Functional Independence Assessment in Children and Adolescents with Achondroplasia: A Multicenter Cross-Sectional Study Using the WeeFIM Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants and Recruitment
2.3. Data Collection Procedures
2.4. Outcome Measures
2.5. Demographic and Clinical Variables
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
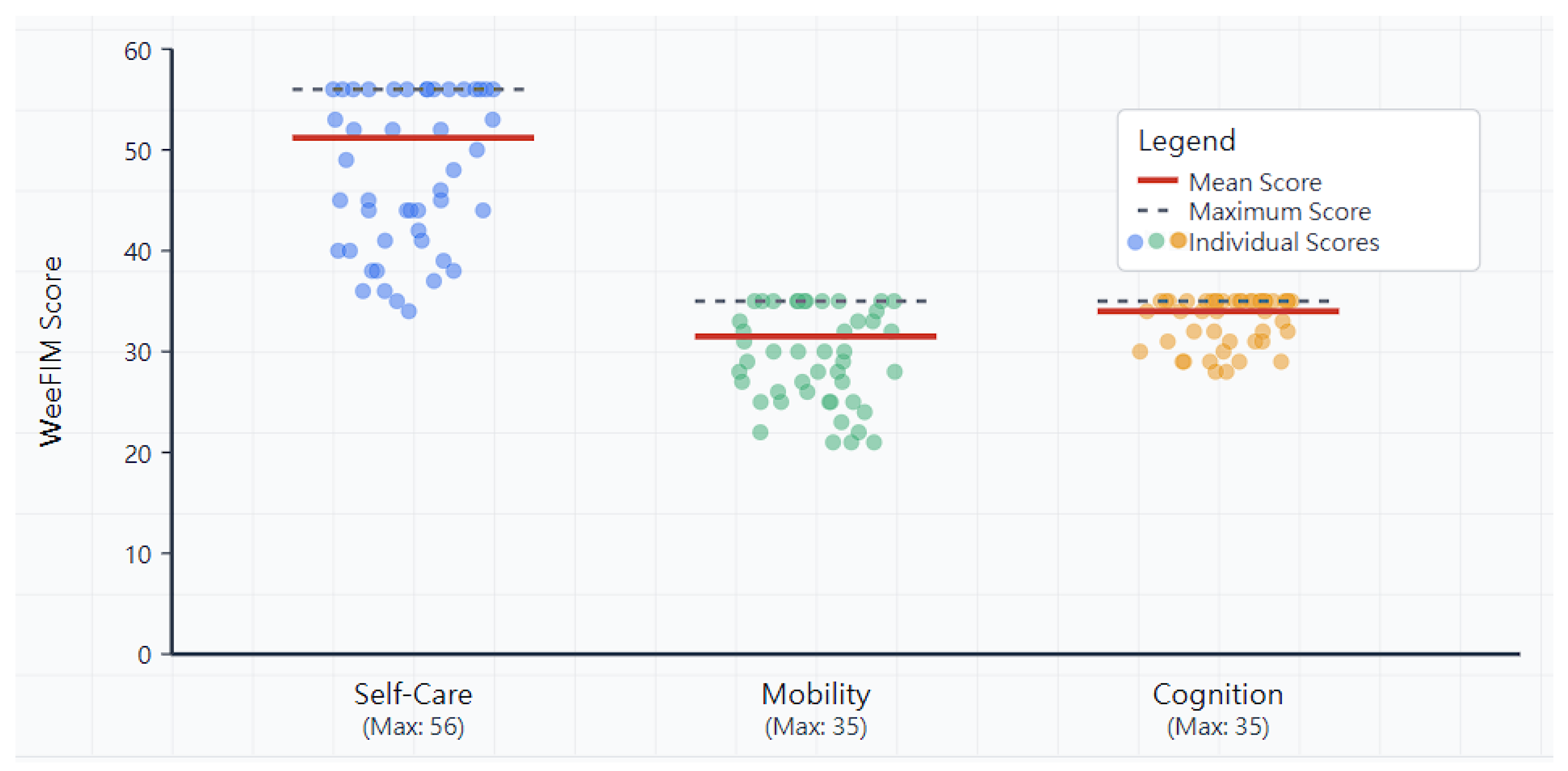
