Zipper Pattern: An Investigation into Psychotic Criminal Detection Using EEG Signals
Abstract
1. Introduction
1.1. Related Works
1.2. Literature Gaps
- Most research has focused on achieving high classification performance on EEG signal datasets [32]. To this end, deep learning (DL) architectures have been widely employed [33,34]. However, while DL models often achieve high accuracy, they come with drawbacks such as exponential time complexity and reliance on expensive hardware or cloud services, making them less suitable for lightweight intelligent applications [35]. This underscores the need for efficient and accurate feature engineering models [36].
- In addition to classification performance, interpretability/explainability is a critical aspect that has received limited attention in the literature [36]. Despite advances in artificial intelligence, explainable artificial intelligence (XAI) methods remain underexplored, particularly in applications related to EEG signal processing.
- There is a lack of AI-based psychotic criminal detection models in the literature.
1.3. Motivation and Study Outline
1.4. Innovations and Contributions
- A new EEG psychotic criminal dataset was curated. In this regard, we have created a new testbed for psychotic criminal detection.
- A new generation channel-based feature extraction function has been presented. The introduced feature extractor is termed ZPat.
- A new ZPat-based XFE model has been developed.
- To the best of our knowledge, the recommended ZPat-based XFE model is the first XFE model for psychotic criminal/criminal detection.
