analyses were also performed for the subgroup of patients with fractures located at the thoracolumbar junction (T11-L2). All data were analyzed with the R software version 4.3.2 for Windows (Vienna, Austria). The level of statistical significance was established for p values less than 0.05.
3. Results
3.1. Main Characteristics of Patients and Vertebral Fractures in Our Sample
Of the 90 patients included in this study (mean age, 72.5 years; 74.4% women), the most frequent cause observed for OVFs was falling from standing height (80%), the level at which the OVF was most frequently located was L1 (37.8%), and the most frequent fracture types according to AO Spine, DGOU, Sugita and Genant’s morphological and Genant’s numerical classifications were, respectively, A1 (52.2%), OF2 (74.4%), bow-shaped (36.7%), biconcave (48.9%), and 0.5 and grade 1 (42.2%). Significant differences were observed in the distribution of categories in the AO Spine classification (p < 0.001), with a higher prevalence of Grade 0.5 fractures in the CT group compared to the PV group. In total, 20 patients (22.2%) developed one or more MFs on follow-up (15 in the conservatively treated group and 5 in the PV-treated group; p = 0.022). Table 1 shows the descriptive analysis of the socio-demographic variables, causes, location and type of the fractures included in this study. Supplementary Table S1 shows the same descriptive analysis for the subgroup of patients with OVFs located at the thoracolumbar junction.

Table 1.
Descriptive analysis of the socio-demographic variables, causes, location and types of the fractures. Data are expressed as mean ± standard deviation or absolute (relative) frequencies for quantitative and qualitative variables, respectively. * Significant p-value.
Regarding the radiological variables measured, we observed baseline differences between the CT and PV groups in fractured vertebra density and fractured-to-healthy vertebral density ratio (higher in the PV group, p = 0.001 and p = 0.030, respectively), loss of posterior height measured in X-rays and CT (higher loss in the PV group in both, p = 0.009 and p = 0.031, respectively), and loss of anterior height measured in CT (higher loss in the PV group, p = 0.007). In addition, a higher loss of middle vertebral height was observed at the end of follow-up in the CT group (p = 0.019). The 12 other radiological variables showed no statistically significant differences between groups, although several trends were observed, indicating greater height loss in the PV group, in general. Table 2 shows the descriptive analysis of the radiological variables assessed in this study. Supplementary Table S2 shows the same descriptive analysis for the subgroup of patients with OVFs located at the thoracolumbar junction.

Table 2.
Descriptive analysis of the radiological variables assessed in the study with bivariate descriptive analysis based on the treatment that was applied. Data are expressed as mean ± standard deviation or absolute (relative) frequencies for quantitative and qualitative variables, respectively. * Significant p-value.
3.2. Variables Associated with the Development of Metachronous Vertebral Fractures
In the bivariate contrastive analyses comparing patients who developed MFs versus those who did not, in addition to the type of treatment applied (p = 0.022), we observed significant differences in the presence of intravertebral cleft (p = 0.004), difference in loss of posterior height between standing X-rays and CT (p = 0.042), and AO Spine fracture type classification (p = 0.028). Table 3 shows the results of the bivariate analyses. Supplementary Table S3 shows the same contrastive analyses for the subgroup of patients with OVFs located at the thoracolumbar junction.

Table 3.
Factors associated with the development of new osteoporotic vertebral fractures. Data are expressed as mean ± standard deviation or absolute (relative) frequencies. * Significant p-value.
3.3. Predictors for the Development of Metachronous Vertebral Fractures
The results of the univariate logistic regression analysis for MFs were statistically significant for the treatment group, intravertebral cleft, and difference in loss of posterior height between X-rays and CT. For the Genant’s classification (numerical), the results showed a trend toward significance (p = 0.064). In the multivariate logistic regression analyses, we analyzed two models, one with the three significant variables (“treatment”, “intravertebral cleft”, “difference in loss of posterior height between X-rays and CT”) and another one with the four variables (i.e., adding Genant’s classification). A comparison between the model with three variables and the model with four variables showed that AIC decreased from 87.26 to 81.74 and BIC decreased from 97.26 to 94.24, indicating that the inclusion of the latter variable improved model fit, with an optimal balance between quality of fit and model complexity. Table 4 shows the results of the univariate and multivariate regression analyses. Supplementary Table S4 shows the same contrastive analyses for the subgroup of patients with OVFs located at the thoracolumbar junction.

