Emerging Radioligands as Tools to Track Multi-Organ Senescence
Abstract
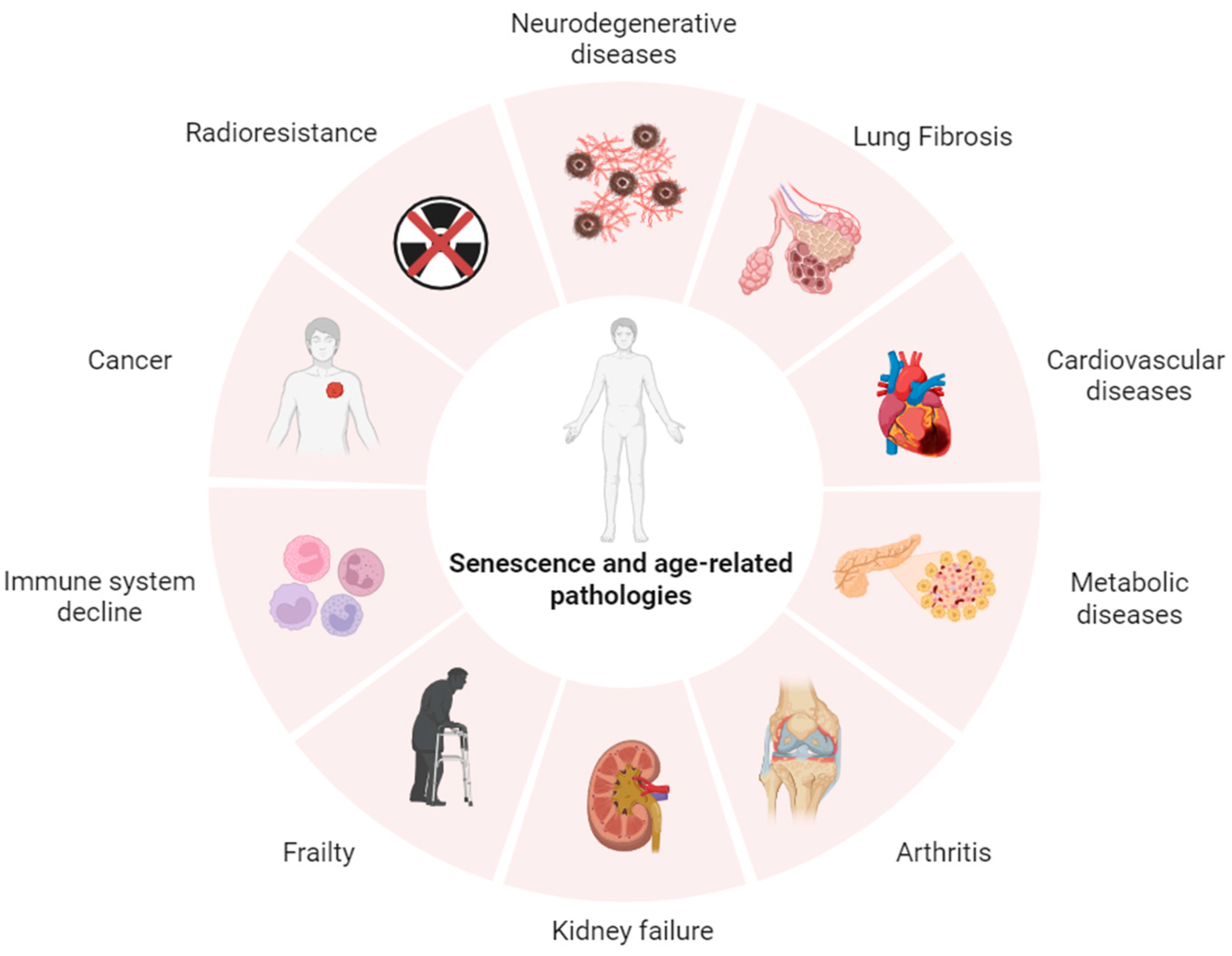
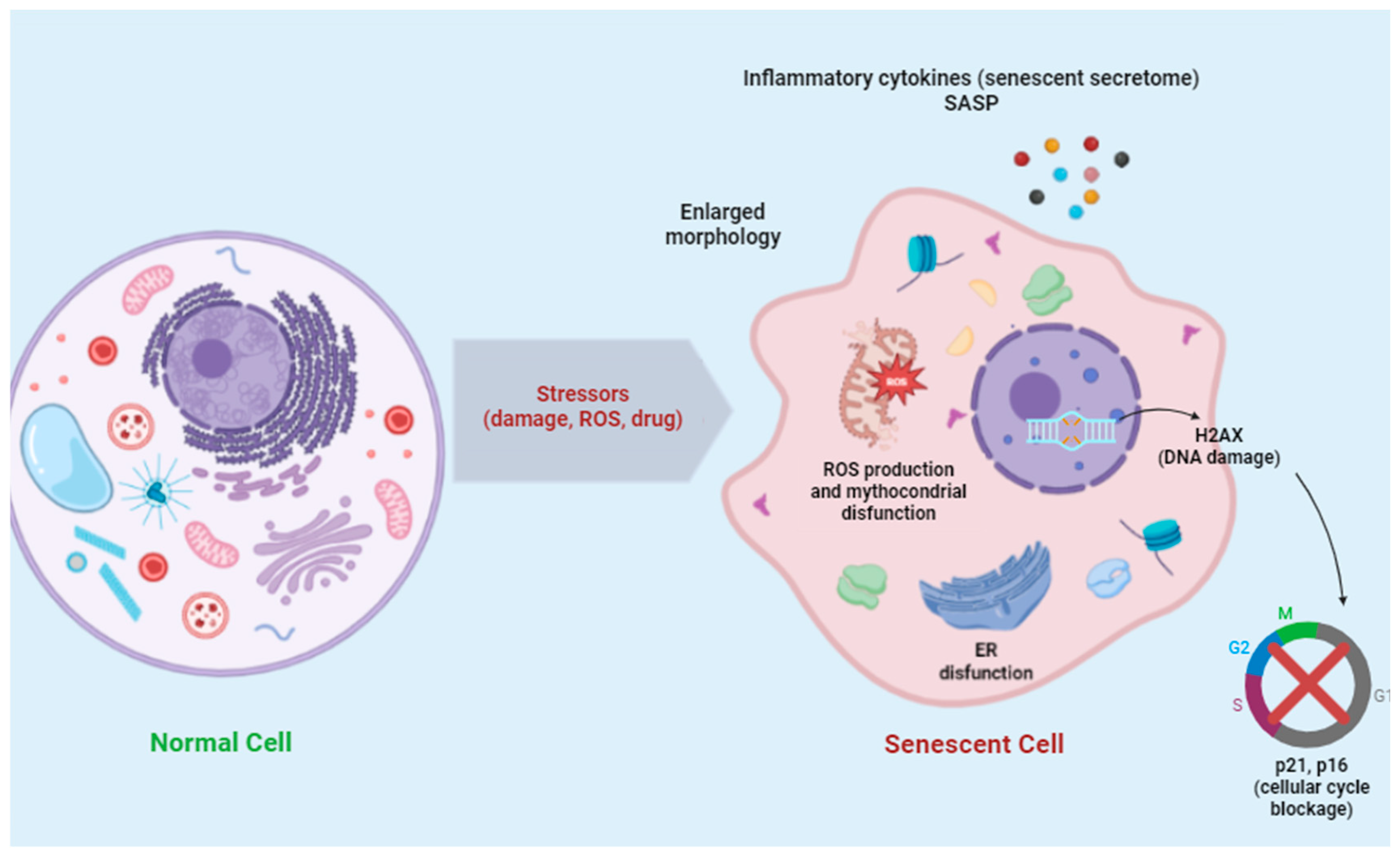
1. Introduction
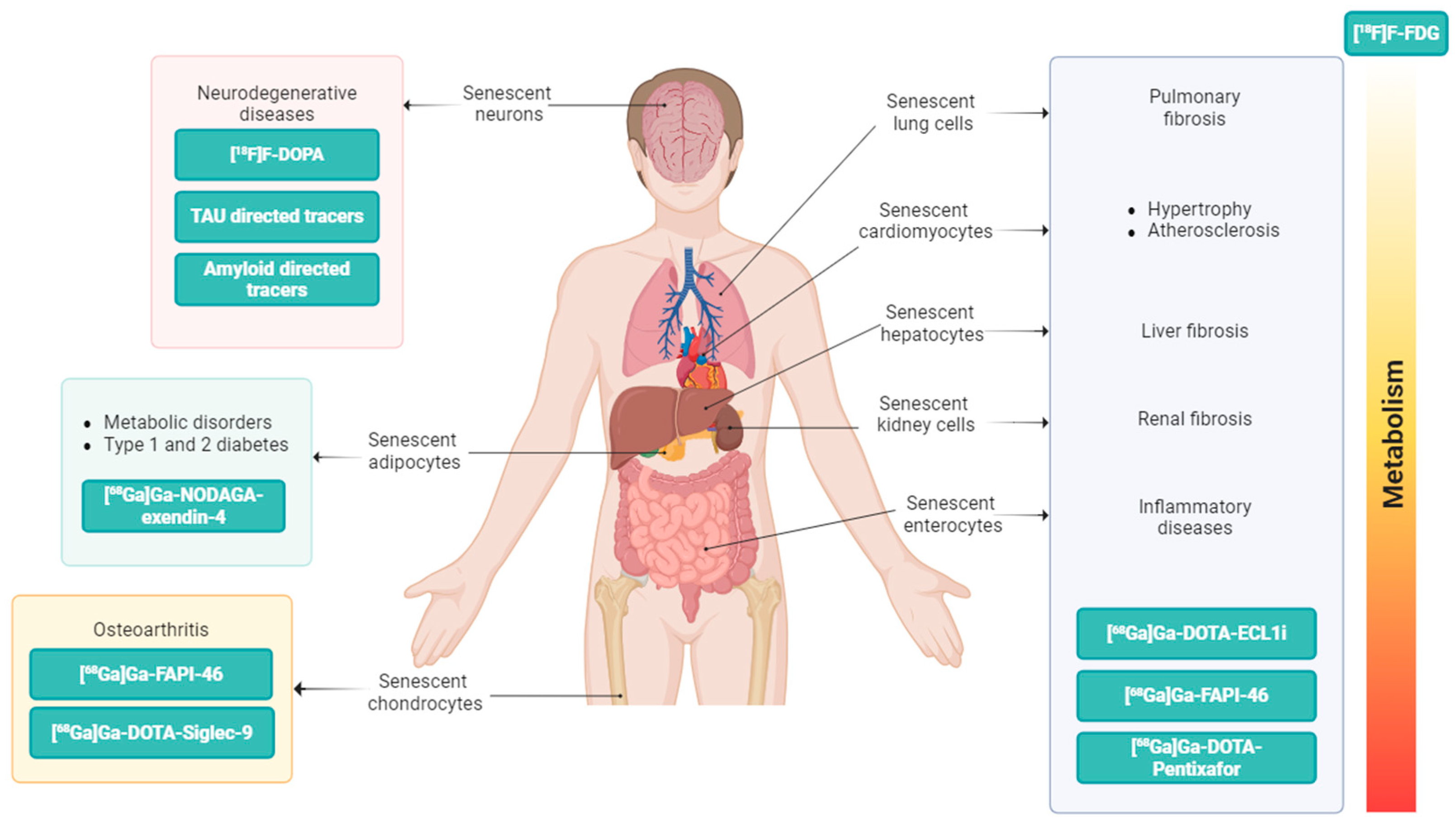
2. The PET Imaging Approach for In Vivo Detection of Senescence Processes
2.1. PET Tracers Targeting Lysosomal β-Galactosidase (β-Gal)
2.2. PET Tracers Targeting Lipofuscin
2.3. PET Tracers Targeting Chemokines
2.4. PET Tracers Targeting Siglecs Family
2.5. PET Tracers Targeting GLP-1 Receptor
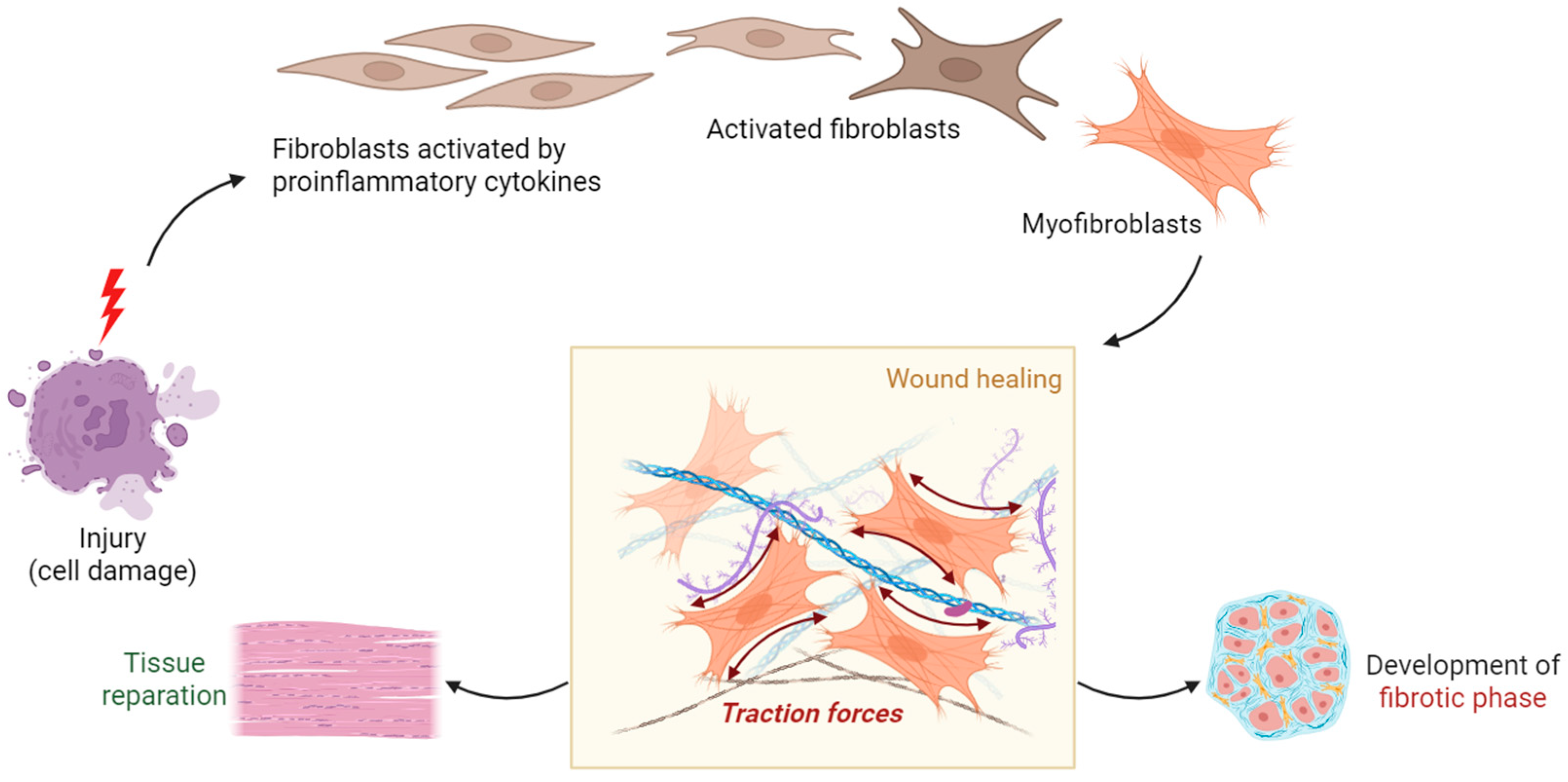
2.6. PET Tracers Targeting Fibroblast Activation Protein
| PET Radioligand | Molecular Target and Biological Pathways | Target Organs and/or Main Pathologies | Stage of Development | Refs. |
|---|---|---|---|---|
| [18F]FDG | GLUTs (Glucose metabolism) | Multi-organ | Clinically established/Multicenter trials | [47,48,49,50,51,52,53,54,55,56,57] |
| [18F]F-PyGal | β-Gal (DNA damage and oxidative stress) | Osteoarthritis Oncopathologies | Early human feasibility | [64,65] |
| [68Ga]Ga-βGal | ||||
| [18F]F-FET-SBB | Lipofuscin (Lysosomal degradation of damaged mitochondria) | Oncopathologies | Preclinical only | [71] |
| [68Ga]Ga-DOTA- ECL1i | CCR2 (CCL2/CCR2 axis) | Lung, Liver, Heart, Kidney | Preclinical only | [83,84,85] |
| [68Ga]Ga-DOTA- Pentixafor | CXCR4 (CXCL12/CXCR4 axis) | Brain, Lung, Liver, Heart, Kidney | Clinically established/Multicenter trials | [95,96,97] |
| [68Ga]Ga-DOTA- Siglec-9 | VAP-1 (Immune response and evasion) | Rheumatoid Arthritis, Oncopathologies | Early human feasibility | [109,110,111,112,113] |
| [18F]F-FDR-Siglec-9 | Preclinical only | |||
| [68Ga]Ga-NODAGA- Exendin-4 | GLP-1R (Intracellular pathways) | Brain, Heart, Pancreas | Multicenter trials | [143,144,145,146,147,148] |
| [68Ga]Ga-FAPI-04 | FAP (Fibroblast activity) | Lung, Liver, Heart, Kidney | Clinically established/ Multicenter trials | [166,167,168,169,170,171,172,173,174,175,176,177,178,179] |
| [68Ga]Ga-FAPI-46 |
| PET Radioligand | Specificity | Sensitivity/ Affinity | Detection Limit | Biodistribution Variability | Refs. |
|---|---|---|---|---|---|
| [18F]FDG | Low-moderate (ubiquitous glucose uptake) | High (docking affinity for HXK −14.11 kcal/mol; for GLUT1 −10.37 kcal/mol) | 0.3 MBq/kg | High inter-organ uptake (brain, heart, BAT, tumors) | [47,48,49,50,51,52,53,54,55,56,57] |
| [18F]F-PyGal | High | Moderate–high (docking affinity NS) | 3.7 MBq/mouse | Mainly osteoarthritic joints, oncopathologies; limited off-target uptake | [64,65] |
| [68Ga]Ga-βGal | |||||
| [18F]F-FET-SBB | Moderate | High PET sensitivity (docking affinity NS) | NS | High liver accumulation; needs further animal validation | [71] |
| [68Ga]Ga-DOTA- ECL1i | High | High for monocyte/macrophage recruitment (docking affinity −72.3 kJ·mol−1) | 12 MBq/mouse | Lung, liver, heart, kidney | [83,84,85] |
| [68Ga]Ga-DOTA- Pentixafor | High | High (binding affinity ~24.8 ±2.5 nM) | 1.4 to 5.0 MBq/kg | Broad Rapid renal clearance Low non-specific background uptake | [95,96,97] |
| [68Ga]Ga-DOTA- Siglec-9 | Moderate–high | High regional uptake at the site of inflamed joints (docking affinity NS) | 150 MBq | Rapid blood clearance and renal excretion | [109,110,111,112,113] |
| [18F]F-FDR-Siglec-9 | |||||