- The presented ZPat-based XFE model has a cognitive structure designed to yield high classification accuracy. In this work, the model achieved over 95% classification accuracy with LORO and 10-fold CVs. Thus, the ZPat-based XFE model is a highly accurate feature engineering model with linear time complexity. In this respect, this research contributes to machine learning.
- By integrating the DLob symbolic language, explainable results have been obtained from the identities of the selected features. A cortical connectome diagram has been generated using DLob. In this context, the introduced ZPat-based XFE model contributes to medical sciences, providing insights into the psychotic criminal/criminal brain by extracting a cortical map. Moreover, this model also contributes to forensic science.
2. Materials and Methods
2.1. Materials
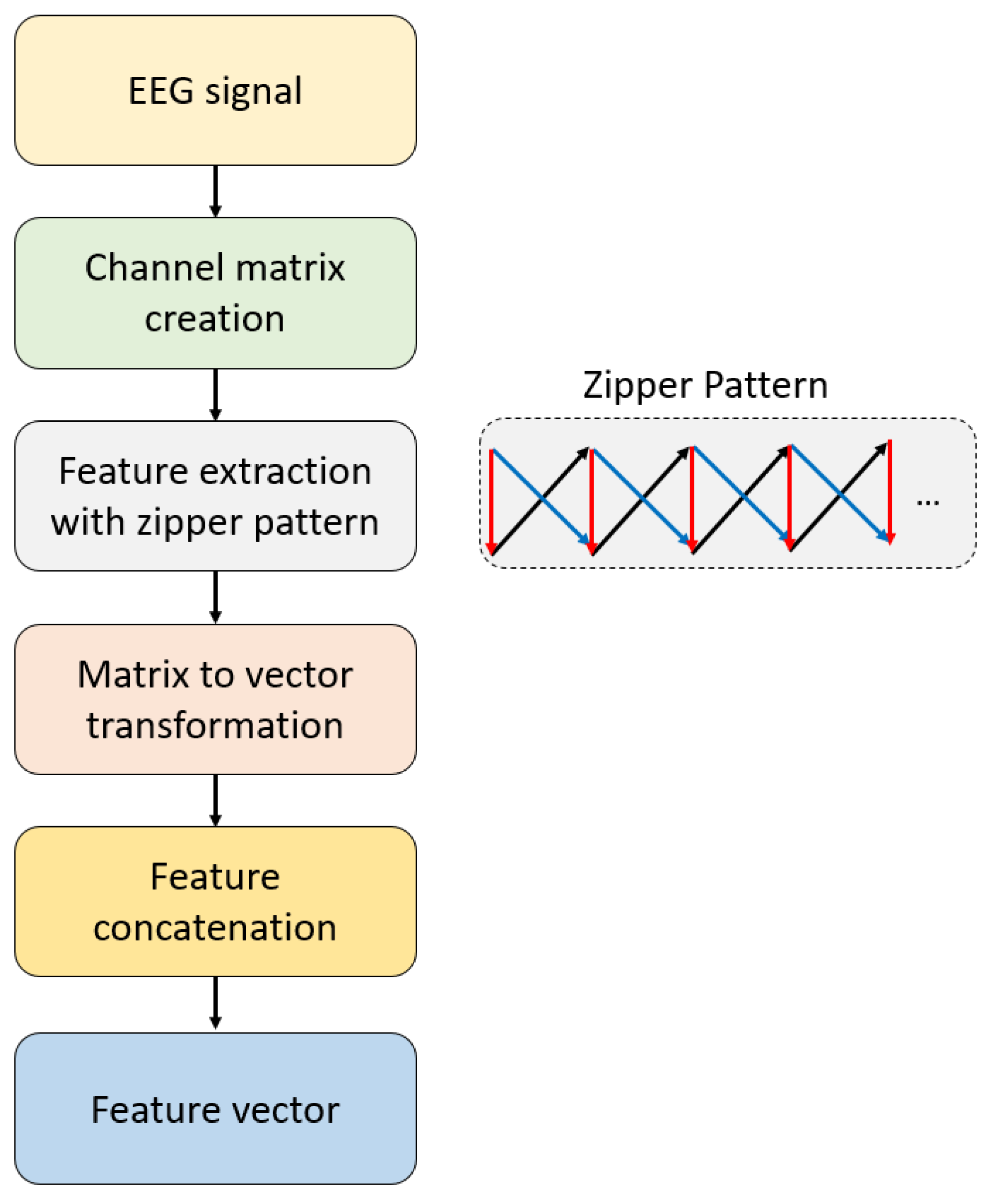
2.2. Zipper Pattern
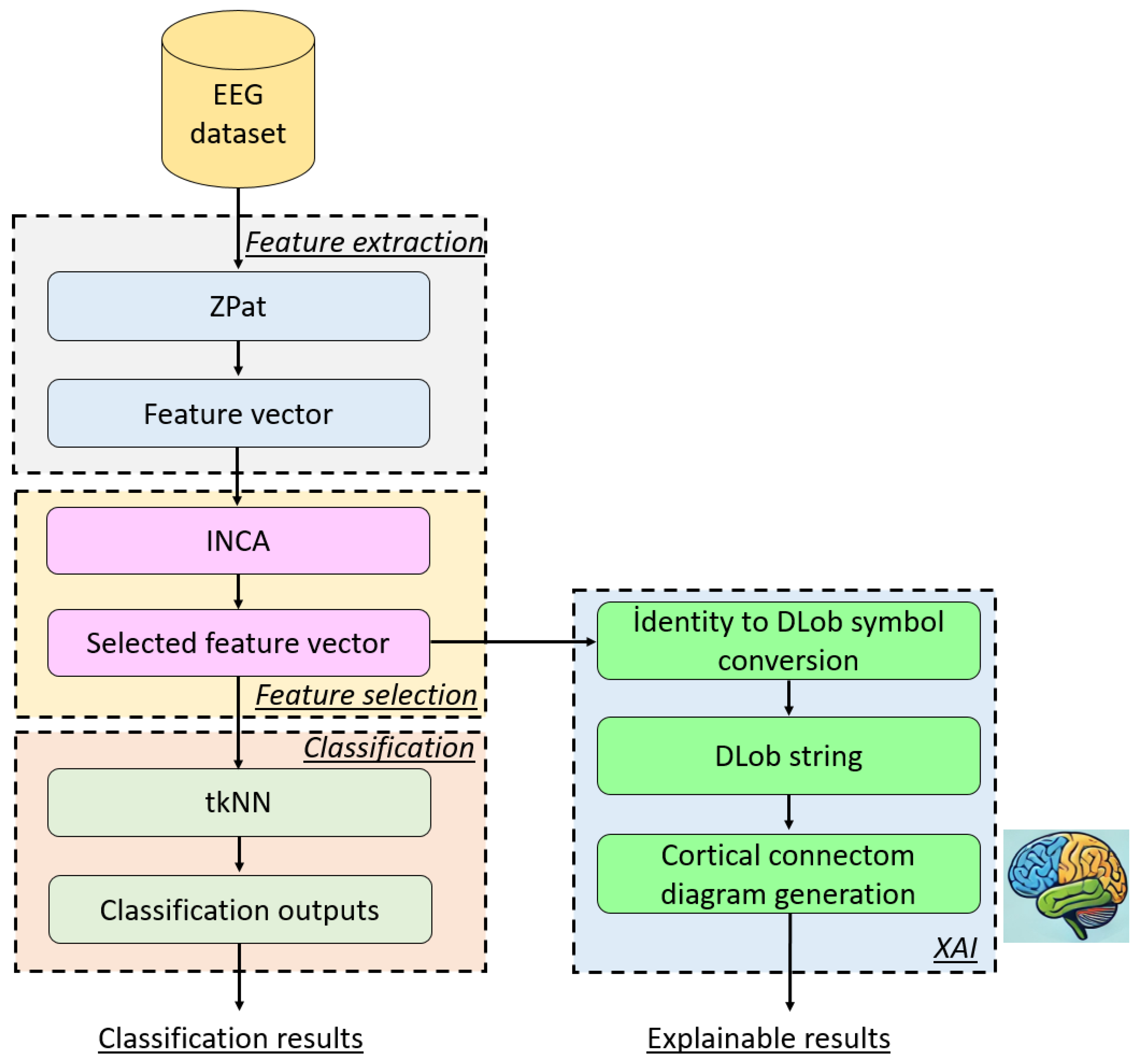
2.3. The Recommended XFE Model
- -
- Parameter-based outcome generation: In this step, an iterative parameter changing has been applied to the kNN classifier and more than one parameter-based outcome has been generated. For this study, the changed parameters are (i) distances, (ii) weights, and (iii) k values. By changing these parameters, 60 parameter-based outcomes have been created.
- -
- Majority voting: in order to created voted results, iterative majority voting (IMV) [47] has been used and 58 voted outcomes has been created from the generated 60 outcomes.
- -
- Greedy algorithm: In this work, the generated 118 (=60 + 58) outcomes have been selected using the maximum classification accuracy. In this step, the maximum accurate outcome has been selected as the final outcome.