3.2. WeeFIM Domain Scores
3.3. Self-Care Domain
3.4. Mobility Domain
3.5. Cognitive Domain
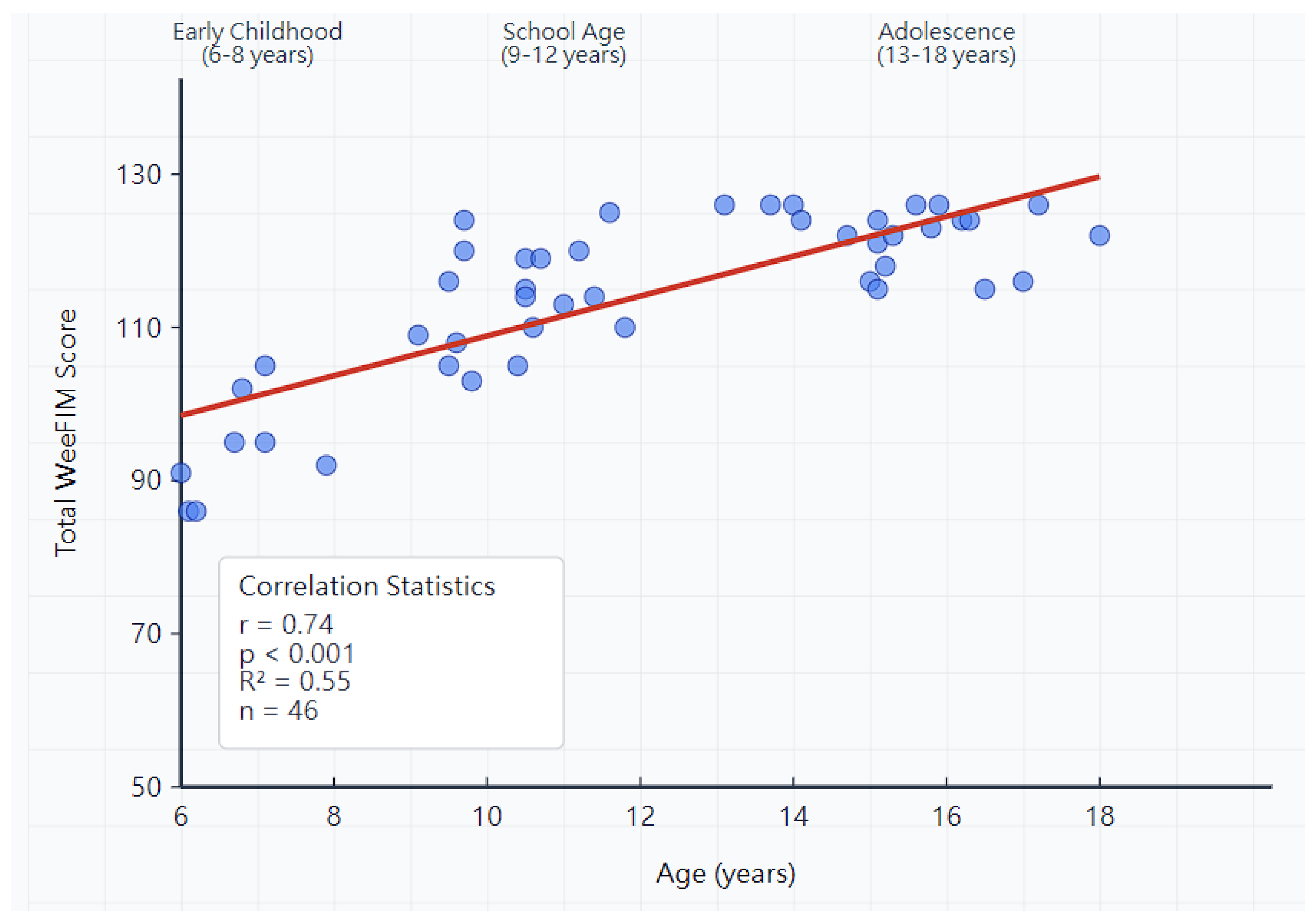
3.6. Age-Related Patterns
3.7. Functional Independence Categories
3.8. Factors Associated with Functional Outcomes
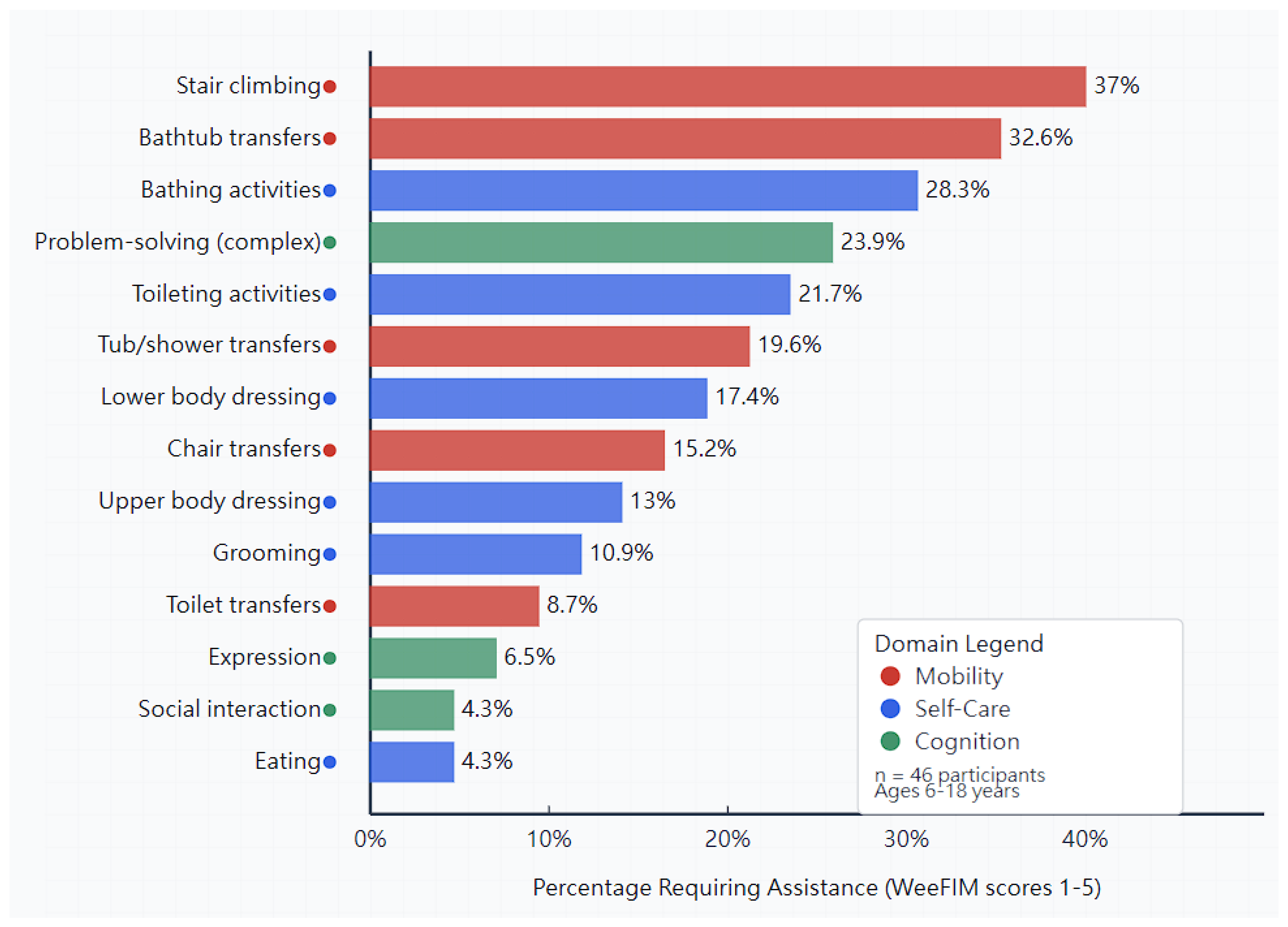
3.9. Specific Challenging Activities
4. Discussion
4.1. Cognitive Function Preservation
4.2. Mobility Challenges and Adaptive Strategies
4.3. Self-Care Independence and Development
4.4. Impact of Early Intervention Services
4.5. Environmental Factors and Accessibility
4.6. Developmental Functional Patterns
4.7. Clinical Implications and Future Directions
4.8. Study Limitations
- a.
- Methodological Limitations:
- b.
- Sampling and Generalizability Concerns:
- c.
- Assessment Tool Appropriateness:
- d.
