Ultrasound Risk Stratification of Autonomously Functioning Thyroid Nodules: Cine Loop Video Sequences Versus Static Image Captures
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Thyroid Ultrasound and Scintigraphy Examinations
2.3. Data Assessment and Observer
2.4. Data Analysis and Statistics
3. Results
3.1. Patient Characteristics and Clinical Data
3.2. Ultrasound Features
3.3. Risk Stratification According to TIRADS
3.4. Technical Ultrasound Features
3.5. Assessment Obstacles
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACR | American College of Radiology |
| AFTN | autonomously functioning thyroid nodule |
| CL | cine loop (ultrasound video sequences) |
| FNC | fine-needle cytology |
| PACS | Picture Archiving and Communication System |
| RSS | Risk stratification systems |
| SD | Standard deviation |
| SIC | static image captures (on ultrasound) |
| SOP | Standard Operation Procedure |
| T3 | thyroxin |
| T4 | triiodothyronine |
| TIRADS | Thyroid Imaging Reporting and Data System |
| TN | thyroid nodule |
| TS | Tc-99m-pertechnetate thyroid scintigraphy |
| TSH | thyroid-stimulating hormone |
| US | ultrasound |
References
- Schenke, S.A.; Kreissl, M.C.; Grunert, M.; Hach, A.; Haghghi, S.; Kandror, T.; Peppert, E.; Rosenbaum-Krumme, S.; Ruhlmann, V.; Stahl, A.; et al. Distribution of Functional Status of Thyroid Nodules and Malignancy Rates of Hyperfunctioning and Hypofunctioning Thyroid Nodules in Germany. Nuklearmedizin 2022, 61, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Johner, S.A.; Thamm, M.; Schmitz, R.; Remer, T. Examination of iodine status in the German population: An example for methodological pitfalls of the current approach of iodine status assessment. Eur. J. Nutr. 2016, 55, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Boers, T.; Braak, S.J.; Rikken, N.E.T.; Versluis, M.; Manohar, S. Ultrasound imaging in thyroid nodule diagnosis, therapy, and follow-up: Current status and future trends. J. Clin. Ultrasound 2023, 51, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Kobaly, K.; Kim, C.S.; Mandel, S.J. Contemporary Management of Thyroid Nodules. Annu. Rev. Med. 2022, 73, 517–528. [Google Scholar] [CrossRef]
- Piticchio, T.; Russ, G.; Radzina, M.; Frasca, F.; Durante, C.; Trimboli, P. Head-to-head comparison of American, European, and Asian TIRADSs in thyroid nodule assessment: Systematic review and meta-analysis. Eur. Thyroid J. 2024, 13, e230242. [Google Scholar] [CrossRef]