3. Experimental Results
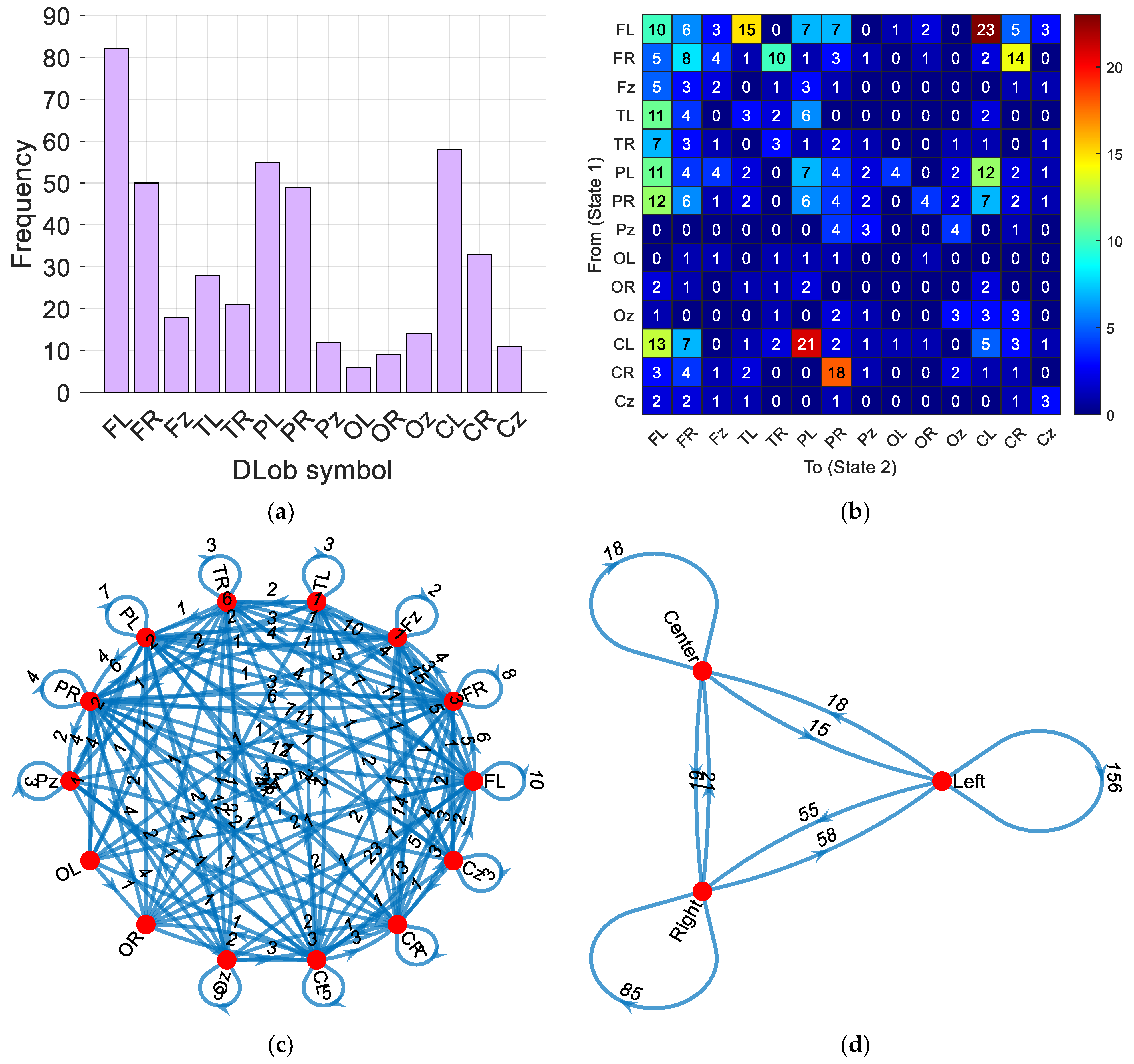
- “FRFRFLFRCLCRPRFLCLFLFLCROzTRTROzOzOzCRFzPLFLFLPRPROzPRFLCLFLCLPzPRPLFzFLCLCLPLCLCLPLFLORCLOLFzFzCRPRCLPRPLCLPLPzPzOzPzPRFRFRFLPRORPLPLOzCLFLTLPLPLPROzCRCLPLFLCLPLFLCLCRPRCLPLCLCLFLPLOLTRCzFRCRFLFLFLFRCRPRORPLFLCzCzFLCLFLCLFRCRPRPzOzCLPLPzOzCLPRPLTLTLPLPRCRPRCLTLTLFLTLFLTLFRTRPLFLTLFRTRPRFRFRCLPLCLPLPRPzCRCRFLTLPLTLFLORCLTRTRFRTRPRFLFLPRPRFRFRFRCRFRCRPzPRFLCLTRFLTLPLPLOLPLOLPRPRCzCzFRCRFRCROzCRFRCRTLTRFLCLFLTLFLTLFLFRPzPzOzOzFLTLFRTRFLCLPLCRPRCLPLFzFLFRCRPRORTRTRCLPLFLCLFLCLCLPLOzPRTLFRFzPLCLPLCLFLCLFLCLFLPRORTLFLPRPLFLPLFLPLOLFRPRFLFLFzPLFLPLCLFLCRPRFLFLPLCzCzPRFzFRPRFLFLTLPLPRCLFRTRPzPzPRFLCLPLCLPLFRCRFLCLORFRFRPLPLFRTRFRTRFLPLFRCRFRFLPLPLFzFRTLPLPLFRTRFLTLCLFRTRFLCLFRFzFLCRPRFRFLTLFLFRFRPRFLFRCRTLFLTLFLPRPRFLCzFLCRPRTLTLFLPRFLTLFLFLFLFzFzCzFzFLCLFLCLFRFzPRFRFLCRPRPLFzFRFRORFLFzTRFRFzFLCLFRCRPRCLFRTRFLTLCLCRPRPLPLCLPLFLOLORFLCzCRPRCLCLPLCLPLCLPLCLPLCRPRCRPRFRCRPRFLCLFLCLCzTLTRFz”
4. Discussion
- -
- A new channel-based feature extractor, ZPat, has been presented in this research.