Table 4.
Univariate and multivariate logistic regression analyses for the prediction of metachronous fractures including the variables that showed statistically significant differences in the bivariate analyses. CM, Conservative management. OR, odds ratio. cOR, crude OR. aOR: adjusted OR. 95%CI, 95% confidence interval. U p-value of the univariate regression analysis. M p-value of the multivariate regression analysis. * Significant p-value. ^ For this variable, apart from sex and age, adjustment for the six variables that showed statistically significant differences in the bivariate descriptive analyses of Table 1 and Table 2 was made.
3.4. Discriminative Power of the Models for Predicting Metachronous Fractures
The results of the ROC curve analyses for each of the significant models are shown in Table 5 and represented in Figure 3. For the variable “loss of posterior height (X-rays–CT)”, the optimal cutoff of 0.240 (Youden index = 0.328) showed a sensitivity of 60% and a specificity of 72.9% to predict MFs. Supplementary Table S5 and Supplementary Figure S1 show the same analyses and graphical representation for the subgroup of patients with OVFs located at the thoracolumbar junction. For the variable “loss of posterior height (CT)”, the optimal cutoff of 0.161 (Youden index = 0.447) showed a high sensitivity (92.9%) but a low specificity (51.9%) to predict MFs.

Table 5.
Receiver-operating characteristic curve analysis for the variables of the multivariate model to predict the development of new fractures. The combined model with 3 variables includes the variables “treatment”, “intravertebral cleft”, “difference in loss of posterior height between X-rays and CT”. The combined model with 4 variables includes these variables as well as “Genant’s classification (numerical)”. AUC, area under the curve. 95%CI, 95% confidence interval. * Significant p-value.
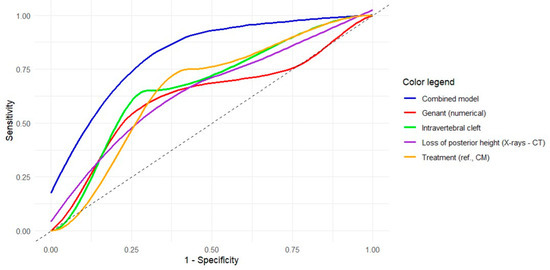
Figure 3.
Receiver operating characteristic (ROC) curves of the univariate and multivariate models obtained for the prediction of metachronous vertebral fractures. The colored curves represent the ROC curves for each significant independent variable based on the univariate logistic regression results (see legend). The blue curve refers to the multivariate model with 4 variables. The gray diagonal line corresponds to the reference of a random classification (line of no discrimination).
Figure 4 and Figure 5 show illustrative examples of patients from our sample with and without MFs following OVFs at the thoracolumbar junction. Other illustrative examples in patients with OVFs in locations different from the thoracolumbar junction are provided in Supplementary Figures S2 and S3.
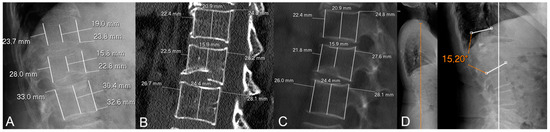
Figure 4.
Illustrative example of a patient without metachronous fractures in our sample. (A) Initial X-rays. (B) Initial CT (MPR set at 1 mm). (C) Initial CT (MPR set at 100 mm for easier comparison with measurements on X-rays). (D) Follow-up X-rays. This is a 77-year-old woman with a biconcave, grade 1 (Genant’s classification), OF2 (DGOU’s classification) fracture of L1. The loss of posterior vertebral height (PVH) on CT was negative and the difference in loss of PVH was close to 0 (0.1%). She was treated with vertebroplasty (note a slight cement leak at T12-L1 intervertebral disc) and developed no metachronous fractures. In addition, follow-up X-rays 3 years later showed low-to-moderate local kyphosis (monosegmental Cobb angle of 15°) with preservation of sagittal balance (orange vertical line in (D)).
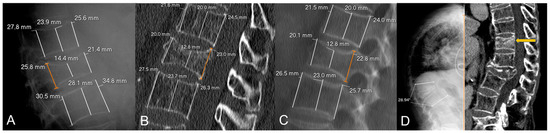
Figure 5.