- Joo, L.; Lee, M.K.; Lee, J.Y.; Ha, E.J.; Na, D.G. Diagnostic Performance of Ultrasound-Based Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Meta-Analysis. Endocrinol. Metab. 2023, 38, 117–128. [Google Scholar] [CrossRef]
- Schmidt, T.N.; Freesmeyer, M.; Kühnel, C.; Gühne, F.; Rosenbaum, L.; Drescher, R.; Seifert, P. Risk Stratification of Thyroid Nodules Using Ultrasound Cine-Loop Video Sequences. Cancers 2025, 17, 2616. [Google Scholar] [CrossRef]
- Rosario, P.W.; de Castro Nicolau, T. The value of ultrasonography for the indication of fine-needle aspiration in autonomous thyroid nodules. Diagn. Cytopathol. 2021, 49, 363–366. [Google Scholar] [CrossRef]
- Schenke, S.; Seifert, P.; Zimny, M.; Winkens, T.; Binse, I.; Gorges, R. Risk Stratification of Thyroid Nodules Using the Thyroid Imaging Reporting and Data System (TIRADS): The Omission of Thyroid Scintigraphy Increases the Rate of Falsely Suspected Lesions. J. Nucl. Med. 2019, 60, 342–347. [Google Scholar] [CrossRef]
- Noto, B.; Eveslage, M.; Pixberg, M.; Gonzalez Carvalho, J.M.; Schafers, M.; Riemann, B.; Kies, P. Prevalence of hyperfunctioning thyroid nodules among those in need of fine needle aspiration cytology according to ATA 2015, EU-TIRADS, and ACR-TIRADS. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1518–1526. [Google Scholar] [CrossRef]
- Trimboli, P.; Bojunga, J.; Deandrea, M.; Frasca, F.; Imperiale, A.; Leoncini, A.; Paone, G.; Pitoia, F.; Rotondi, M.; Sadeghi, R.; et al. Reappraising the role of thyroid scintigraphy in the era of TIRADS: A clinically-oriented viewpoint. Endocrine 2024, 85, 1035–1040. [Google Scholar] [CrossRef]
- Schenke, S.A.; Gorges, R.; Seifert, P.; Zimny, M.; Kreissl, M.C. Update on diagnosis and treatment of hyperthyroidism: Ultrasonography and functional imaging. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 102–112. [Google Scholar] [CrossRef]
- Esfandiari, N.H.; Papaleontiou, M. Biochemical Testing in Thyroid Disorders. Endocrinol. Metab. Clin. N. Am. 2017, 46, 631–648. [Google Scholar] [CrossRef]
- Mounsey, A.; Paulus, R.; Roten, W. Hyperthyroidism: Diagnosis and Treatment. Am. Fam. Physician 2025, 112, 146–152. [Google Scholar]