- -
- By integrating INCA, tkNN, and DLob into the presented ZPat, a new XFE model has been developed.
- -
- The recommended ZPat-based XFE model is a lightweight model as it has linear time complexity.
- -
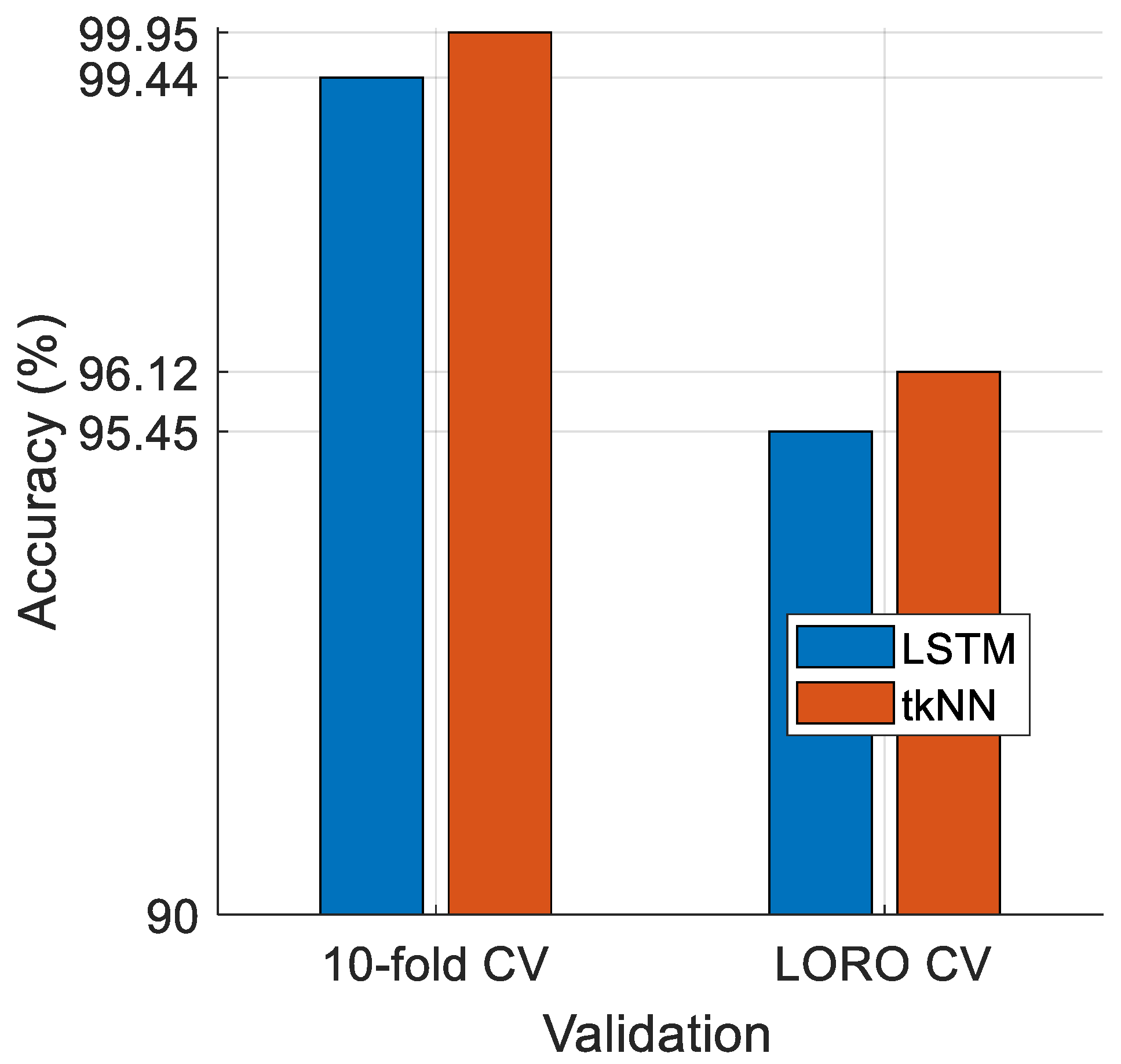
- In this research, two validation techniques were used in the classification phase: 10-fold CV and LORO CV.
- -
- The introduced ZPat-driven XFE model achieved over 95% classification accuracy, attaining 99.95% with 10-fold CV and 96.64% with LORO CV. This high performance is attributed to the self-organized nature of the model, which utilizes the INCA selector and tkNN classifier.
- -
- We compared the performance of the tkNN classifier with LSTM (a deep classifier), and tkNN attained higher classification accuracy than LSTM.
- -
- A DLob string was generated in this research. By deploying this string, cortical and hemispheric connectome diagrams were created to provide interpretable results.
- -
- The information entropy of the DLob string (3.4575 out of a maximum of 3.8074) reflected the complex neural activation patterns involved in psychotic criminal detection.
- -
- According to the DLob string, the frontal, central, and parietal lobes were the most commonly activated lobes. Central lobe activations implied hemisphere transitions, coordination of muscle movements, and planning of complex movements. Frontal lobe activation was associated with impaired executive functions and decision making. Parietal lobe activity indicated altered sensory integration and spatial awareness.