- Data Collection Variability:
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waller, D.K.; Correa, A.; Vo, T.M.; Wang, Y.; Hobbs, C.; Langlois, P.H.; Pearson, K.; Romitti, P.A.; Shaw, G.M.; Hecht, J.T. The population-based prevalence of achondroplasia and thanatophoric dysplasia in selected regions of the US. Am. J. Med. Genet. A 2008, 146A, 2385–2389. [Google Scholar] [CrossRef]
- Oberklaid, F.; Danks, D.M.; Jensen, F.; Stace, L.; Rosshandler, S. Achondroplasia and hypochondroplasia. Comments on frequency, mutation rate, and radiological features in skull and spine. J. Med. Genet. 1979, 16, 140–146. [Google Scholar] [CrossRef] [PubMed]
- National Human Genome Research Institute. About Achondroplasia; National Institutes of Health: Bethesda, MD, USA, 2019. Available online: https://www.genome.gov/Genetic-Disorders/Achondroplasia (accessed on 20 September 2025).
- Lee, Y.C.; Song, I.W.; Pai, Y.J.; Chen, S.D.; Chen, Y.T. Knock-in human FGFR3 achondroplasia mutation as a mouse model for human skeletal dysplasia. Sci. Rep. 2017, 7, 43220. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.A.; Hall, J.G.; Hecht, J.T. Achondroplasia. Lancet 2007, 370, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Pauli, R.M. Achondroplasia: A comprehensive clinical review. Orphanet J. Rare Dis. 2019, 14, 1. [Google Scholar] [CrossRef]
- Hoover-Fong, J.E.; Schulze, K.J.; McGready, J.; Barnes, H.; Scott, C.I. Age-appropriate body mass index in children with achondroplasia: Interpretation in relation to indexes of height. Am. J. Clin. Nutr. 2008, 88, 364–371. [Google Scholar] [CrossRef]
- Richette, P.; Bardin, T.; Stheneur, C. Achondroplasia: From genotype to phenotype. Jt. Bone Spine 2008, 75, 125–130. [Google Scholar] [CrossRef]
- Ireland, P.J.; Donaghey, S.; Mcgill, J.; Zankl, A.; Ware, R.S.; Pacey, V.; Ault, J.; Savarirayan, R.; Sillence, D.; Thompson, E.; et al. Development in children with achondroplasia: A prospective clinical cohort study. Dev. Med. Child Neurol. 2012, 54, 532–537. [Google Scholar] [CrossRef]
- Hoover-Fong, J.; Scott, C.I.; Jones, M.C.; Committee on Genetics. Health supervision for people with achondroplasia. Pediatrics 2020, 145, e20201010. [Google Scholar] [CrossRef]
- Savarirayan, R.; Ireland, P.; Irving, M.; Thompson, D.; Alves, I.; Baratela, W.A.R.; Betts, J.; Bober, M.B.; Boero, S.; Briddell, J.; et al. International Consensus Statement on the diagnosis, multidisciplinary management and lifelong care of individuals with achondroplasia. Nat. Rev. Endocrinol. 2022, 18, 173–189. [Google Scholar] [CrossRef]
- Kubota, T.; Adachi, M.; Kitaoka, T.; Hasegawa, K.; Ohata, Y.; Fujiwara, M.; Michigami, T.; Mochizuki, H.; Ozono, K. Clinical Practice Guidelines for Achondroplasia. Clin. Pediatr. Endocrinol. 2020, 29, 25–42. [Google Scholar] [CrossRef]
- Ireland, P.J.; McGill, J.; Zankl, A.; Ware, R.S.; Pacey, V.; Ault, J.; Savarirayan, R.; Sillence, D.; Thompson, E.M.; Townshend, S.; et al. Functional performance in young Australian children with achondroplasia. Dev. Med. Child Neurol. 2011, 53, 944–950. [Google Scholar] [CrossRef]
- Maghnie, M.; Semler, O.; Guillen-Navarro, E.; Selicorni, A.; Heath, K.E.; Haeusler, G.; Hagenäs, L.; Merker, A.; Leiva-Gea, A.; González, V.L.; et al. Lifetime impact of achondroplasia study in Europe (LIAISE): Findings from a multinational observational study. Orphanet J. Rare Dis. 2023, 18, 56. [Google Scholar] [CrossRef]