- Seifert, P.; Kuhnel, C.; Reissmann, I.; Winkens, T.; Freesmeyer, M. Standardized acquisition and documentation of cine loops on conventional thyroid ultrasound. Laryngorhinootologie 2024, 103, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Seifert, P.; Maikowski, I.; Winkens, T.; Kuhnel, C.; Guhne, F.; Drescher, R.; Freesmeyer, M. Ultrasound Cine Loop Standard Operating Procedure for Benign Thyroid Diseases-Evaluation of Non-Physician Application. Diagnostics 2021, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Schenke, S.A.; Petersen, M.; Gorges, R.; Ruhlmann, V.; Zimny, M.; Richter, J.P.; Groener, D.; Baumgarten, J.; Kreissl, M.C.; Stahl, A.R.; et al. Interobserver Agreement in Ultrasound Risk Stratification Systems for Thyroid Nodules on Static Images Versus Cine-Loop Video Sequences. Diagnostics 2024, 14, 2138. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, M.; Hohberg, M.; Lassmann, M.; Verburg, F.A.; Luster, M.; Schmidt, M. DGN-Handlungsempfehlung (S1-Leitlinie) Schilddrüsenszintigraphie mit Tc-99m Pertechnetat und I-123 Natriumiodid. AWMF-Registernummer: 031-011. 2022. Available online: https://register.awmf.org/assets/guidelines/031-011l_S1_Schilddruesenszintigraphie-Tc-99m-Pertechnetat-I-123_Natriumiodid__2023-04.pdf (accessed on 11 March 2025).
- Durante, C.; Hegedus, L.; Na, D.G.; Papini, E.; Sipos, J.A.; Baek, J.H.; Frasoldati, A.; Grani, G.; Grant, E.; Horvath, E.; et al. International Expert Consensus on US Lexicon for Thyroid Nodules. Radiology 2023, 309, e231481. [Google Scholar] [CrossRef]
- Borges, A.P.; Antunes, C.; Caseiro-Alves, F.; Donato, P. Analysis of 665 thyroid nodules using both EU-TIRADS and ACR TI-RADS classification systems. Thyroid Res. 2023, 16, 12. [Google Scholar] [CrossRef]
- Mohan, S.L.; Govindarajalou, R.; Naik, D.; Saxena, S.K.; Toi, P.C.; Shankar, G.V. Determining the Best Thyroid Imaging Reporting and Data System: A Prospective Study Comparing the Diagnostic Performance of ACR, EU, and K TIRADS in the Evaluation of Thyroid Nodules. Indian J. Radiol. Imaging 2024, 34, 220–231. [Google Scholar] [CrossRef]
- Chen, J.; Ye, D.; Lv, S.; Li, X.; Ye, F.; Huang, Y.; Su, Z.; Lin, Y.; Xie, T.; Wen, X. Benign thyroid nodules classified as ACR TI-RADS 4 or 5: Imaging and histological features. Eur. J. Radiol. 2024, 175, 111261. [Google Scholar] [CrossRef]
- Staibano, P.; Ham, J.; Chen, J.; Zhang, H.; Gupta, M.K. Inter-Rater Reliability of Thyroid Ultrasound Risk Criteria: A Systematic Review and Meta-Analysis. Laryngoscope 2023, 133, 485–493. [Google Scholar] [CrossRef]