- -
- The left hemisphere was the most activated hemisphere for psychotic criminal detection. Dominance of the left hemisphere reflected challenges in language, logic, mathematics, verbal memory, and planning tasks.
- -
- The gathered dataset was collected from a single medical center and includes 64 participants with 4098 EEG segments. The EEG brain cap used has 32 channels. In this regard, a more diverse and larger dataset could be collected.
- -
- The introduced ZPat-driven model can be implemented on other EEG datasets to demonstrate its general classification ability.
- -
- Collaboration with more medical centers is being planned to collect larger and more diverse EEG datasets.
- -
- The presented ZPat-driven XFE model will be tested on other EEG datasets to demonstrate the general classification ability of the model.
- -
- New generation EEG abnormality detection assistants can be developed using the recommended ZPat-based XFE model.
- -
- The presented model can be integrated into EEG devices to automatically create cortical and hemispheric connectome diagrams, simplifying the translation of EEG signals.
- -
- New neuro digital forensic systems will be developed to extract behavioral analyses based on brain mapping by utilizing the recommended ZPat-driven XFE model.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verdolini, N.; Pacchiarotti, I.; Köhler, C.A.; Reinares, M.; Samalin, L.; Colom, F.; Tortorella, A.; Stubbs, B.; Carvalho, A.F.; Vieta, E.; et al. Violent criminal behavior in the context of bipolar disorder: Systematic review and meta-analysis. J. Affect. Disord. 2018, 239, 161–170. [Google Scholar] [CrossRef]
- Winstok, Z.; Sowan-Basheer, W. Does psychological violence contribute to partner violence research? A historical, conceptual and critical review. Aggress. Violent Behav. 2015, 21, 5–16. [Google Scholar] [CrossRef]
- Bravo-Queipo-de-Llano, B.; Sainz, T.; Sáez, C.D.; Miras, E.B.; Barriocanal, M.B.; Olmo, J.A.C.; Martori, A.F.; Baranda, A.G. Violence as a Health Problem. An. Pediatría 2024, 100, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Latalova, K.; Kamaradova, D.; Prasko, J. Suicide in bipolar disorder: A review. Psychiatr. Danub. 2014, 26, 108–114. [Google Scholar] [PubMed]
- Schanda, H.; Stompe, T.; Ortwein-Swoboda, G. Increasing criminality in patients with schizophrenia: Fiction, logical consequence or avoidable side effect of the mental health reforms? Neuropsychiatr. Klin. Diagn. Ther. Rehabil. Organ Ges. Osterr. Nervenarzte Psychiater 2010, 24, 170–181. [Google Scholar]
- Kirkbride, J.B.; Anglin, D.M.; Colman, I.; Dykxhoorn, J.; Jones, P.B.; Patalay, P.; Pitman, A.; Soneson, E.; Steare, T.; Wright, T.; et al. The social determinants of mental health and disorder: Evidence, prevention and recommendations. World Psychiatry 2024, 23, 58–90. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.; Kuja-Halkola, R.; Fazel, S.; Sariaslan, A.; Du Rietz, E.; Lichtenstein, P.; Brikell, I. Post-traumatic stress disorder and the risk of violent crime conviction in Sweden: A nationwide, register-based cohort study. Lancet Public Health 2023, 8, e432–e441. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.K.; March, S.; Barua, P.D.; Gadre, V.M.; Acharya, U.R. Application of data fusion for automated detection of children with developmental and mental disorders: A systematic review of the last decade. Inf. Fusion 2023, 99, 101898. [Google Scholar] [CrossRef]
- Tully, J.; Hafferty, J.; Whiting, D.; Dean, K.; Fazel, S. Forensic mental health: Envisioning a more empirical future. Lancet Psychiatry 2024, 11, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A. Parents and genes and their effects on alcohol, drugs, and crime in triparental families. Am. J. Psychiatry 2015, 172, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Antenora, F.; Riba, M.; Belvederi Murri, M.; Biancosino, B.; Zerbinati, L.; Grassi, L. Aggressive behavior and psychiatric inpatients: A narrative review of the literature with a focus on the European experience. Curr. Psychiatry Rep. 2021, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zeev, D.; Scherer, E.A.; Brian, R.M.; Mistler, L.A.; Campbell, A.T.; Wang, R. Use of multimodal technology to identify digital correlates of violence among inpatients with serious mental illness: A pilot study. Psychiatr. Serv. 2017, 68, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Rozel, J.S.; Mulvey, E.P. The link between mental illness and firearm violence: Implications for social policy and clinical practice. Annu. Rev. Clin. Psychol. 2017, 13, 445–469. [Google Scholar] [CrossRef]
- Mahato, S.; Paul, S. Classification of depression patients and normal subjects based on electroencephalogram (EEG) signal using alpha power and theta asymmetry. J. Med. Syst. 2020, 44, 28. [Google Scholar] [CrossRef]