| [68Ga]Ga-NODAGA- Exendin-4 | High | High (binding affinity 36.0 nM) | 105.6 ± 2.3 MBq; peptide dose, 4–7 μg | Rapid blood clearance | [143,144,145,146,147,148] |
| [68Ga]Ga-FAPI-04 | High | High (binding affinity 0.04 nM) | 1.85–3.7 MBq/kg | Persistent uptake in fibrosis areas | [166,167,168,169,170,171,172,173,174,175,176,177,178,179] |
| [68Ga]Ga-FAPI-46 |
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayflick, L. The Limited In Vitro Lifetime Of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yousefzadeh, M.J.; Flores, R.R.; Zhu, Y.; Schmiechen, Z.C.; Brooks, R.W.; Trussoni, C.E.; Cui, Y.; Angelini, L.; Lee, K.A.; McGowan, S.J.; et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021, 594, 100–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez-Meljem, J.M.; Apps, J.R.; Fraser, H.C.; Martinez-Barbera, J.P. Paracrine roles of cellular senescence in promoting tumourigenesis. Br. J. Cancer 2018, 118, 1283–1288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; van Deursen, J.M.; Baker, D.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Uryga, A.K.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 2015, 132, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Mazzucato, C.; Andle, J.; Lee, T.B., Jr.; Midha, A.; Talemal, L.; Chipashvili, V.; Hollister-Lock, J.; van Deursen, J.; Weir, G.; Bonner-Weir, S. Acceleration of β Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell Metab. 2019, 30, 129–142.e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Livingston, M.J.; Ma, Z.; Hu, X.; Wen, L.; Ding, H.F.; Zhou, D.; Dong, Z. Tubular cell senescence promotes maladaptive kidney repair and chronic kidney disease after cisplatin nephrotoxicity. JCI Insight 2023, 8, e166643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcozzi, S.; Bigossi, G.; Giuliani, M.E.; Giacconi, R.; Piacenza, F.; Cardelli, M.; Brunetti, D.; Segala, A.; Valerio, A.; Nisoli, E.; et al. Cellular senescence and frailty: A comprehensive insight into the causal links. Geroscience 2023, 45, 3267–3305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoetz, U.; Klein, D.; Hess, J.; Shnayien, S.; Spoerl, S.; Orth, M.; Mutlu, S.; Hennel, R.; Sieber, A.; Ganswindt, U.; et al. Early senescence and production of senescence-associated cytokines are major determinants of radioresistance in head-and-neck squamous cell carcinoma. Cell Death Dis. 2021, 12, 1162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, J.; Li, Y.; Wan, C.; Sun, Y.; Dai, X.; Huang, J.; Hu, Y.; Gao, Y.; Wu, B.; Zhang, Z.; et al. Targeting senescence-like fibroblasts radiosensitizes non-small cell lung cancer and reduces radiation-induced pulmonary fibrosis. JCI Insight 2021, 6, e146334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; El Jurdi, N.; Thyagarajan, B.; Prizment, A.; Blaes, A.H. Accelerated Aging in Cancer Survivors: Cellular Senescence, Frailty, and Possible Opportunities for Interventions. Int. J. Mol. Sci. 2024, 25, 3319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Cué, C.; Rueda, N. Cellular Senescence in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohanna, M.; Giuliano, S.; Bonet, C.; Imbert, V.; Hofman, V.; Zangari, J.; Bille, K.; Robert, C.; Bressac-de Paillerets, B.; Hofman, P.; et al. Senescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS). Genes Dev. 2011, 25, 1245–1261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsumura, T.; Zerrudo, Z.; Hayflick, L. Senescent human diploid cells in culture: Survival, DNA synthesis and morphology. J. Gerontol. 