- Topcuoglu, O.M.; Uzunoglu, B.; Orhan, T.; Basaran, E.B.; Gormez, A.; Sarica, O. A real-world comparison of the diagnostic performances of six different TI-RADS guidelines, including ACR-/Kwak-/K-/EU-/ATA-/C-TIRADS. Clin. Imaging 2025, 117, 110366. [Google Scholar] [CrossRef]
- Hoang, J.K.; Middleton, W.D.; Tessler, F.N. Update on ACR TI-RADS: Successes, Challenges, and Future Directions, From the AJR Special Series on Radiology Reporting and Data Systems. AJR Am. J. Roentgenol. 2021, 216, 570–578. [Google Scholar] [CrossRef]
- Grani, G.; Sponziello, M.; Filetti, S.; Durante, C. Thyroid nodules: Diagnosis and management. Nat. Rev. Endocrinol. 2024, 20, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, R.; Yang, S.; Lu, L.; Ma, R.; Xiao, Z.; Lin, N.; Huang, Y.; Chen, L. Comparison of the C-TIRADS, ACR-TIRADS, and ATA guidelines in malignancy risk stratification of thyroid nodules. Quant. Imaging Med. Surg. 2023, 13, 4514–4525. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, M.; Turk, G.; Bilgili, M.; Akay, E.; Koc, A. Comparison of Diagnostic Performances of ATA Guidelines, ACR-TIRADS, and EU-TIRADS and Modified K-TIRADS: A Single Center Study of 4238 Thyroid Nodules. Exp. Clin. Endocrinol. Diabetes 2025, 133, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.K. Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Bojunga, J. Ultrasound of Thyroid Nodules. Ultraschall Med. 2018, 39, 488–511. [Google Scholar] [CrossRef]
- Dormagen, J.B.; Gaarder, M.; Drolsum, A. Standardized cine-loop documentation in abdominal ultrasound facilitates offline image interpretation. Acta Radiol. 2015, 56, 3–9. [Google Scholar] [CrossRef]
- Sopuschek, M.P.; Freesmeyer, M.; Winkens, T.; Kuhnel, C.; Petersen, M.; Guhne, F.; Werner, A.; Seifert, P. Standard operating procedure (SOP) for cervical ultrasound cine loop video sequences in the follow-up of differentiated thyroid carcinoma (DTC). Endocrine 2025, 87, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Shayganfar, A.; Hashemi, P.; Esfahani, M.M.; Ghanei, A.M.; Moghadam, N.A.; Ebrahimian, S. Prediction of thyroid nodule malignancy using thyroid imaging reporting and data system (TIRADS) and nodule size. Clin. Imaging 2020, 60, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chung, S.R.; Choi, S.H.; Kim, K.W. Accuracy of thyroid imaging reporting and data system category 4 or 5 for diagnosing malignancy: A systematic review and meta-analysis. Eur. Radiol. 2020, 30, 5611–5624. [Google Scholar] [CrossRef] [PubMed]