- Dev, A.; Roy, N.; Islam, M.K.; Biswas, C.; Ahmed, H.U.; Amin, M.A.; Sarker, F.; Vaidyanathan, R.; Mamun, K.A. Exploration of EEG-based depression biomarkers identification techniques and their applications: A systematic review. IEEE Access 2022, 10, 16756–16781. [Google Scholar] [CrossRef]
- Tasci, G.; Loh, H.W.; Barua, P.D.; Baygin, M.; Tasci, B.; Dogan, S.; Tuncer, T.; Palmer, E.E.; Tan, R.-S.; Acharya, U.R. Automated accurate detection of depression using twin Pascal’s triangles lattice pattern with EEG Signals. Knowl. Based Syst. 2023, 260, 110190. [Google Scholar] [CrossRef]
- Yun, S. Advances, challenges, and prospects of electroencephalography-based biomarkers for psychiatric disorders: A narrative review. J. Yeungnam Med. Sci. 2024, 41, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Khodayari-Rostamabad, A.; Reilly, J.P.; Hasey, G.; Debruin, H.; MacCrimmon, D. Diagnosis of psychiatric disorders using EEG data and employing a statistical decision model. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 4006–4009. [Google Scholar] [CrossRef]
- Yasin, S.; Hussain, S.A.; Aslan, S.; Raza, I.; Muzammel, M.; Othmani, A. EEG based Major Depressive disorder and Bipolar disorder detection using Neural Networks: A review. Comput. Methods Programs Biomed. 2021, 202, 106007. [Google Scholar] [CrossRef] [PubMed]
- Siuly, S.; Khare, S.K.; Bajaj, V.; Wang, H.; Zhang, Y. A computerized method for automatic detection of schizophrenia using EEG signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.D.; Haveman, Y.; Sergiou, C.S.; Choy, O. Neuroprediction of violence and criminal behavior using neuro-imaging data: From innovation to considerations for future directions. Aggress. Violent Behav. 2024, 80, 102008. [Google Scholar] [CrossRef]
- Park, S.M.; Jeong, B.; Oh, D.Y.; Choi, C.-H.; Jung, H.Y.; Lee, J.-Y.; Lee, D.; Choi, J.-S. Identification of major psychiatric disorders from resting-state electroencephalography using a machine learning approach. Front. Psychiatry 2021, 12, 707581. [Google Scholar] [CrossRef] [PubMed]
- Machetanz, L.; Huber, D.; Lau, S.; Kirchebner, J. Model building in forensic psychiatry: A machine learning approach to screening offender patients with SSD. Diagnostics 2022, 12, 2509. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Khan, A. Artificial intelligence in forensic psychiatry: Admissibility and relevance before courts. Int. J. Syst. Assur. Eng. Manag. 2024, 15, 1638–1649. [Google Scholar] [CrossRef]
- Srinivasan, S.; Johnson, S.D. A novel approach to schizophrenia Detection: Optimized preprocessing and deep learning analysis of multichannel EEG data. Expert Syst. Appl. 2024, 246, 122937. [Google Scholar] [CrossRef]
- Chen, H.; Lei, Y.; Li, R.; Xia, X.; Cui, N.; Chen, X.; Liu, J.; Tang, H.; Zhou, J.; Huang, Y.; et al. Resting-state EEG dynamic functional connectivity distinguishes non-psychotic major depression, psychotic major depression and schizophrenia. Mol. Psychiatry 2024, 29, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, J.; Gao, J.; Zeng, X.; Min, X.; Zhan, H.; Zheng, H.; Hu, H.; Yang, Y.; Wei, S. Identification of Methamphetamine Abusers Can Be Supported by EEG-Based Wavelet Transform and BiLSTM Networks. Brain Topogr. 2024, 37, 1217–1231. [Google Scholar] [CrossRef]
- Pettorruso, M.; Di Lorenzo, G.; Benatti, B.; d’Andrea, G.; Cavallotto, C.; Carullo, R.; Mancusi, G.; Di Marco, O.; Mammarella, G.; D’Attilio, A.; et al. Overcoming treatment-resistant depression with machine-learning based tools: A study protocol combining EEG and clinical data to personalize glutamatergic and brain stimulation interventions (SelecTool Project). Front. Psychiatry 2024, 15, 1436006. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Yairi, I.E. Multi-Input CNN-LSTM deep learning model for fear level classification based on EEG and peripheral physiological signals. Front. Psychol. 2023, 14, 1141801. [Google Scholar] [CrossRef] [PubMed]
- Baumgartl, H.; Dikici, F.; Sauter, D.; Buettner, R. Detecting Antisocial Personality Disorder Using a Novel Machine Learning Algorithm Based on Electroencephalographic Data. In Proceedings of the PACIS 2020 Proceedings: 24th Pacific Asia Conference on Information Systems, Dubai, United Arab Emirates, 20–24 June 2020; Volume 48, pp. 1–14. [Google Scholar]
- van Dongen, J.D.; Franken, I.H. Neuroscience in forensic psychiatry and psychology: An introduction to the special issue. Int. J. Forensic Ment. Health 2019, 18, 179–186. [Google Scholar] [CrossRef]
- Sharma, R.; Meena, H.K. Emerging Trends in EEG Signal Processing: A Systematic Review. SN Comput. Sci. 2024, 5, 415. [Google Scholar] [CrossRef]
- Bengio, Y. Deep learning of representations for unsupervised and transfer learning. In Proceedings of the ICML Workshop on Unsupervised and Transfer Learning, Bellevue, WA, USA, 2 July 2011; JMLR Inc.: New York, NY, USA, 2012; pp. 17–36. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Khamparia, A.; Singh, K.M. A systematic review on deep learning architectures and applications. Expert Syst. 2019, 36, e12400. [Google Scholar] [CrossRef]