1979, 34, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Hong, S.M.; Lee, Y.K.; Min, S.; Yoon, G. Metabolic features and regulation in cell senescence. BMB Rep. 2019, 52, 5–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000, 113 Pt 20, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.F.; Saretzki, G.; von Zglinicki, T. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007, 35, 7505–7513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, M.H.; Ploegh, H.L.; Weissman, J.S. Road to ruin: Targeting proteins for degradation in the endoplasmic reticulum. Science 2011, 334, 1086–1090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruggiano, A.; Foresti, O.; Carvalho, P. Quality control: ER-associated degradation: Protein quality control and beyond. J. Cell Biol. 2014, 204, 869–879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pluquet, O.; Pourtier, A.; Abbadie, C. The unfolded protein response and cellular senescence. A review in the theme: Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am. J. Physiol. Cell Physiol. 2015, 308, C415–C425. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Chevet, E.; Oakes, S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Kaufman, R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005, 74, 739–789. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- James, E.L.; Michalek, R.D.; Pitiyage, G.N.; de Castro, A.M.; Vignola, K.S.; Jones, J.; Mohney, R.P.; Karoly, E.D.; Prime, S.S.; Parkinson, E.K. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J. Proteome Res. 2015, 14, 1854–1871. [Google Scholar] [CrossRef] [PubMed]
- Flor, A.C.; Wolfgeher, D.; Wu, D.; Kron, S.J. A signature of enhanced lipid metabolism, lipid peroxidation and aldehyde stress in therapy-induced senescence. Cell Death Discov. 2017, 3, 17075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiley, C.D.; Flynn, J.M.; Morrissey, C.; Lebofsky, R.; Shuga, J.; Dong, X.; Unger, M.A.; Vijg, J.; Melov, S.; Campisi, J. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 2017, 16, 1043–1050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogrodnik, M.; Carlos Acosta, J.; Adams, P.D.; d’Adda di Fagagna, F.; Baker, D.J.; Bishop, C.L.; Chandra, T.; Collado, M.; Gil, J.; Gorgoulis, V.; et al. Guidelines for minimal information on cellular senescence experimentation in vivo. Cell 2024, 187, 4150–4175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zwerschke, W.; Mazurek, S.; Stöckl, P.; Hütter, E.; Eigenbrodt, E.; Jansen-Dürr, P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem. J. 2003, 376 Pt 2, 403–411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiley, C.D.; Campisi, J. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab. 2016, 23, 1013–1021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Behmoaras, J.; Gil, J. Similarities and interplay between senescent cells and macrophages. J. Cell Biol. 2021, 220, e202010162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, G.D.; Yang, J.; Li, X.X.; Song, X.Y.; Hayashi, T.; Tashiro, S.I.; Onodera, S.; Song, S.J.; Ikejima, T. Blocking the utilization of glucose induces the switch from senescence to apoptosis in pseudolaric acid B-treated human lung cancer cells in vitro. Acta Pharmacol. Sin. 2017, 38, 1401–1411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, S.J.; Moon, J.S.; Lee, C.M.; Choi, A.M.; Stout-Delgado, H.W. Glucose Transporter 1-Dependent Glycolysis Is Increased during Aging-Related Lung Fibrosis, and Phloretin Inhibits Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 521–531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Subtirelu, R.C.; Teichner, E.M.; Su, Y.; Al-Daoud, O.; Patel, M.; Patil, S.; Writer, M.; Werner, T.; Revheim, M.E.; Høilund-Carlsen, P.F.; et al. Aging and Cerebral Glucose Metabolism: 18F-FDG-PET/CT Reveals Distinct Global and Regional Metabolic Changes in Healthy Patients. Life 2023, 13, 2044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishibashi, K.; Onishi, A.; Fujiwara, Y.; Oda, K.; Ishiwata, K.; Ishii, K. Longitudinal effects of aging on 18F-FDG distribution in cognitively normal elderly individuals. Sci. Rep. 2018, 8, 11557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, C.M.; Bang, J.I.; Lee, S.Y.; Lee, J.K.; Chai, J.W.; Oh, S.W. An Analysis of Age-Related Body Composition Changes and Metabolic Patterns in Korean Adults Using FDG-PET/CT Health Screening Data. Diabetes Metab. J. 2025, 49, 92–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berbée, J.F.; Boon, M.R.; Khedoe, P.P.; Bartelt, A.; Schlein, C.; Worthmann, A.; Kooijman, S.; Hoeke, G.; Mol, I.M.; John, C.; et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015, 6, 6356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koksharova, E.; Ustyuzhanin, D.; Philippov, Y.; Mayorov, A.; Shestakova, M.; Shariya, M.; Ternovoy, S.; Dedov, I. The Relationship Between Brown Adipose Tissue Content in Supraclavicular Fat Depots and Insulin Sensitivity in Patients with Type 2 Diabetes Mellitus and Prediabetes. Diabetes Technol. Ther. 2017, 19, 96–102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohade, C.; Osman, M.; Pannu, H.K.; Wahl, R.L. Uptake in supraclavicular area fat (“USA-Fat”): Description on 18F-FDG PET/CT. J. Nucl. Med. 2003, 44, 170–176. [Google Scholar] [PubMed]
- Carpentier, A.C. Tracers and Imaging of Fatty Acid and Energy Metabolism of Human Adipose Tissues. Physiology 2024, 39, 61–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cypess, A.M.; Haft, C.R.; Laughlin, M.R.; Hu, H.H. Brown fat in humans: Consensus points and experimental guidelines. Cell Metab. 2014, 20, 408–415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devesa, A.; Fuster, V.; Vazirani, R.; García-Lunar, I.; Oliva, B.; España, S.; Moreno-Arciniegas, A.; Sanz, J.; Perez-Herreras, C.; Bueno, H.; et al. Cardiac Insulin Resistance in Subjects with Metabolic Syndrome Traits and Early Subclinical Atherosclerosis. Diabetes Care 2023, 46, 2050–2057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruffini, L.; Zilioli, A.; Cervati, V.; Lauretani, F.; Misirocchi, F.; Maggio, M.; Migliari, S.; Graziani, T.; Cidda, C.; Baldari, G.; et al. PET and SPECT imaging as a solid guide to detect and discriminate atypical phenotypes of neurodegenerative disorders. Eur. J. Clin. Exp. Med. 2024, 22, 201–221. [Google Scholar] [CrossRef]