- Kyrilli, A.; Tacelli, N.; Russo, L.; Lebrun, L.; Salmon, I.; Russ, G.; Moreno-Reyes, R.; Corvilain, B. Autonomously functioning thyroid nodules present intermediate malignancy risk according to European Thyroid Imaging Reporting and Data System (EU-TIRADS) and yield indeterminate cytology results. Eur. Thyroid J. 2023, 12, e230135. [Google Scholar] [CrossRef]
- Rago, T.; Vitti, P. Risk Stratification of Thyroid Nodules: From Ultrasound Features to TIRADS. Cancers 2022, 14, 717. [Google Scholar] [CrossRef]
- Castellana, M.; Virili, C.; Paone, G.; Scappaticcio, L.; Piccardo, A.; Giovanella, L.; Trimboli, P. Ultrasound systems for risk stratification of thyroid nodules prompt inappropriate biopsy in autonomously functioning thyroid nodules. Clin. Endocrinol. 2020, 93, 67–75. [Google Scholar] [CrossRef]
- Papini, E.; Monpeyssen, H.; Frasoldati, A.; Hegedus, L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur. Thyroid J. 2020, 9, 172–185. [Google Scholar] [CrossRef]
- Giovanella, L.; Avram, A.M.; Iakovou, I.; Kwak, J.; Lawson, S.A.; Lulaj, E.; Luster, M.; Piccardo, A.; Schmidt, M.; Tulchinsky, M.; et al. EANM practice guideline/SNMMI procedure standard for RAIU and thyroid scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2514–2525. [Google Scholar] [CrossRef]
- Sollmann, L.; Eveslage, M.; Danzer, M.F.; Schafers, M.; Heitplatz, B.; Conrad, E.; Hescheler, D.; Riemann, B.; Noto, B. Additional Value of Pertechnetate Scintigraphy to American College of Radiology Thyroid Imaging Reporting and Data Systems and European Thyroid Imaging Reporting and Data Systems for Thyroid Nodule Classification in Euthyroid Patients. Cancers 2024, 16, 4184. [Google Scholar] [CrossRef]
- Baad, M.; Lu, Z.F.; Reiser, I.; Paushter, D. Clinical Significance of US Artifacts. Radiographics 2017, 37, 1408–1423. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.K.; Katyal, S.; Blackwood, M.S. US artifacts. Radiographics 2009, 29, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, E.K.; Kim, S.J.; Kwak, J.Y. Thyroid ultrasonography: Pitfalls and techniques. Korean J. Radiol. 2014, 15, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.X.; Mulvana, H.; Gauthier, T.; Lim, A.K.; Cosgrove, D.O.; Eckersley, R.J.; Stride, E. Quantitative contrast-enhanced ultrasound imaging: A review of sources of variability. Interface Focus 2011, 1, 520–539. [Google Scholar] [CrossRef]