Illustrative example of a patient with metachronous fracture in our sample. (A) Initial X-rays. (B) Initial CT (MPR set at 1 mm). (C) Initial CT (MPR set at 100 mm for easier comparison with measurements on X-rays). (D) Follow-up X-rays. This is a 74-year-old woman with a biconcave, grade 2 (Genant’s classification), OF2 (DGOU’s classification) involving L1. The loss of posterior vertebral height (PVH) on CT was 9.5% and the difference in loss of PVH between X-rays and CT was 2%. She was managed conservatively and developed vertebral collapse and hyperkyphosis (monosegmental Cobb angle close to 30°) with loss of sagittal balance (sagittal vertical axis shown as an orange vertical line at the left panel in (D)). At 2-year follow-up, she developed L1 collapse and a metachronous fracture of T11 (yellow arrow in (D)).
4. Discussion
This study aimed to identify factors associated with the development of new incident OVFs (i.e., MFs), with particular focus on a comparison between the differential effects of PV and CM. As a secondary objective, we analyzed factors associated with MFs in patients with fractures located at the thoracolumbar junction. In the whole sample, we found that the presence of an intravertebral cleft and a difference in posterior height loss between X-rays and CT in initial imaging examinations were significantly associated with a higher risk of developing MFs, and a trend toward significance for the type of fracture according to Genant’s numerical classification was observed. Conversely, PV was associated with a significantly lower risk of subsequent OVFs, indicating a protective effect. A combined model including four variables showed a moderate-to-high discriminant value for predicting the development or not of MFs. In fractures located at the thoracolumbar junction, we classified the fracture type according to DGOU; a higher loss of posterior height at CT and the presence of intravertebral cleft increased the risk of developing MFs, while PV showed a protective effect. A multivariate model combining three of these variables also showed a moderate-to-high discriminant value for predicting the development or not of MFs. However, there are some limitations that need to be considered, particularly derived from the retrospective nature of this study and the relatively limited sample size, which probably underpowered the statistical analyses.
Regarding radiological measurements, first of all, we found that the presence of intravertebral cleft increased the chance of developing MFs, with higher OR values in fractures located at the thoracolumbar junction. This is consistent with previous findings. For instance, a study by Trout et al. found that patients treated with PV for fractures with clefts had a nearly twofold (OR, 1.90; 95%CI, 1.04–3.49) increased risk of subsequent fractures compared to those without clefts [37]. Our results showed an even more pronounced association of over a fourfold increase in such a risk. Similarly, Wang et al. identified intravertebral clefts as a significant risk factor for adjacent-level symptomatic fractures after vertebral augmentation, with a higher incidence of new fractures within six months post-procedure in patients with clefts [38]. However, to our knowledge, no specific studies have assessed the risk of MFs in OVFs with intravertebral clefts managed conservatively, although in these patients, clefts are a known risk factor for increasing vertebral collapse [31]. Considering that PV was found to have a significant protective effect for MFs in our sample, further studies in patients managed conservatively are warranted to determine how the presence of clefts in acute OVFs influences the prognosis and risk of MFs in these patients.
Secondly, we observed that involvement of the posterior wall increases the risk of developing MFs. In the whole sample, the loss of posterior vertebral height between standing X-rays and CT showed an inverse relationship with the risk of MFs, with an optimal cutoff point of 2.4%. Interestingly, this pattern is opposite to the one found in our previous study for predicting vertebral collapse [32]. Although the significance of this finding from a pathophysiological perspective is unclear, we hypothesize that increased tendency of the posterior wall to collapse could favor a more homogeneous distribution of load forces, decreasing kyphotic angulation in the mid or long term, which is a known risk factor for the development of MFs. In fact, Okamoto et al. demonstrated through finite element analysis that kyphotic deformity significantly increases compressive stresses on adjacent vertebrae, thereby elevating the risk of subsequent fractures [39]. Similarly, Huang et al. found that hyperkyphotic posture, which can result from increased local kyphosis, is associated with a higher risk of MFs [40]. The association between vertebral collapse and MFs considering kyphotic changes in the light of these findings should also be explored in future studies. Notably, this trend was also observed in the subgroup of patients with fractures located at the thoracolumbar junction, but it did not reach statistical significance, probably owing to the more reduced sample size. Conversely, for these patients, our results showed that a higher loss of posterior vertebral height at initial CT was a predictor for developing MFs. Overall, these findings highlight the relevance of posterior wall involvement in spinal stability and call for more profound analyses in future studies.