- Tiepolt, S.; Patt, M.; Aghakhanyan, G.; Meyer, P.M.; Hesse, S.; Barthel, H.; Sabri, O. Current radiotracers to image neurodegenerative diseases. EJNMMI Radiopharm. Chem. 2019, 4, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni, R.; Nitsch, R.M. Recent Developments in Positron Emission Tomography Tracers for Proteinopathies Imaging in Dementia. Front. Aging Neurosci. 2022, 13, 751897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reed, M.B.; Murgaš, M.; Lanzenberger, R.; Hahn, A. The Value of Functional PET in Quantifying Neurotransmitter Dynamics. J. Nucl. Med. 2025, 66, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Manca, R.; De Marco, M.; Soininen, H.; Ruffini, L.; Venneri, A. Changes in neurotransmitter-related functional connectivity along the Alzheimer’s disease continuum. Brain Commun. 2025, 7, fcaf008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, N.; Choi, J.Y.; Ryu, Y.H. The development status of PET radiotracers for evaluating neuroinflammation. Nucl. Med. Mol. Imaging 2024, 58, 160–176, Erratum in Nucl. Med. Mol. Imaging 2025, 59, 92. https://doi.org/10.1007/s13139-024-00880-3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Kuca, K.; You, L.; Nepovimova, E.; Heger, Z.; Valko, M.; Adam, V.; Wu, Q.; Jomova, K. The role of cellular senescence in neurodegenerative diseases. Arch. Toxicol. 2024, 98, 2393–2408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lauretani, F.; Ruffini, L.; Ticinesi, A.; Nouvenne, A.; Maggio, M.; Meschi, T. Accuracy of Quantitative Positron Emission Tomography Assessment for Differentiating Cerebral Age-related from Pathological Amyloid Deposition: A Preliminary Report from a Case-series Study. World J. Nucl. Med. 2018, 17, 106–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riessland, M.; Ximerakis, M.; Jarjour, A.A.; Zhang, B.; Orr, M.E. Therapeutic targeting of senescent cells in the CNS. Nat. Rev. Drug Discov. 2024, 23, 817–837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in ageing skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, Z.M.; Cui, L. Detecting cellular senescence in vivo: Imagining imaging better. Aging Cancer 2023, 4, 97–110. [Google Scholar] [CrossRef]
- Xiang, X.; Dong, C.; Zhou, L.; Liu, J.; Rabinowitz, Z.M.; Zhang, Y.; Guo, H.; He, F.; Chen, X.; Wang, Y.; et al. Novel PET Imaging Probe for Quantitative Detection of Senescence In Vivo. J. Med. Chem. 2024, 67, 5924–5934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suryadevara, V.; Baratto, L.; von Kruechten, R.; Malik, N.; Singh, S.B.; Dreisbach, A.M.; Shokri Varniab, Z.; Tanyildizi, Y.; Liang, T.; Cotton, J.; et al. [18F]FPyGal PET TRACER DETECTS SENESCENCE IN HUMAN OSTEOARTHRITIC SPECIMENS. Osteoarthr. Imaging 2024, 4 (Suppl. 1), 100205. [Google Scholar] [CrossRef]
- Kakimoto, Y.; Okada, C.; Kawabe, N.; Sasaki, A.; Tsukamoto, H.; Nagao, R.; Osawa, M. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci. Rep. 2019, 9, 3304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gray, D.A.; Woulfe, J. Lipofuscin and aging: A matter of toxic waste. Sci. Aging Knowl. Environ. 2005, 2005, re1. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, E.A.; Tsimaratou, K.; Evangelou, K.; Fernandez Marcos, P.J.; Zoumpourlis, V.; Trougakos, I.P.; Kletsas, D.; Bartek, J.; Serrano, M.; Gorgoulis, V.G. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging 2013, 5, 37–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nyström, T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Muñoz-Espín, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brickute, D.; Chen, C.; Braga, M.; Barnes, C.; Wang, N.; Allott, L.; Aboagye, E.O. Design, synthesis, and evaluation of a novel PET imaging agent targeting lipofuscin in senescent cells. RSC Adv. 2022, 12, 26372–26381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yasuda, T.; Koiwa, M.; Yonemura, A.; Miyake, K.; Kariya, R.; Kubota, S.; Yokomizo-Nakano, T.; Yasuda-Yoshihara, N.; Uchihara, T.; Itoyama, R.; et al. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell Rep. 2021, 34, 108779. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Schafer, M.J.; Noren Hooten, N.; Atkinson, E.J.; Evans, M.K.; Baker, D.J.; Quarles, E.K.; Robbins, P.D.; Ladiges, W.C.; LeBrasseur, N.K.; et al. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell 2018, 17, e12706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martini, H.; Iacovoni, J.S.; Maggiorani, D.; Dutaur, M.; Marsal, D.J.; Roncalli, J.; Itier, R.; Dambrin, C.; Pizzinat, N.; Mialet-Perez, J.; et al. Aging induces cardiac mesenchymal stromal cell senescence and promotes endothelial cell fate of the CD90 + subset. Aging Cell 2019, 18, e13015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lefèvre, L.; Iacovoni, J.S.; Martini, H.; Bellière, J.; Maggiorani, D.; Dutaur, M.; Marsal, D.J.; Decaunes, P.; Pizzinat, N.; Mialet-Perez, J.; et al. Kidney inflammaging is promoted by CCR2+ macrophages and tissue-derived micro-environmental factors. Cell. Mol. Life Sci. 2021, 78, 3485–3501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Yang, K.; Chen, F.; Liu, Q.; Ni, J.; Cao, W.; Hua, Y.; He, F.; Liu, Z.; Li, L.; et al. Role of the CCL2-CCR2 axis in cardiovascular disease: Pathogenesis and clinical implications. Front. Immunol. 2022, 13, 975367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luciano-Mateo, F.; Cabré, N.; Baiges-Gaya, G.; Fernández-Arroyo, S.; Hernández-Aguilera, A.; Elisabet Rodríguez-Tomàs, E.; Arenas, M.; Camps, J.; Menéndez, J.A.; Joven, J. Systemic overexpression of C-C motif chemokine ligand 2 promotes metabolic dysregulation and premature death in mice with accelerated aging. Aging 2020, 12, 20001–20023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stahl, E.C.; Delgado, E.R.; Alencastro, F.; LoPresti, S.T.; Wilkinson, P.D.; Roy, N.; Haschak, M.J.; Skillen, C.D.; Monga, S.P.; Duncan, A.W.; et al. Inflammation and Ectopic Fat Deposition in the Aging Murine Liver Is Influenced by CCR2. Am. J. Pathol. 2020, 190, 372–387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, A.; Krappe, J.; Mühe, A.M.; Lindenmeyer, M.T.; Eltrich, N.; Luckow, B.; Vielhauer, V. C-C chemokine receptor type 2 mediates glomerular injury and interstitial fibrosis in focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 2020, 35, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Migliari, S.; Scarlattei, M.; Baldari, G.; Ruffini, L. Scale down and optimized automated production of [68Ga]68Ga-DOTA-ECL1i PET tracer targeting CCR2 expression. EJNMMI Radiopharm. Chem. 2023, 8, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Li, W.; Luehmann, H.P.; Zhao, Y.; Detering, L.; Sultan, D.H.; Hsiao, H.M.; Krupnick, A.S.; Gelman, A.E.; Combadiere, C.; et al. Noninvasive Imaging of CCR2+ Cells in Ischemia-Reperfusion Injury After Lung Transplantation. Am. J. Transplant. 