- Sassaroli, E.; Crake, C.; Scorza, A.; Kim, D.S.; Park, M.A. Image quality evaluation of ultrasound imaging systems: Advanced B-modes. J. Appl. Clin. Med. Phys. 2019, 20, 115–124. [Google Scholar] [CrossRef]

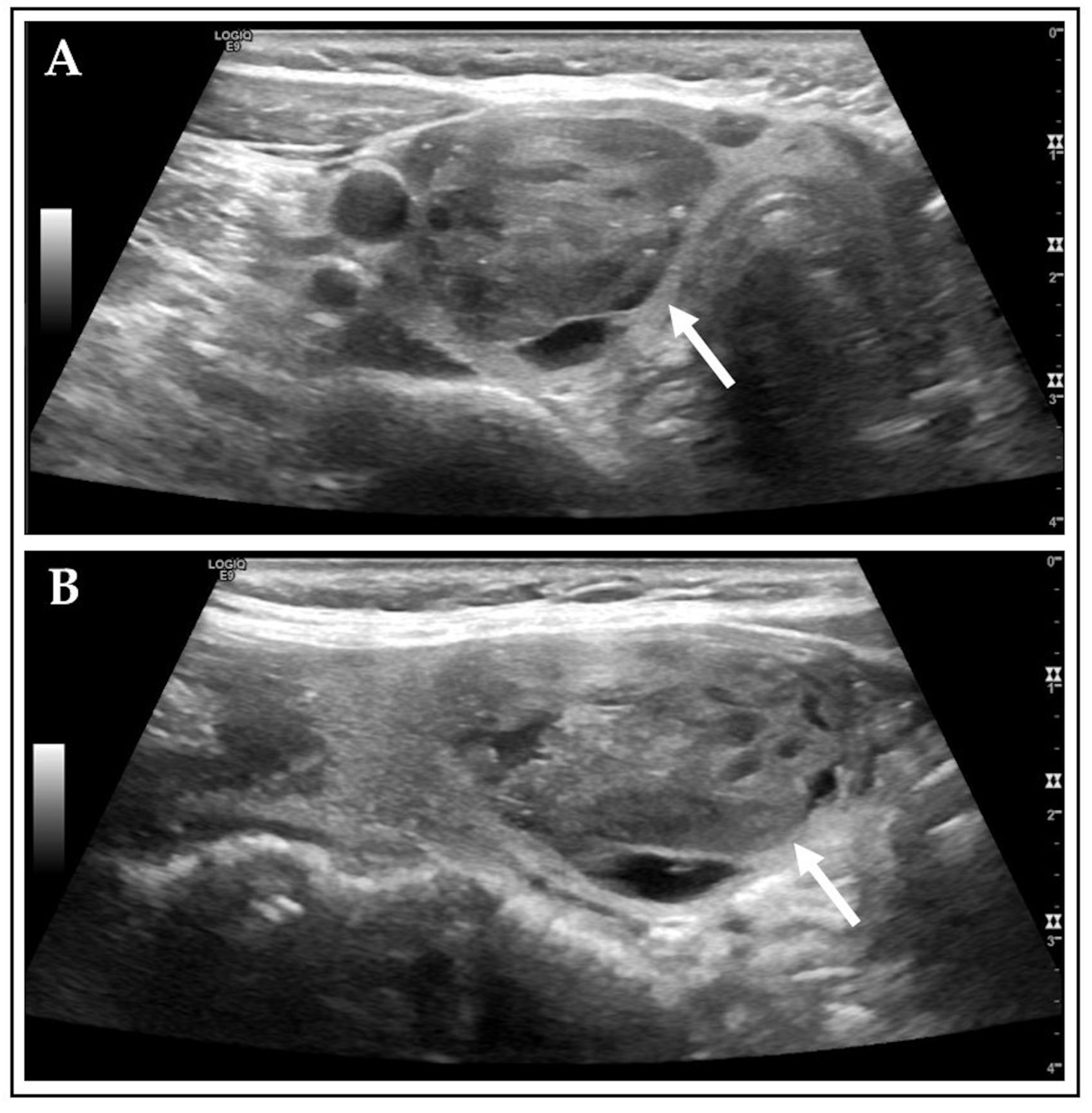
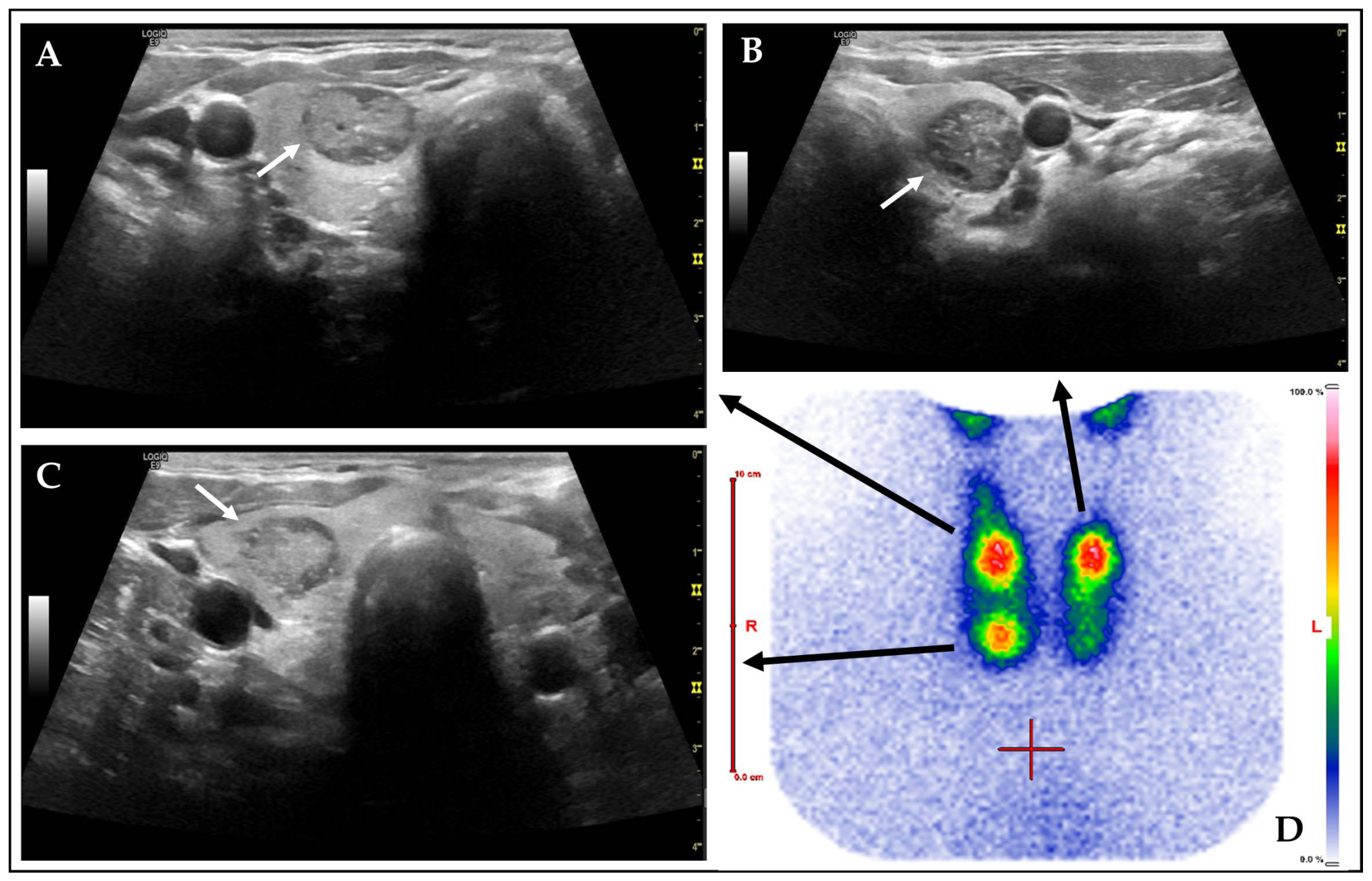
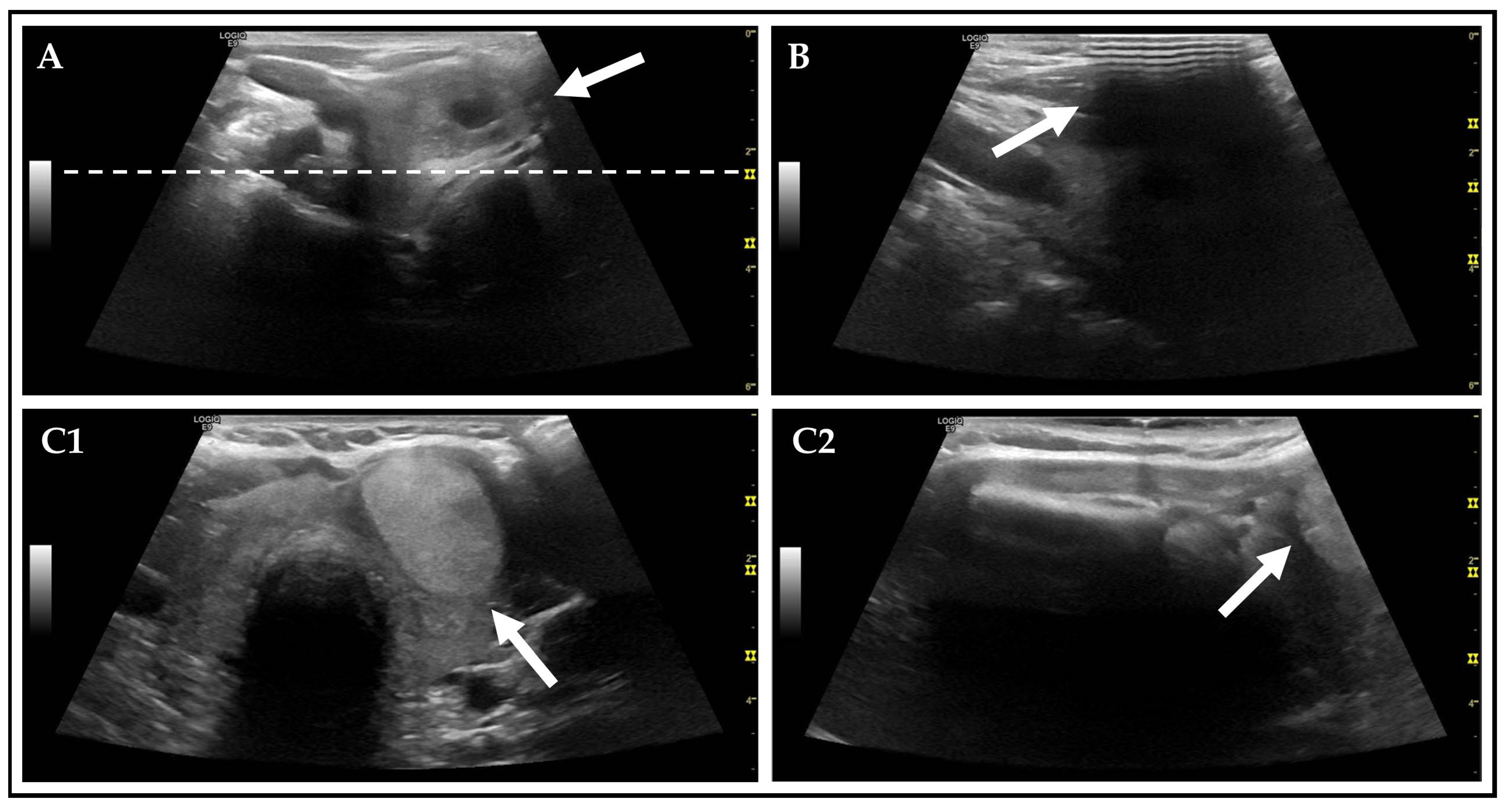
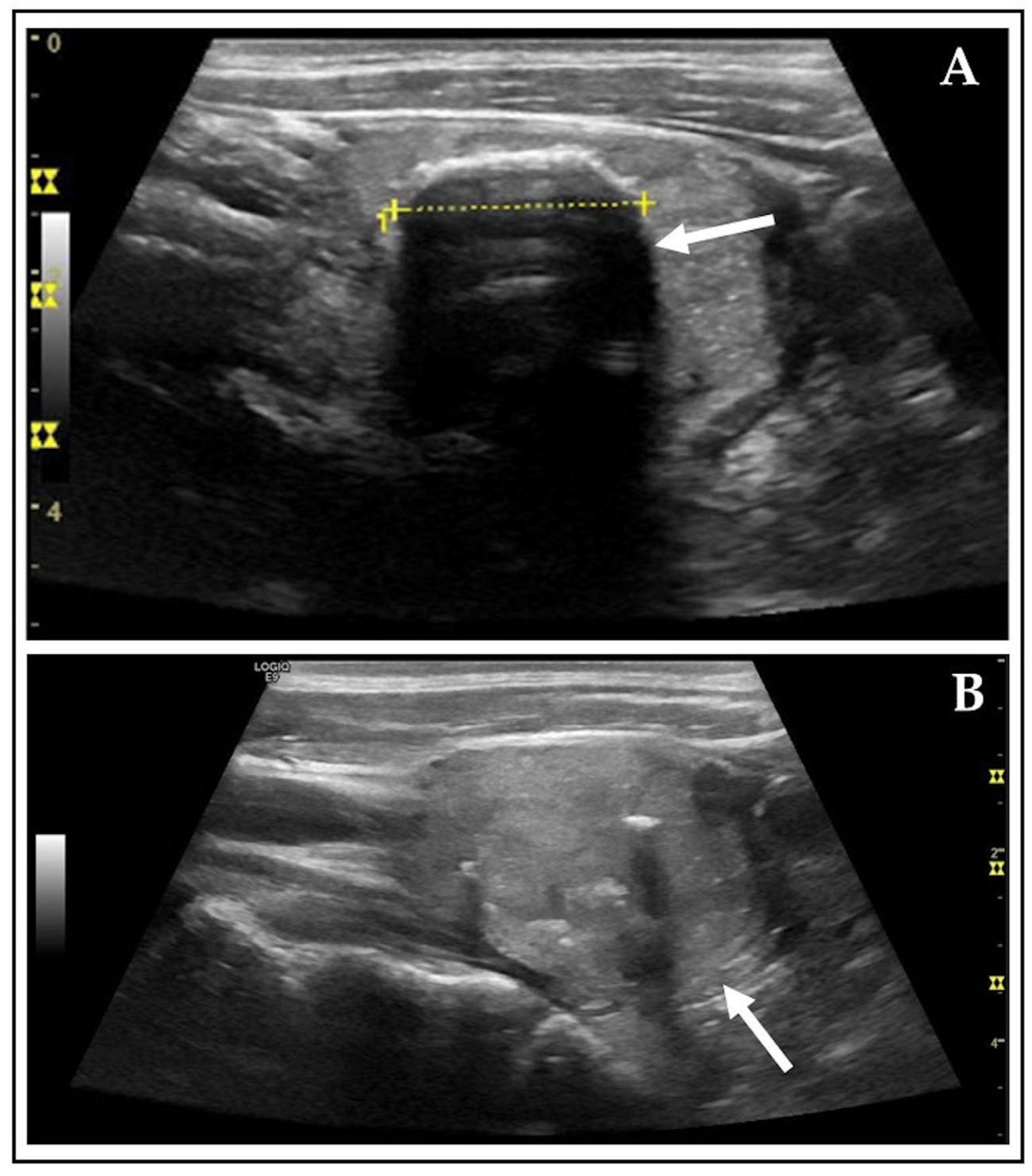
| Category | n (%)/Mean ± SD (Median, Range) | Unit |
|---|---|---|
| Patient’s demographics | ||
| Total number of patients | 407 (100.0) | |
| Female | 257 (63.1) | |
| Male | 150 (36.9) | |
| Age | 62 ± 11 (61, 21–86) | years |
| Thyroid function | ||
| TSH level | 0.2 ± 0.5 (0.1, 0.01–5.2) | mU/L |
| Euthyroid | 93 (22.9) | |
| Hyperthyroid | 313 (76.9) | |
| Hypothyroid | 1 (0.2) | |
| Thyroid medication | ||
| None | 179 (44.0) | |
| Thyreostatics | 89 (21.9) | |
| L-thyroxine permanently 1 | 10 (2.5) | |
| L-thyroxine premedication | 129 (31.7) | |
| Ultrasound | ||
| Thyroid volume | 31.5 ± 17.1 (27.0, 6.0–122.0) | mL |
| Number of identified AFTNs | 424 (100.0) | |
| Unifocal | 393 (96.6) | |
| Bifocal | 11(2.7) | |
| Trifocal | 3 (0.7) | |
| Largest diameter of AFTNs | ||
| CL | 30 ± 10 (29, 9–62) | mm |
| SIC | 30 ± 10 (30, 9–64) | mm |
| US Features | CL n (%) | SIC n (%) | Chi2 test 1 | Spearman’s Correlation 2 |
|---|---|---|---|---|
| Composition | ||||
| Cystic or almost completely cystic | 2 (0.5) | 0 (0.0) | p = 0.499 | rs = 0.722 p < 0.001 |
| Spongiform | 5 (1.2) | 2 (0.5) | p = 0.451 | |
| Mixed cystic and solid | 275 (64.9) | 270 (63.7) | p = 0.720 | |
| Solid or almost completely solid | 140 (33.0) | 148 (34.9) | p = 0.562 | |
| No assessment possible | 2 (0.5) | 4 (0.9) | p = 0.686 | |
| Echogenicity | ||||
| Anechoic | 0 (0.0) | 0 (0.0) | - | rs = 0.681 p < 0.001 |
| Hyperechoic or isoechoic | 209 (49.3) | 221 (52.1) | p = 0.451 | |
| Hypoechoic | 205 (48.3) | 200 (47.2) | p = 0.720 | |
| Very hypoechoic | 10 (2.4) | 3 (0.7) | p = 0.562 | |
| Shape | ||||
| Wider-than-tall | 362 (85.4) | 353 (83.3) | p = 0.395 | rs = 0.547 |
| Taller-than-wide | 62 (14.6) | 71 (16.7) | p = 0.395 | p < 0.001 |
| Margin | ||||
| Smooth | 389 (91.7) | 400 (94.3) | p = 0.138 | rs = 0.483 p < 0.001 |
| Ill-defined | 35 (8.3) | 24 (5.7) | p = 0.138 | |
| Lobulated or irregular | 0 (0.0) | 0 (0.0) | - | |