On the other hand, we observed an association between the categories of Genant’s numerical classification and MFs in the whole sample, with a higher likelihood of developing MFs as the degrees of this classification increase. However, it should be taken into account that there was an asymmetric distribution in the fracture categories between the treatment groups, with a significantly higher number of OVFs grade 0.5 in the CM group. Therefore, this finding may represent a confounding effect, explainable by the fact that OVFs with minimal height loss are more prone to be treated conservatively rather than with PV (at least in the short or mid term). This is supported by the results of our multivariate analysis, where the inclusion of the Genant’s classification, which showed a trend toward significance in the univariate regression analysis, improved model fitting. The other OVF classifications analyzed showed no significant associations with the risk of developing MFs, in line with other previous studies [41,42]. Interestingly, although we observed a similar trend for the Genant’s numerical classification in the subgroup of patients with OVFs at the thoracolumbar junction, it did not reach statistical significance (p = 0.058). Conversely, the DGOU classification, which showed a trend toward significance in the whole sample, did, probably due to the fact that only OF2 and OF3 fractures (i.e., no OF4 fractures) were present in patients with OVFs at the thoracolumbar junction. A higher risk of developing MFs was found in OF3 fractures compared to OF2 fractures, emphasizing the role of posterior wall involvement, in line with our previously described findings. These findings underscore the need to find classification systems with greater prognostic capacity for the development of OVFs.
Finally, probably the most interesting result that we observed is that PV was associated with a significant decrease in the risk of developing metachronous OVFs compared to CM. Specifically, the incidence of new incident OVFs in patients treated with PV occurred in 5 cases (11.1%), while in the CM group, it occurred in 15 cases (33.3%), with statistically significant differences and a protective cOR of 0.250 and aOR of 0.050. These outcomes were analogous in the subgroup of patients with fractures located at the thoracolumbar junction, with 3 (9.7%) and 11 (29.7) MFs in patients treated with PV and CM, respectively (p = 0.042), and cOR and aOR values of 0.253 and 0.120, respectively. Of note, for aOR, we adjusted for variables that showed significant differences between patients managed with CM and PV (potential confounders), apart from sex and age. The main biological rationale for this finding lies in the decreased destabilization of the initial fracture focus and corroborates the results of previous studies [43,44] while disagreeing with authors who argued that the risk of developing MFs in adjacent vertebrae increases due to the stiffness and biomechanical change caused by PV [45,46]. As in previous meta-analyses [44,47], our findings defy the hypothesis that PV contributes to increased risk of subsequent vertebral fracture but emphasize the need for adequately designed randomized controlled trials to confirm these findings.
The main limitations of this study lie in its retrospective nature, in not having assessed some potential confounders such as bone mineral density, and in the relatively limited sample size, which probably underpowered some statistical analyses and precluded level-by-level contrastive analyses, particularly in the subgroup of patients with fractures located at the thoracolumbar junction. Notably, the discrepancies observed in the predictivity of some radiological variables in the whole sample as compared to patients with fractures in the thoracolumbar junction, which probably owed to variations in both the sample sizes and the specific features of vertebrae in different spinal segments, merit further investigation. The main advantages of this study lie in the high number of radiological variables measured by expert musculoskeletal radiologists, its two-centered nature, and the long follow-up (mean follow-up over 2 years). Overall, it is necessary to validate our results in prospective studies with larger sample sizes.
5. Conclusions
In osteoporotic vertebral fractures of the thoracic and lumbar spine, the presence of an intravertebral cleft, a difference in loss of posterior vertebral height between standing X-rays and CT equal to or lower than 2.4%, and the application of conservative treatment instead of vertebroplasty were identified as potential predictors for the development of metachronous vertebral fractures. In fractures located at the thoracolumbar junction, the most relevant predictors were intravertebral cleft, posterior height loss compared to adjacent vertebrae at initial CT over 1.6%, OF3 fractures of the DGOU’s classification, and conservative management. These findings could improve our understanding of the course of osteoporotic vertebral fractures and improve patient selection for treatment-based strategies. However, some limitations of our study, particularly derived from its retrospective nature, call for prospective validation in future studies with larger sample sizes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics15020160/s1, Supplementary Table S1. Descriptive analysis of the socio-demographic variables, causes, location and types of the fractures located at the thoracolumbar junction; Supplementary Table S2. Descriptive analysis of the radiological variables assessed in the study for fractures located at the thoracolumbar junction with bivariate descriptive analysis based on the treatment that was applied; Supplementary Table S3. Factors associated with the development of new osteoporotic vertebral fractures in patients with fractures located at the thoracolumbar junction; Supplementary Table S4. Univariate and multivariate logistic regression analyses for the prediction of metachronous fractures in patients with vertebral fractures located at the thoracolumbar junction, including the variables that showed statistically significant differences in the bivariate analyses; Supplementary Table S5. Receiver-operating characteristics curve analysis for the variables of the multivariate model to predict the development of new fractures. The combined model includes the variables “treatment”, DGOU classification and loss of posterior height; Supplementary Figure S1. ROC curves of the univariate and multivariate models obtained for the prediction of metachronous vertebral fractures in patients with fractures located at the thoracolumbar junction; Supplementary Figure S2. Illustrative example of a patient without metachronous fractures in our sample; Supplementary Figure S3. Illustrative example of a patient who developed a metachronous fracture in our sample.