- Malakouti, S.M. Heart disease classification based on ECG using machine learning models. Biomed. Signal Process. Control 2023, 84, 104796. [Google Scholar] [CrossRef]
- Tuninger, E.E.; Levander, S.; Bernce, R.; Johansson, G. Criminality and aggression among psychotic in-patients: Frequency and clinical correlates. Acta Psychiatr. Scand. 2001, 103, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, S.; Calem, M.; Shimel, R.; Williams, A.; Harleston, D.; Morgan, C.; Dazzan, P.; Fearon, P.; Morgan, K.; Lappin, J.; et al. Criminal offending and distinguishing features of offenders among persons experiencing a first episode of psychosis. Early Interv. Psychiatry 2011, 5, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, T.; Dogan, S.; Özyurt, F.; Belhaouari, S.B.; Bensmail, H. Novel Multi Center and Threshold Ternary Pattern Based Method for Disease Detection Method Using Voice. IEEE Access 2020, 8, 84532–84540. [Google Scholar] [CrossRef]
- Dogan, S.; Baygin, M.; Tasci, B.; Loh, H.W.; Barua, P.D.; Tuncer, T.; Tan, R.-S.; Acharya, U.R. Primate brain pattern-based automated Alzheimer’s disease detection model using EEG signals. Cogn. Neurodyn. 2023, 17, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, T.; Dogan, S.; Tasci, I.; Baygin, M.; Barua, P.D.; Acharya, U.R. Lobish: Symbolic Language for Interpreting Electroencephalogram Signals in Language Detection Using Channel-Based Transformation and Pattern. Diagnostics 2024, 14, 1987. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, T.; Dogan, S.; Baygin, M.; Tasci, I.; Mungen, B.; Tasci, B.; Barua, P.D.; Acharya, U. Directed Lobish-based explainable feature engineering model with TTPat and CWINCA for EEG artifact classification. Knowl. Based Syst. 2024, 305, 112555. [Google Scholar] [CrossRef]
- Goldberger, J.; Hinton, G.E.; Roweis, S.; Salakhutdinov, R.R. Neighbourhood components analysis. Adv. Neural Inf. Process. Syst. 2004, 17, 513–520. [Google Scholar]
- Maillo, J.; Ramírez, S.; Triguero, I.; Herrera, F. kNN-IS: An Iterative Spark-based design of the k-Nearest Neighbors classifier for big data. Knowl. Based Syst. 2017, 117, 3–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Dogan, S.; Tuncer, T.; Barua, P.D.; Acharya, U.R. Automated EEG-based language detection using directed quantum pattern technique. Appl. Soft. Comput. 2024, 7, 112301. [Google Scholar] [CrossRef]
- Dogan, A.; Akay, M.; Barua, P.D.; Baygin, M.; Dogan, S.; Tuncer, T.; Dogru, A.H.; Acharya, U.R. PrimePatNet87: Prime pattern and tunable q-factor wavelet transform techniques for automated accurate EEG emotion recognition. Comput. Biol. Med. 2021, 138, 104867. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Rakic, P.S. Cellular basis of working memory. Neuron 1995, 14, 477–485. [Google Scholar] [CrossRef]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Öhman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, K.; Løberg, E.-M.; Specht, K.; Steen, V.M.; van Wageningen, H.; Jørgensen, H.A. Auditory hallucinations in schizophrenia: The role of cognitive, brain structural and genetic disturbances in the left temporal lobe. Front. Hum. Neurosci. 2008, 2, 131. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.C.; Paradiso, S.; O’Leary, D.S. “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 1998, 24, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.-W.; Yang, J.-J. EEG-Based Schizophrenia Diagnosis through Time Series Image Conversion and Deep Learning. Electronics 2022, 11, 2265. [Google Scholar] [CrossRef]
- Nsugbe, E.; Samuel, O.W.; Asogbon, M.G.; Li, G. Intelligence Combiner: A Combination of Deep Learning and Handcrafted Features for an Adolescent Psychosis Prediction using EEG Signals. In Proceedings of the 2022 IEEE International Workshop on Metrology for Industry 4.0 & IoT (MetroInd4.0&IoT), Trento, Italy, 7–9 June 2022; pp. 92–97. [Google Scholar] [CrossRef]
- Gengeç Benli, Ş. Classification of First-Episode Psychosis with EEG Signals: ciSSA and Machine Learning Approach. Biomedicines 2023, 11, 3223. [Google Scholar] [CrossRef]
- Redwan, S.M.; Uddin, M.P.; Ulhaq, A.; Sharif, M.I.; Krishnamoorthy, G. Power spectral density-based resting-state EEG classification of first-episode psychosis. Sci. Rep. 2024, 14, 15154. [Google Scholar] [CrossRef] [PubMed]
- Ravan, M.; Noroozi, A.; Sanchez, M.M.; Borden, L.; Alam, N.; Flor-Henry, P.; Colic, S.; Khodayari-Rostamabad, A.; Minuzzi, L.; Hasey, G. Diagnostic deep learning algorithms that use resting EEG to distinguish major depressive disorder, bipolar disorder, and schizophrenia from each other and from healthy volunteers. J. Affect. Disord. 2024, 346, 285–298. [Google Scholar] [CrossRef] [PubMed]