- Aldhouse, N.V.J.; Kitchen, H.; Johnson, C.; Marshall, C.; Pegram, H.; Pease, S.; Collins, S.; Baker, C.L.; Beaverson, K.; Crews, C.; et al. Key measurement concepts and appropriate clinical outcome assessments in pediatric achondroplasia clinical trials. Orphanet J. Rare Dis. 2022, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, K.J.; Msall, M.E.; Lyon, N.; Duffy, L.C.; Ziviani, J.; Granger, C.V.; Braun, S.; Feidler, R.C. The WeeFIM instrument: Its utility in detecting change in children with developmental disabilities. Arch. Phys. Med. Rehab. 2000, 81, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Lee, H.; Kostanjsek, N.; Fornari, A.; Raggi, A.; Martinuzzi, A.; Yáñez, M.; Almborg, A.H.; Fresk, M.; Besstrashnova, Y.; et al. 20 years of ICF-International Classification of Functioning, Disability and Health: Uses and applications around the world. Int. J. Environ. Res. Public Health 2022, 19, 11321. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.J.; Gray, K.; Gibbons, P.J.; Grayson, J.; Sullivan, J.; Amorim, A.B.; Burns, J.; McKay, M.J. Evaluating the use of PROMs in paediatric orthopaedic registries. Children 2023, 10, 1552. [Google Scholar] [CrossRef]
- Fragala-Pinkham, M.A.; Dumas, H.M.; Lombard, K.A.; O’Brien, J.E. Responsiveness of the Pediatric Evaluation of Disability Inventory-Computer Adaptive Test in measuring functional outcomes for inpatient pediatric rehabilitation. J. Pediatr. Rehabil. Med. 2016, 9, 215–222. [Google Scholar] [CrossRef]
- Chokshi, K.P.; Tedla, J.S.; Narayan, A.; Ganesan, S.; Reddy, R.S. Functional independence measure (WeeFIM) reference values in Indian children aged 3-7 years: A cross-sectional study. Natl. Med. J. India 2021, 34, 73–78. [Google Scholar]
- King, J.A.; Vachhrajani, S.; Drake, J.M.; Rutka, J.T. Neurosurgical implications of achondroplasia. J. Neurosurg. Pediatr. 2009, 4, 297–306. [Google Scholar] [CrossRef]
- Savarirayan, R.; Irving, M.; Bacino, C.A.; Bostwick, B.; Charrow, J.; Cormier-Daire, V.; Le Quan Sang, K.H.; Dickson, P.; Harmatz, P.; Phillips, J.; et al. C-type natriuretic peptide analogue therapy in children with achondroplasia. N. Engl. J. Med. 2019, 381, 25–35. [Google Scholar] [CrossRef]
- Legeai-Mallet, L.; Savarirayan, R. Novel therapeutic approaches for the treatment of achondroplasia. Bone 2020, 141, 115579. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Savarirayan, R.; Tofts, L.; Irving, M.; Wilcox, W.; Bacino, C.A.; Hoover-Fong, J.; Ullot Font, R.; Harmatz, P.; Rutsch, F.; Bober, M.B.; et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: A randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet 2020, 396, 684–692. [Google Scholar] [CrossRef]
- Duggan, S. Vosoritide: First Approval. Drugs 2021, 81, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Savarirayan, R.; Wilcox, W.R.; Harmatz, P.; Phillips, J., 3rd; Polgreen, L.E.; Tofts, L.; Ozono, K.; Arundel, P.; Irving, M.; Bacino, C.A.; et al. Vosoritide therapy in children with achondroplasia aged 3-59 months: A multinational, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Child Adolesc. Health 2024, 8, 40–50. [Google Scholar] [CrossRef]
- Savarirayan, R.; Hoover-Fong, J.; Ozono, K.; Backeljauw, P.; Cormier-Daire, V.; DeAndrade, K.; Ireland, P.; Irving, M.; Llerena Junior, J.; Maghnie, M.; et al. International consensus guidelines on the implementation and monitoring of vosoritide therapy in individuals with achondroplasia. Nat. Rev. Endocrinol. 2025, 21, 314–324. [Google Scholar] [CrossRef]
- Reincke, S.; Semler, O.; Junghänel-Welzing, S.; Stasek, S.; Rehberg, M.; Pfeiffer, E.; Hoyer-Kuhn, H. Real-world Outcome of Vosoritide Treatment in Children with Achondroplasia: A 12-month Retrospective Observational Study. J. Endocr. Soc. 2025, 9, bvaf041. [Google Scholar] [CrossRef]
- Ireland, P.; Pacey, V.; Zankl, A.; Edwards, P.; Johnson, L.; Savarirayan, R. Optimal management of complications associated with achondroplasia. Appl. Clin. Genet. 2014, 7, 117–125. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar] [CrossRef]
- Australasian Rehabilitation Outcomes Centre (AROC). Training & Credentialing: FIM/WeeFIM; University of Wollongong: Wollongong, Australia, 2024; Available online: https://www.uow.edu.au/ahsri/aroc/fim-weefim/training-credentialing/ (accessed on 20 September 2025).