Author Contributions
Conceptualization, A.J.L.R.-B. and F.R.S.; formal analysis, A.J.L.R.-B. and M.R.I.; investigation, A.J.L.R.-B., F.G.S., L.B.C., D.L.G. and P.M.J.G.; methodology, A.J.L.R.-B., J.M.B., M.R.I. and F.R.S.; project administration, A.J.L.R.-B.; resources, J.M.B. and F.R.S.; software, A.J.L.R.-B.; supervision, A.J.L.R.-B. and F.R.S.; writing—original draft, A.J.L.R.-B. and L.B.C.; writing—review and editing, P.M.J.G., D.L.G. and J.M.B. All authors will be informed about each step of manuscript processing including submission, revision, revision reminder, etc. via emails from our system or assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Ciencia, Innovación y Universidades (MICINN) under Grant Ref PID2023-151336OB-I00.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by our Institutional Review Board (Provincial Ethics Committee of Granada; code TFG-FX-2019; 7 June 2019).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to restrictions imposed by the Ethics Committee, which approved the study protocol.
Acknowledgments
Some of the results presented in this article were part of a master’s thesis of one of the authors (L.B.C.), which was co-directed by the first and senior authors, presented at the University of Granada in July 2024.
Conflicts of Interest
The first and senior authors host a special issue in Diagnostics. They were not involved in the review process or editorial decisions related to the manuscript.
References
- Xie, L.; Zhao, Z.G.; Zhang, S.J.; Hu, Y. Bin Percutaneous Vertebroplasty versus Conservative Treatment for Osteoporotic Vertebral Compression Fractures: An Updated Meta-Analysis of Prospective Randomized Controlled Trials. Int. J. Surg. 2017, 47, 25–32. [Google Scholar] [CrossRef]
- Yamauchi, K.; Adachi, A.; Kameyama, M.; Murakami, M.; Sato, Y.; Kato, C.; Kato, T. A Risk Factor Associated with Subsequent New Vertebral Compression Fracture after Conservative Therapy for Patients with Vertebral Compression Fracture: A Retrospective Observational Study. Arch. Osteoporos. 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.A.; McCabe, E.; Bergin, D.; Kearns, S.R.; McCabe, J.P.; Armstrong, C.; Heaney, F.; Carey, J.J. Osteoporotic Vertebral Fractures Are Common in Hip Fracture Patients and Are Under-Recognized. J. Clin. Densitom. 2021, 24, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, R.; Zhang, S.D.; Wu, X.T. Comparison of Acute Single versus Multiple Osteoporotic Vertebral Compression Fractures in Radiographic Characteristic and Bone Fragility. J. Orthop. Surg. Res. 2023, 18, 1–10. [Google Scholar] [CrossRef]
- Kano, S.; Tanikawa, H.; Mogami, Y.; Shibata, S.I.; Takanashi, S.; Oji, Y.; Aoki, T.; Oba, H.; Ikegami, S.; Takahashi, J. Comparison between Continuous and Discontinuous Multiple Vertebral Compression Fractures. Eur. Spine J. 2012, 21, 1867–1872. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Harsløf, T. Medical Treatment of Osteoporotic Vertebral Fractures. Ther. Adv. Musculoskelet. Dis. 2011, 3, 17–29. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, E.H.; Lee, J.C.; Choi, S.W.; Kim, H.S.; Cha, J.S.; Shin, B.J. Management of Osteoporotic Vertebral Fracture: Review Update 2022. Asian Spine J. 2022, 16, 934–946. [Google Scholar] [CrossRef]