| Class | (1) Psychotic Criminal | (2) Control | Total/Overall |
|---|---|---|---|
| Number of EEG segments | 1350 | 2748 | 4098 |
| Number of records | 93 | 37 | 130 |
| Number of participants | 27 (=25 M + 2 F) | 37 (=29 M + 8 F) | 64 (=54 M + 10 F) |
| Age range | from 19 to 47 | from 22 to 59 | from 19 to 59 |
| Phase | Method | Parameters |
|---|---|---|
| Feature extraction | ZPat | Size of the vector: 2 × 32, Number of transition tables: 3, Size of each transition table: 32 × 32, Number of generated features: 3072. |
| Feature selection | INCA | Number of selected feature vectors: 401 (=500 − 100 + 1) Classification accuracy generator: kNN classifier Final feature vector selection method: maximum classification accuracy (greedy algorithm) Length of the final selected feature vector: 174. |
| Classification | tkNN | Distance metrics: L1 and L2 norms, Weights: Inverse, Equal, and Squared inverse. k values: 1–10, Number of generated parameter-based outcomes: 60, Validation methods: 10-fold CV and LORO CV, Number of generated voted outcomes: 58, Total outcomes: 118, Voting function: iterative majority voting (IMV), Selection of the final feature vector: outcome with maximum classification accuracy (greedy algorithm), Parameters of IMV: Ordering: classification accuracy in descending order, Voting function: mode, Loop range: from 3 to 60, Number of generated outcomes: 58 (=60 − 3 + 1). |
| XAI | DLob | Number of unique DLob symbols used: 15, Length of the generated DLob string: 446 DLob symbols, Statistical methods: transition table and information entropy. |
| Metric | 10-Fold CV | LORO CV |
|---|---|---|
| Accuracy | 99.95 | 96.12 |
| Sensitivity/recall | 99.85 | 97.11 |
| Specificity | 100 | 95.63 |
| Precision | 100 | 91.61 |
| F1 score | 99.93 | 94.28 |
| Geometric mean | 99.93 | 96.37 |
| Research | Method | Type of Data Used | Sample Size | Results (%) |
|---|---|---|---|---|
| Ko et al. (2022) [52] | EEG Time Series Conversion; Gramian Angular Field (GAF); Recurrence Plot (RP); CNN | EEG (9 channels, N100 stimulus) | 81 participants (49 SZH, 32 controls) | GAF: accuracy 93.20, sensitivity 93.90, specificity 92.10 RP: accuracy 90.00, sensitivity 90.9, specificity 88.60 |
| Nsugbe et al. (2022) [53] | EEG; Spectrogram and Scalogram transformations; CNN (SqueezeNet, AlexNet, ResNet18); Handcrafted Features | EEG (60 s, 128 Hz) | 20 participants (10 SZH, 10 controls) | Accuracy: 98.30 |
| Benli et al. (2023) [54] | ciSSA (Circulant Singular Spectrum Analysis), SVM, ANN, Ensemble Methods | Resting-state EEG (64 channels, 3 min, 250 Hz) | 138 participants (78 first episode psychosis, 60 controls) | Accuracy: 96.23, recall: 96.60, specificity: 95.60, F1 Score: 96.66 |
| Redwan et al. (2024) [55] | ML methods (Random Forest, GPC, SVM, MLP, AdaBoost); PSD analysis | Resting-state EEG signals | 72 subjects (44 first episode psychosis patients, 28 controls) | Accuracy: 95.51, recall: 95.30, Specificity: 95.78, F1 Score: 95.26 |
| Ravan et al. (2024) [56] | Resting-state EEG, ReLORETA, Deep Learning Algorithms (3D CNN) | Resting EEG (256 Hz, 10 20 electrode positions) | 409 participants (105 MDD, 27 MDD A, 35 MDD P, 71 BD DE, 49 BD ME, 122 SCZ, 239 healthy controls) | Average accuracy: 94.17 (between HC and psychiatric disorders); 93.99 (pairwise classification among disorders). |
| Our method | ZPat-based XFE Model, INCA Feature Selection, tkNN Classifier, DLob Explainable AI | EEG (32 channel, 15 s segments, 256 Hz) | 64 participants (27 psychotic criminals, 37 controls) | (10-fold CV), Accuracy: 99.95 (LORO CV). Accuracy: 96.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasci, G.; Barua, P.D.; Tanko, D.; Keles, T.; Tas, S.; Sercek, I.; Kaya, S.; Yildirim, K.; Talu, Y.; Tasci, B.; et al. Zipper Pattern: An Investigation into Psychotic Criminal Detection Using EEG Signals. Diagnostics 2025, 15, 154. https://doi.org/10.3390/diagnostics15020154
Tasci G, Barua PD, Tanko D, Keles T, Tas S, Sercek I, Kaya S, Yildirim K, Talu Y, Tasci B, et al. Zipper Pattern: An Investigation into Psychotic Criminal Detection Using EEG Signals. Diagnostics. 2025; 15(2):154. https://doi.org/10.3390/diagnostics15020154
Chicago/Turabian StyleTasci, Gulay, Prabal Datta Barua, Dahiru Tanko, Tugce Keles, Suat Tas, Ilknur Sercek, Suheda Kaya, Kubra Yildirim, Yunus Talu, Burak Tasci, and et al. 2025. "Zipper Pattern: An Investigation into Psychotic Criminal Detection Using EEG Signals" Diagnostics 15, no. 2: 154. https://doi.org/10.3390/diagnostics15020154
APA StyleTasci, G., Barua, P. D., Tanko, D., Keles, T., Tas, S., Sercek, I., Kaya, S., Yildirim, K., Talu, Y., Tasci, B., Ozsoy, F., Gonen, N., Tasci, I., Dogan, S., & Tuncer, T. (2025). Zipper Pattern: An Investigation into Psychotic Criminal Detection Using EEG Signals. Diagnostics, 15(2), 154. https://doi.org/10.3390/diagnostics15020154