- Sugiyama, M.; Aoki, S.; Kawate, N.; Hashimoto, K. Limitation of developmental test to measure functional independence of children: Relationship between the Japanese version of WeeFIM II® and KSPD. J. Pediatr. Rehabil. Med. 2022, 15, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mwenge, M.M.; Figge, C.J.; Metz, K.; Kane, J.C.; Kohrt, B.A.; Pedersen, G.A.; Sikazwe, I.; Van Wyk, S.S.; Mulemba, S.M.; Murray, L.K. Improving inter-rater reliability of the enhancing assessment of common therapeutic factors (ENACT) measure through training of raters. J. Public Health Afr. 2022, 13, 2201. [Google Scholar] [CrossRef] [PubMed]
- Portney, L.G. Foundations of Clinical Research: Applications to Evidence-Based Practice, 4th ed.; F.A. Davis Company: Philadelphia, PA, USA, 2020. [Google Scholar]
- Wong, V.; Wong, S.; Chan, K.; Wong, W. Functional Independence Measure (WeeFIM) for Chinese children: Hong Kong Cohort. Pediatrics 2002, 109, E36. [Google Scholar] [CrossRef]
- Chen, C.C.; Bode, R.K.; Granger, C.V.; Heinemann, A.W. Psychometric properties and developmental differences in children’s ADL item hierarchy: A study of the WeeFIM instrument. Am. J. Phys. Med. Rehabil. 2005, 84, 671–679. [Google Scholar] [CrossRef]
- Australian Health Outcomes Consortium. FIM/WeeFIM Assessment Guidelines; University of Wollongong: Wollongong, Australia, 2025; Available online: https://www.uow.edu.au/australasian-health-outcomes-consortium/aroc/fim-weefim/ (accessed on 20 September 2025).
- Maddux, A.B.; Cox-Martin, M.; Dichiaro, M.; Bennett, T.D. The association between the Functional Status Scale and the Pediatric Functional Independence Measure in children who survive traumatic brain injury. Pediatr. Crit. Care Med. 2018, 19, 1046–1053. [Google Scholar] [CrossRef]
- Ziviani, J.; Ottenbacher, K.; Shephard, K.; Foreman, S.; Astbury, W.; Ireland, P. Concurrent validity of the Functional Independence Measure for Children (WeeFIMTM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries. Phys. Occup. Ther. Pediatr. 2001, 21, 91–101. [Google Scholar] [CrossRef]
- Kim, G.W.; Kim, H.; Jeon, J.Y.; Jang, J.S. Validity and Reliability of Functional Independence Measure for Children (WeeFIM) for Children with Cerebral Palsy. Inquiry 2022, 59, 469580211072454. [Google Scholar]
- Grilli, L.; Feldman, D.E.; Majnemer, A.; Couture, M.; Azoulay, L.; Swaine, B. Associations between a functional independence measure (WeeFIM) and the pediatric quality of life inventory (PedsQL4.0) in young children with physical disabilities. Qual. Life Res. 2006, 15, 1023–1031. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 28.0; IBM Corp: Armonk, NY, USA, 2021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 5th ed.; Sage Publications: London, UK, 2018. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 7th ed.; Pearson: Boston, MA, USA, 2019. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 8th ed.; Cengage Learning: Boston, MA, USA, 2019; Volume 8. [Google Scholar]
- Localio, A.R.; Henegan, J.A.; Chang, S.; Meibohm, A.R.; Ross, E.A.; Goodman, S.N.; Couper, D.; Guallar, E.; Griswold, M.E. Standardization and prediction to control confounding: Estimating risk differences and ratios for clinical interpretations and decision making. Ann. Intern. Med. 2025, 178, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Wigg, K.; Tofts, L.; Benson, S.; Porter, M. The neuropsychological function of children with achondroplasia. Am. J. Med. Genet. A 2016, 170, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Siracusano, M.; El Malhany, N.; Cerminara, C.; Pitzianti, M.; Terribili, M. Cognitive phenotype and language skills in children with achondroplasia. Minerva Pediatr. 2019, 71, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Committee on Bioethics. Informed consent in decision-making in pediatric practice. Pediatrics 2016, 138, e20161485. [Google Scholar] [CrossRef]