- Anselmetti, G.C.; Bernard, J.; Blattert, T.; Court, C.; Fagan, D.; Fransen, H.; Fransen, P.; Sabharwal, T.; Schils, F.; Schupfner, R.; et al. Criteria for the Appropriate Treatment of Osteoporotic Vertebral Compression Fractures. Pain. Physician 2013, 16, 519–530. [Google Scholar] [CrossRef]
- Patel, D.; Liu, J.; Ebraheim, N.A. Managements of Osteoporotic Vertebral Compression Fractures: A Narrative Review. World J. Orthop. 2022, 13, 564–573. [Google Scholar] [CrossRef]
- Tu, P.H.; Liu, Z.H.; Lee, S.T.; Chen, J.F. Treatment of Repeated and Multiple New-Onset Osteoporotic Vertebral Compression Fractures with Teriparatide. J. Clin. Neurosci. 2012, 19, 532–535. [Google Scholar] [CrossRef]
- Jang, H.-D.; Kim, E.-H.; Lee, J.C.; Choi, S.-W.; Kim, K.; Shin, B.-J. Current Concepts in the Management of Osteoporotic Vertebral Fractures: A Narrative Review. Asian Spine J. 2020, 14, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Slavici, A.; Rauschmann, M.; Fleege, C. Conservative Management of Osteoporotic Vertebral Fractures: An Update. Eur. J. Trauma. Emerg. Surg. 2017, 43, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Saracen, A.; Kotwica, Z. Treatment of Multiple Osteoporotic Vertebral Compression Fractures by Percutaneous Cement Augmentation. Int. Orthop. 2014, 38, 2309–2312. [Google Scholar] [CrossRef]
- Galibert, P.; Deramond, H.; Rosat, P.; Le Gars, D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987, 33, 166–168. [Google Scholar]
- Kushchayev, S.V.; Wiener, P.C.; Teytelboym, O.M.; Arrington, J.A.; Khan, M.; Preul, M.C. Percutaneous Vertebroplasty: A History of Procedure, Technology, Culture, Specialty, and Economics. Neuroimaging Clin. N. Am. 2019, 29, 481–494. [Google Scholar] [CrossRef]
- Ruiz Santiago, F.; Pérez Abela, A.L.; Almagro Ratia, M.M. El Final de Las Vertebroplastias. Radiologia 2012, 54, 532–538. [Google Scholar] [CrossRef]
- Zhai, W.; Jia, Y.; Wang, J.; Cheng, L.; Zeng, Z.; Yu, Y.; Chen, L. The Clinical Effect of Percutaneous Kyphoplasty for the Treatment of Multiple Osteoporotic Vertebral Compression Fractures and the Prevention of New Vertebral Fractures. Int. J. Clin. Exp. Med. 2015, 8, 13473–13481. [Google Scholar]
- Kallmes, D.F.; Comstock, B.A.; Heagerty, P.J.; Turner, J.A.; Wilson, D.J.; Diamond, T.H.; Edwards, R.; Gray, L.A.; Stout, L.; Owen, S.; et al. A Randomized Trial of Vertebroplasty for Osteoporotic Spinal Fractures. N. Engl. J. Med. 2009, 361, 569–579. [Google Scholar] [CrossRef]
- Buchbinder, R.; Osborne, R.H.; Ebeling, P.R.; Wark, J.D.; Mitchell, P.; Wriedt, C.; Graves, S.; Staples, M.P.; Murphy, B. A Randomized Trial of Vertebroplasty for Painful Osteoporotic Vertebral Fractures. N. Engl. J. Med. 2009, 361, 557–568. [Google Scholar] [CrossRef]
- Dai, C.; Liang, G.; Zhang, Y.; Dong, Y.; Zhou, X. Risk Factors of Vertebral Re-Fracture after PVP or PKP for Osteoporotic Vertebral Compression Fractures, Especially in Eastern Asia: A Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2022, 17, 161. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Yang, J.S.; Hao, D.J.; Liu, T.J.; Jing, Q.M. Risk Factors for New Vertebral Fracture after Percutaneous Vertebroplasty for Osteoporotic Vertebral Compression Fractures. Clin. Interv. Aging 2021, 16, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, R.; Johnston, R.V.; Rischin, K.J.; Homik, J.; Jones, C.A.; Golmohammadi, K.; Kallmes, D.F. Percutaneous Vertebroplasty for Osteoporotic Vertebral Compression Fracture. Cochrane Database Syst. Rev. 2018, 2018, CD006349. [Google Scholar]