2016, 16, 3016–3023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heo, G.S.; Kopecky, B.; Sultan, D.; Ou, M.; Feng, G.; Bajpai, G.; Zhang, X.; Luehmann, H.; Detering, L.; Su, Y.; et al. Molecular Imaging Visualizes Recruitment of Inflammatory Monocytes and Macrophages to the Injured Heart. Circ. Res. 2019, 124, 881–890. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lavine, K.J.; Sultan, D.; Luehmann, H.; Detering, L.; Zhang, X.; Heo, G.S.; Zhang, X.; Hoelscher, M.; Harrison, K.; Combadière, C.; et al. CCR2 Imaging in Human ST-Segment Elevation Myocardial Infarction. Nat. Cardiovasc. Res. 2023, 2, 874–880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yap, M.L.; Peter, K. Molecular Positron Emission Tomography in Cardiac Ischemia/Reperfusion. Circ. Res. 2019, 124, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Sammartano, A.; Migliari, S.; Scarlattei, M.; Baldari, G.; Ruffini, L. Synthesis, validation and quality controls of [68Ga]-DOTA-Pentixafor for PET imaging of chemokine receptor CXCR4 expression. Acta Biomed. 2020, 91, e2020097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wagner, P.L.; Hyjek, E.; Vazquez, M.F.; Meherally, D.; Liu, Y.F.; Chadwick, P.A.; Rengifo, T.; Sica, G.L.; Port, J.L.; Lee, P.C.; et al. CXCL12 and CXCR4 in adenocarcinoma of the lung: Association with metastasis and survival. J. Thorac. Cardiovasc. Surg. 2009, 137, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Gundavarapu, S.; Mishra, N.C.; Singh, S.P.; Langley, R.J.; Saeed, A.I.; Feghali-Bostwick, C.A.; McIntosh, J.M.; Hutt, J.; Hegde, R.; Buch, S.; et al. HIV gp120 induces mucus formation in human bronchial epithelial cells through CXCR4/α7-nicotinic acetylcholine receptors. PLoS One 2013, 8, e77160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mannelli, F.; Cutini, I.; Gianfaldoni, G.; Bencini, S.; Scappini, B.; Pancani, F.; Ponziani, V.; Bonetti, M.I.; Biagiotti, C.; Longo, G.; et al. CXCR4 expression accounts for clinical phenotype and outcome in acute myeloid leukemia. Cytometry B Clin. Cytom. 2014, 86, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Lee, Y.; Choi, U.; Moeckel, G.; Karihaloo, A. Chemokine receptor Cxcr4 contributes to kidney fibrosis via multiple effectors. Am. J. Physiol. Renal Physiol. 2015, 308, F459–F472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, M.; Cui, S.; Li, C.; Jothy, S.; Haase, V.; Steer, B.M.; Marsden, P.A.; Pippin, J.; Shankland, S.; Rastaldi, M.P.; et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat. Med. 2006, 12, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Blanc, R.S.; Kallenbach, J.G.; Bachman, J.F.; Mitchell, A.; Paris, N.D.; Chakkalakal, J.V. Inhibition of inflammatory CCR2 signaling promotes aged muscle regeneration and strength recovery after injury. Nat. Commun. 