- Trotter, T.L.; Hall, J.G.; Committee on Genetics. Health supervision for children with achondroplasia. Pediatrics 2005, 116, 771–783. [Google Scholar] [CrossRef]
- Fredwall, S.O.; Maanum, G.; Johansen, H.; Snekkevik, H.; Savarirayan, R.; Lidal, I.B. Current knowledge of medical complications in adults with achondroplasia: A scoping review. Clin. Genet. 2020, 97, 179–197. [Google Scholar] [CrossRef]
- Jennings, S.E.; Ditro, C.P.; Bober, M.B.; Mackenzie, W.G.; Rogers, K.J.; Conway, L.; Duker, A.L. Prevalence of mental health conditions and pain in adults with skeletal dysplasia. Qual. Life Res. 2019, 28, 1457–1464. [Google Scholar] [CrossRef]
- Savarirayan, R.; Baratela, W.; Butt, T.; Cormier-Daire, V.; Irving, M.; Miller, B.S.; Mohnike, K.; Ozono, K.; Rosenfeld, R.; Selicorni, A.; et al. Literature review and expert opinion on the impact of achondroplasia on medical complications and health-related quality of life and expectations for long-term impact of vosoritide: A modified Delphi study. Orphanet J. Rare Dis. 2022, 17, 224. [Google Scholar] [CrossRef]
- Pfeiffer, K.M.; Brod, M.; Smith, A.; Gianettoni, J.; Viuff, D.; Ota, S.; Charlton, R.W. Assessing physical symptoms, daily functioning, and well-being in children with achondroplasia. Am. J. Med. Genet. A 2021, 185, 33–45. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, S.; Zhang, R.; Guo, S.; Sheng, Q.; Wang, K.; Shan, Y.; Liao, L.; Dong, J. Review of published 467 achondroplasia patients: Clinical and mutational spectrum. Orphanet J. Rare Dis. 2024, 19, 29. [Google Scholar] [CrossRef]
- Murton, M.C.; Drane, E.L.A.; Goff-Leggett, D.M.; Shediac, R.; O’Hara, J.; Irving, M.; Butt, T.J. Burden and Treatment of Achondroplasia: A Systematic Literature Review. Adv. Ther. 2023, 40, 3639–3680. [Google Scholar] [CrossRef]
- Savarirayan, R.; Irving, M.; Wilcox, W.R.; Bacino, C.A.; Hoover-Fong, J.E.; Harmatz, P.; Polgreen, L.E.; Palm, K.; Prada, C.E.; Kubota, T.; et al. Sustained growth-promoting effects of vosoritide in children with achondroplasia from an ongoing phase 3 extension study. Med 2025, 6, 100566. [Google Scholar] [CrossRef]
| Characteristic | n (%) or Mean ± SD |
|---|---|
| Age at assessment (years) | 13.8 ± 3.6 |
| Range | 6–18 |
| Age groups | |
| Early childhood (6–8 years) | 8 (17.4) |
| School age (9–12 years) | 18 (39.1) |
| Adolescence (13–18 years) | 20 (43.5) |
| Gender | |
| Male | 26 (56.5) |
| Female | 20 (43.5) |
| Age at diagnosis | |
| Infancy (<1 year) | 36 (78.3) |
| Early childhood (1–3 years) | 8 (17.4) |
| School age (4–11 years) | 1 (2.2) |
| Adolescence (12–18 years) | 1 (2.2) |
| Genetic testing performed | 32 (69.6) |
| FGFR3 mutation confirmed | 32 (100.0) |
| Early intervention services | 38 (82.6) |
| Physical therapy | 35 (76.1) |
| Occupational therapy | 32 (69.6) |
| Speech therapy | 26 (56.5) |
| Early intervention details | |
| Age at therapy initiation (years) | 2.9 ± 1.5 |
| Duration of physical therapy (months) | 29.2 ± 15.8 |
| Duration of occupational therapy (months) | 24.8 ± 13.2 |