- Clark, W.; Bird, P.; Gonski, P.; Diamond, T.H.; Smerdely, P.; McNeil, H.P.; Schlaphoff, G.; Bryant, C.; Barnes, E.; Gebski, V. Safety and Efficacy of Vertebroplasty for Acute Painful Osteoporotic Fractures (VAPOUR): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2016, 388, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Carli, D.; Venmans, A.; Lodder, P.; Donga, E.; van Oudheusden, T.; Boukrab, I.; Schoemaker, K.; Smeets, A.; Schonenberg, C.; Hirsch, J.; et al. Vertebroplasty versus Active Control Intervention for Chronic Osteoporotic Vertebral Compression Fractures: The VERTOS V Randomized Controlled Trial. Radiology 2023, 308, e222535. [Google Scholar] [CrossRef]
- Láinez Ramos-Bossini, A.J.; López Zúñiga, D.; Ruiz Santiago, F. Percutaneous Vertebroplasty versus Conservative Treatment and Placebo in Osteoporotic Vertebral Fractures: Meta-Analysis and Critical Review of the Literature. Eur. Radiol. 2021, 31, 8542–8553. [Google Scholar] [CrossRef]
- Papanastassiou, I.D.; Filis, A.; Aghayev, K.; Kokkalis, Z.T.; Gerochristou, M.A.; Vrionis, F.D. Adverse Prognostic Factors and Optimal Intervention Time for Kyphoplasty/Vertebroplasty in Osteoporotic Fractures. Biomed. Res. Int. 2014, 2014, 925683. [Google Scholar] [CrossRef]
- Gazzotti, M.R.; Roco, C.M.; Pradella, C.O.; Nascimento, O.A.; Porto, E.F.; Adas, M.; Lazaretti-Castro, M.; Jardim, J.R. Frequency of Osteoporosis and Vertebral Fractures in Chronic Obstructive Pulmonary Disease (COPD) Patients. Arch. Bronconeumol. 2019, 55, 252–257. [Google Scholar] [CrossRef]
- Gifre, L.; Massó, E.; Fusaro, M.; Haarhaus, M.; Ureña, P.; Cozzolino, M.; Mazzaferro, S.; Calabia, J.; Peris, P.; Bover, J. Vertebral Fractures in Patients with CKD and the General Population: A Call for Diagnosis and Action. Clin. Kidney J. 2024, 17, sfae191. [Google Scholar] [CrossRef]
- Ruiz Santiago, F.; Láinez Ramos-Bossini, A.J.; Wáng, Y.X.J.; López Zúñiga, D. The Role of Radiography in the Study of Spinal Disorders. Quant. Imaging Med. Surg. 2020, 10, 2322–2355. [Google Scholar] [CrossRef]
- Ruiz Santiago, F.; Láinez Ramos-Bossini, A.; Wáng, Y.X.J.; Martínez Barbero, J.; García Espinosa, J.; Martínez Martínez, A. The Value of Magnetic Resonance Imaging and Computed Tomography in the Study of Spinal Disorders. Quant. Imaging Med. Surg. 2022, 12, 3947–3986. [Google Scholar] [CrossRef]
- Muratore, M.; Ferrera, A.; Masse, A.; Bistolfi, A. Osteoporotic Vertebral Fractures: Predictive Factors for Conservative Treatment Failure. A Systematic Review. Eur. Spine J. 2018, 27, 2565–2576. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Santiago, F.; Láinez Ramos-Bossini, A.J.; Moraleda-Cabrera, B. Factors Influencing Vertebral Collapse in Osteoporotic Vertebral Fractures: A Case-Control Study of Symptomatic Patients Attended in the Emergency Department. Arch. Osteoporos. 2023, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Kepler, C.K.; Vaccaro, A.R.; Koerner, J.D.; Dvorak, M.F.; Kandziora, F.; Rajasekaran, S.; Aarabi, B.; Vialle, L.R.; Fehlings, M.G.; Schroeder, G.D.; et al. Reliability Analysis of the AOSpine Thoracolumbar Spine Injury Classification System by a Worldwide Group of Naïve Spinal Surgeons. Eur. Spine J. 2016, 25, 1082–1086. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Oner, C.; Kepler, C.K.; Dvorak, M.; Schnake, K.; Bellabarba, C.; Reinhold, M.; Aarabi, B.; Kandziora, F.; Chapman, J.; et al. AOSpine Thoracolumbar Spine Injury Classification System. Spine (Phila. Pa. 1976) 2013, 38, 2028–2037. [Google Scholar] [CrossRef]