| Extra-thyroidal extension | 0 (0.0) | 0 (0.0) | - | |
| Echogenic Foci | ||||
| None | 52 (12.3) | 102 (24.1) | p < 0.001 | rs = 0.603 p < 0.001 |
| Large comet-tail artifacts | 350 (82.5) | 280 (66.0) | p < 0.001 | |
| Macrocalcifications | 145 (34.2) | 109 (25.7) | p = 0.007 | |
| Peripheral (rim) calcifications | 15 (3.5) | 11 (2.6) | p = 0.426 | |
| Punctate echogenic foci | 41 (9.7) | 33 (7.8) | p = 0.330 | |
| TIRADS | CL n (%) | SIC n (%) | Chi2 Test 1 | Spearman’s Correlation 2 |
|---|---|---|---|---|
| Kwak | ||||
| 3 | 115 (27.1) | 117 (27.6) | p = 0.844 | rs = 0.699 p < 0.001 |
| 4A | 178 (42.0) | 177 (41.7) | p = 0.991 | |
| 4B | 110 (25.9) | 102 (24.1) | p = 0.552 | |
| 4C | 19 (4.5) | 24 (5.7) | p = 0.424 | |
| 4C (3 pts.) | 19 (4.5) | 24 (5.7) | p = 0.424 | |
| 4C (4 pts.) | 0 (0.0) | 0 (0.0) | - | |
| 5 | 0(0.0) | 0 (0.0) | - | |
| No assessment possible 1 | 2 (0.5) | 4 (0.9) | p = 0.686 | |
| ACR | ||||
| TR1 | 1 (0.2) | 0 (0.0) | p = 0.999 | rs = 0.654 p < 0.001 |
| TR2 | 75 (17.7) | 90 (21.2) | p = 0.181 | |
| TR3 | 140 (33.0) | 137 (32.3) | p = 0.864 | |
| TR4 | 165 (38.9) | 150 (35.4) | p = 0.310 | |
| TR4 (4 pts.) | 78 (18.4) | 74 (17.5) | p = 0.769 | |
| TR4 (5 pts.) | 36 (8.5) | 33 (7.8) | p = 0.722 | |
| TR4 (6 pts.) | 51 (12.0) | 43 (10.1) | p = 0.395 | |
| TR5 | 41 (9.7) | 43 (10.1) | p = 0.800 | |
| No assessment possible 1 | 2 (0.5) | 4 (0.9) | p = 0.686 | |
| FNC ACR | ||||
| FNC not recommended | 85 (20.0) | 93 (21.9) | p = 0.500 | rs = 0.445 p < 0.001 |
| FNC recommended | 288 (67.9) | 271 (63.9) | p = 0.218 | |
| Follow up | 47 (11.1) | 49 (11.6) | p = 0.828 | |
| No assessment possible 3,4 | 4 (0.9) | 11 (2.6) | p = 0.115 | |
| Type of Artifacts | CL n (%) | SIC n (%) | Chi2 Test 1 |
|---|---|---|---|
| Transverse | |||
| None | 263 (62.0) | 360 (84.9) | p < 0.001 |
| Poor image quality | 116 (27.4) | 24 (5.7) | p < 0.001 |
| Acoustic shadowing due to… | 64 (15.1) | 41 (9.7) | p = 0.016 |
| macrocalcifications | 44 (10.4) | 37 (8.7) | p = 0.413 |
| peripheral (rim) calcifications | 2 (0.5) | 3 (0.7) | p = 0.999 |
| other reasons 2 | 18 (4.2) | 1 (0.2) | p < 0.001 |
| AFTNs incompletely displayed | 3 (0.7) | 2 (0.5) | p = 0.999 |
| Sagittal | |||
| None | 247 (58.3) | 357 (84.2) | p < 0.001 |
| Poor image quality | 123 (29.0) | 21 (5.0) | p < 0.001 |
| Acoustic shadowing due to… | 77 (18.2) | 49 (11.6) | p = 0.007 |
| macrocalcifications | 49 (11.6) | 43 (10.1) | p = 0.508 |