| Duration of speech therapy (months) | 19.1 ± 12.0 |
| Therapy intensity (sessions/week) | 2.4 ± 0.9 |
| Primary indication for therapy referral | |
| Developmental delay | 29 (76.3) |
| Motor skill enhancement | 26 (68.4) |
| Speech delay | 16 (42.1) |
| Adaptive equipment training | 14 (36.8) |
| Multiple indications | 20 (52.6) |
| Age Group | n | Self-Care | Mobility | Cognition | Total WeeFIM |
|---|---|---|---|---|---|
| Early childhood (6–8 years) | 8 | 43.8 ± 7.2 | 26.4 ± 4.8 | 32.1 ± 2.9 | 98.3 ± 15.2 |
| School age (9–12 years) | 18 | 51.2 ± 5.8 | 31.8 ± 3.2 | 33.8 ± 1.8 | 114.8 ± 12.6 |
| Adolescence (13–18 years) | 20 | 54.1 ± 4.2 | 33.2 ± 2.1 | 34.9 ± 1.2 | 121.4 ± 8.3 |
| Overall | 46 | 51.2 ± 6.8 | 31.5 ± 4.5 | 34.0 ± 2.3 | 113.7 ± 15.2 |
| F-statistic (p-value) | 14.67 (<0.001) | 18.43 (<0.001) | 10.28 (<0.001) | 21.85 (<0.001) |
| Independence Level | Self-Care n (%) | Mobility n (%) | Cognition n (%) |
|---|---|---|---|
| Complete independence (6–7) | 37 (80.4) | 33 (71.7) | 43 (93.5) |
| Supervision (5) | 6 (13.0) | 9 (19.6) | 3 (6.5) |
| Complete dependence (1–4) | 3 (6.5) | 4 (8.7) | 0 (0.0) |
| Variable | β Coefficient | Standard Error | t-Value | p-Value | 95% CI |
|---|---|---|---|---|---|
| Age at assessment | 0.71 | 0.11 | 6.45 | <0.001 | 0.49–0.93 |
| Early intervention | 0.38 | 0.16 | 2.38 | 0.022 | 0.06–0.70 |
| Gender (male) | 0.15 | 0.18 | 0.83 | 0.412 | −0.21–0.51 |
| Age at diagnosis | −0.12 | 0.15 | −0.80 | 0.429 | −0.42–0.18 |
| Genetic testing | 0.09 | 0.17 | 0.53 | 0.601 | −0.25–0.43 |
| Age at therapy initiation | −0.32 | 0.15 | −2.13 | 0.039 | −0.62–0.02 |
| Duration of physical therapy | 0.31 | 0.14 | 2.21 | 0.032 | 0.03–0.59 |
| Duration of occupational therapy | 0.19 | 0.16 | 1.19 | 0.241 | −0.13–0.51 |
| Therapy intensity | 0.21 | 0.13 | 1.62 | 0.113 | −0.05–0.47 |
| Multiple therapy indications | 0.28 | 0.17 | 1.65 | 0.107 | −0.06–0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Fang, H.-H.; Chuang, C.-K.; Niu, D.-M.; Lin, J.-L.; Chao, M.-C.; Chou, Y.-Y.; Chiu, P.C.; Hsu, C.-C.; Chu, T.-H.; et al. Functional Independence Assessment in Children and Adolescents with Achondroplasia: A Multicenter Cross-Sectional Study Using the WeeFIM Scale. Diagnostics 2025, 15, 2532. https://doi.org/10.3390/diagnostics15192532
Lee C-L, Fang H-H, Chuang C-K, Niu D-M, Lin J-L, Chao M-C, Chou Y-Y, Chiu PC, Hsu C-C, Chu T-H, et al. Functional Independence Assessment in Children and Adolescents with Achondroplasia: A Multicenter Cross-Sectional Study Using the WeeFIM Scale. Diagnostics. 2025; 15(19):2532. https://doi.org/10.3390/diagnostics15192532
Chicago/Turabian StyleLee, Chung-Lin, Hung-Hsiang Fang, Chih-Kuang Chuang, Dau-Ming Niu, Ju-Li Lin, Mei-Chyn Chao, Yen-Yin Chou, Pao Chin Chiu, Chia-Chi Hsu, Tzu-Hung Chu, and et al. 2025. "Functional Independence Assessment in Children and Adolescents with Achondroplasia: A Multicenter Cross-Sectional Study Using the WeeFIM Scale" Diagnostics 15, no. 19: 2532. https://doi.org/10.3390/diagnostics15192532
APA StyleLee, C.-L., Fang, H.-H., Chuang, C.-K., Niu, D.-M., Lin, J.-L., Chao, M.-C., Chou, Y.-Y., Chiu, P. C., Hsu, C.-C., Chu, T.-H., Chien, Y.-H., Chiu, H.-C., Chang, Y.-H., Tu, Y.-R., Lo, Y.-T., Lin, H.-Y., & Lin, S.-P. (2025). Functional Independence Assessment in Children and Adolescents with Achondroplasia: A Multicenter Cross-Sectional Study Using the WeeFIM Scale. Diagnostics, 15(19), 2532. https://doi.org/10.3390/diagnostics15192532