- Hounsfield, G.N. Computed Medical Imaging. J. Comput. Assist. Tomogr. 1980, 4, 665–674. [Google Scholar] [CrossRef]
- Trout, A.T.; Kallmes, D.F. Does Vertebroplasty Cause Incident Vertebral Fractures? A Review of Available Data. AJNR Am. J. Neuroradiol. 2006, 27, 1397–1403. [Google Scholar]
- Wang, J.; Chen, M.; Du, J. Therapeutic effect of conservative treatment of refracture in cemented vertebrae after percutaneous vertebroplasty for osteoporotic vertebral compression fractures. Nan Fang. Yi Ke Da Xue Xue Bao 2016, 36, 277–281. [Google Scholar]
- Okamoto, Y.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Sakamoto, J.; Kawahara, N.; Tsuchiya, H. The Effect of Kyphotic Deformity Because of Vertebral Fracture: A Finite Element Analysis of a 10° and 20° Wedge-Shaped Vertebral Fracture Model. Spine J. 2015, 15, 713–720. [Google Scholar] [CrossRef]
- Huang, M.-H.; Barrett-Connor, E.; Greendale, G.A.; Kado, D.M. Hyperkyphotic Posture and Risk of Future Osteoporotic Fractures: The Rancho Bernardo Study. J. Bone Min. Res. 2006, 21, 419–423. [Google Scholar] [CrossRef]
- Ha, K.Y.; Kim, Y.H. Risk Factors Affecting Progressive Collapse of Acute Osteoporotic Spinal Fractures. Osteoporos. Int. 2013, 24, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Tsujio, T.; Nakamura, H.; Terai, H.; Hoshino, M.; Namikawa, T.; Matsumura, A.; Kato, M.; Suzuki, A.; Takayama, K.; Fukushima, W.; et al. Characteristic Radiographic or Magnetic Resonance Images of Fresh Osteoporotic Vertebral Fractures Predicting Potential Risk for Nonunion. Spine (Phila. Pa. 1976) 2011, 36, 1229–1235. [Google Scholar] [CrossRef]
- Farrokhi, M.R.; Alibai, E.; Maghami, Z. Randomized Controlled Trial of Percutaneous Vertebroplasty versus Optimal Medical Management for the Relief of Pain and Disability in Acute Osteoporotic Vertebral Compression Fractures: Clinical Article. J. Neurosurg. Spine 2011, 14, 561–569. [Google Scholar] [CrossRef]
- Láinez Ramos-Bossini, A.J.; Jiménez Gutiérrez, P.M.; Moraleda Cabrera, B.; Bueno Caravaca, L.; González Díez, M.; Ruiz Santiago, F. Risk of New Vertebral Compression Fractures and Serious Adverse Effects after Vertebroplasty: A Critical Review and Meta-Analysis of Randomized Controlled Trials. Quant. Imaging Med. Surg. 2024, 14, 7848–7861. [Google Scholar] [CrossRef]
- Blasco, J.; Martinez-Ferrer, A.; Macho, J.; San Roman, L.; Pomés, J.; Carrasco, J.; Monegal, A.; Guañabens, N.; Peris, P. Effect of Vertebroplasty on Pain Relief, Quality of Life, and the Incidence of New Vertebral Fractures: A 12-Month Randomized Follow-up, Controlled Trial. J. Bone Miner. Res. 2012, 27, 1159–1166. [Google Scholar] [CrossRef]
- Huang, P.-H.; Chen, C.-W.; Hu, M.-H.; Yang, S.-H.; Huang, C.-C. Risk Factors of Failed Conservative Treatment for Adjacent Vertebral Fractures Following Percutaneous Vertebroplasty. Spine (Phila. Pa. 1976) 2024. [Google Scholar] [CrossRef]
- Han, S.L.; Wan, S.L.; Li, Q.T.; Xu, D.T.; Zang, H.M.; Chen, N.J.; Chen, L.Y.; Zhang, W.P.; Luan, C.; Yang, F.; et al. Is Vertebroplasty a Risk Factor for Subsequent Vertebral Fracture, Meta-Analysis of Published Evidence? Osteoporos. Int. 2015, 26, 113–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).