| peripheral (rim) calcifications | 3 (0.7) | 3 (0.7) | p = 0.999 |
| other reasons 2 | 25 (5.9) | 3 (0.7) | p < 0.001 |
| AFTNs incompletely displayed | 73 (17.2) | 5 (1.2) | p < 0.001 |
| Isthmus | 12 (2.8) | 1 (0.2) | p = 0.003 |
| Upper pole | 3 (0.7) | 0 (0.0) | p = 0.249 |
| Lower pole | 39 (9.2) | 3 (0.7) | p < 0.001 |
| Central | 19 (4.5) | 1 (0.2) | p < 0.001 |
| Result | CL n (%) | SIC n (%) | Chi2 Test 1 | Spearman’s Correlation 2 |
|---|---|---|---|---|
| Assessment confidence | ||||
| 1 (Very confident) | 168 (39.6) | 303 (71.5) | p < 0.001 | rs = 0.186 p < 0.001 |
| 2 (Confident) | 184 (43.4) | 107 (25.2) | p < 0.001 | |
| 3 (Unsure) | 65 (15.3) | 14 (3.3) | p < 0.001 | |
| 4 (Very unsure) | 7 (1.7) | 0 (0.0) | p = 0.015 | |
| US feature with most significant uncertainty | ||||
| None | 168 (39.6) | 303 (71.5) | p < 0.001 | rs = 0.133 p = 0.006 |
| Composition | 77 (18.2) | 54 (12.7) | p = 0.029 | |
| Echogenicity | 20 (4.7) | 12 (2.8) | p = 0.149 | |
| Shape | 12 (2.8) | 7 (1.7) | p = 0.246 | |
| Margin | 32 (7.5) | 2 (0.5) | p < 0.001 | |
| Echogenic Foci | 115 (27.1) | 46 (10.8) | p < 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenbaum, L.; Freesmeyer, M.; Schmidt, T.N.; Kühnel, C.; Gühne, F.; Seifert, P. Ultrasound Risk Stratification of Autonomously Functioning Thyroid Nodules: Cine Loop Video Sequences Versus Static Image Captures. Diagnostics 2025, 15, 2525. https://doi.org/10.3390/diagnostics15192525
Rosenbaum L, Freesmeyer M, Schmidt TN, Kühnel C, Gühne F, Seifert P. Ultrasound Risk Stratification of Autonomously Functioning Thyroid Nodules: Cine Loop Video Sequences Versus Static Image Captures. Diagnostics. 2025; 15(19):2525. https://doi.org/10.3390/diagnostics15192525
Chicago/Turabian StyleRosenbaum, Larissa, Martin Freesmeyer, Tabea Nikola Schmidt, Christian Kühnel, Falk Gühne, and Philipp Seifert. 2025. "Ultrasound Risk Stratification of Autonomously Functioning Thyroid Nodules: Cine Loop Video Sequences Versus Static Image Captures" Diagnostics 15, no. 19: 2525. https://doi.org/10.3390/diagnostics15192525
APA StyleRosenbaum, L., Freesmeyer, M., Schmidt, T. N., Kühnel, C., Gühne, F., & Seifert, P. (2025). Ultrasound Risk Stratification of Autonomously Functioning Thyroid Nodules: Cine Loop Video Sequences Versus Static Image Captures. Diagnostics, 15(19), 2525. https://doi.org/10.3390/diagnostics15192525