2020, 11, 4167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaffar, J.; Griffiths, K.; Oveissi, S.; Duan, M.; Foley, M.; Glaspole, I.; Symons, K.; Organ, L.; Westall, G. CXCR4+ cells are increased in lung tissue of patients with idiopathic pulmonary fibrosis. Respir. Res. 2020, 21, 221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griffiths, K.; Habiel, D.M.; Jaffar, J.; Binder, U.; Darby, W.G.; Hosking, C.G.; Skerra, A.; Westall, G.P.; Hogaboam, C.M.; Foley, M. Anti-fibrotic Effects of CXCR4-Targeting i-body AD-114 in Preclinical Models of Pulmonary Fibrosis. Sci. Rep. 2018, 8, 3212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kopp, C.R.; Sharma, S.K.; Krishnaraju, V.S.; Sood, A.; Kumar, R.; Sinha, A.; Dhooria, S.; Singh, J.; Anand, S.; Minz, R.W.; et al. Chemokine receptor CXCR4 based positron emission tomography imaging in systemic sclerosis-related interstitial lung disease. Rheumatology, 2024; keae503, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Derlin, T.; Jaeger, B.; Jonigk, D.; Apel, R.M.; Freise, J.; Shin, H.O.; Weiberg, D.; Warnecke, G.; Ross, T.L.; Wester, H.J.; et al. Clinical Molecular Imaging of Pulmonary CXCR4 Expression to Predict Outcome of Pirfenidone Treatment in Idiopathic Pulmonary Fibrosis. Chest 2021, 159, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Calabretta, R.; Wadsak, W.; Haug, A.R.; Mayerhöfer, M.; Raderer, M.; Zhang, X.; Li, J.; Hacker, M.; Li, X. Imaging Inflammation in Atherosclerosis with CXCR4-Directed [68Ga]PentixaFor PET/MRI-Compared with [18F]FDG PET/MRI. Life 2022, 12, 1039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, Y.; Cao, S.; Liu, J.; Ding, B.; Wang, S.; Pan, J.; Ge, Y.; Cheng, K.; Wang, L.; Ge, L. CXCR4-targeted PET imaging in rheumatoid arthritis: A novel approach for monitoring disease activity and therapeutic response. EJNMMI Res. 2025, 15, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, L.; Li, J.; Jiang, X.; Bai, R. CXCR4/CXCL12 axis: “old” pathway as “novel” target for anti-inflammatory drug discovery. Med. Res. Rev. 2024, 44, 1189–1220. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.C. Immune Factors, Immune Cells and Inflammatory Diseases. Int. J. Mol. Sci. 2024, 25, 2417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonham, L.W.; Karch, C.M.; Fan, C.C.; Tan, C.; Geier, E.G.; Wang, Y.; Wen, N.; Broce, I.J.; Li, Y.; Barkovich, M.J.; et al. International FTD-Genomics Consortium (IFGC); International Parkinson’s Disease Genetics Consortium (IPDGC); International Genomics of Alzheimer’s Project (IGAP). CXCR4 involvement in neurodegenerative diseases. Transl. Psychiatry 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Wang, R. A focus on CXCR4 in Alzheimer’s disease. Brain Circ. 2017, 3, 199–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gabriel, Y.; Voronov-Goldman, M.; Solomon, B. Reversible inhibition of chemokine receptor CXC4 signaling via AMD3100 mitigates neuroinflammation in Alzheimer’s disease. Ageing Neur. Dis. 2025, 5, 1. [Google Scholar] [CrossRef]
- Chaudhary, J.K.; Danga, A.K.; Kumari, A.; Bhardwaj, A.; Rath, P.C. Role of chemokines in aging and age-related diseases. Mech. Ageing Dev. 2025, 223, 112009. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Pearce, O.M.; Wang, X.; Samraj, A.N.; Läubli, H.; Garcia, J.O.; Lin, H.; Fu, X.; Garcia-Bingman, A.; Secrest, P.; et al. Siglec receptors impact mammalian lifespan by modulating oxidative stress. Elife 2015, 4, e06184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bordon, Y. Inflammation: Live long and prosper with Siglecs. Nat. Rev. Immunol. 2015, 15, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Linnartz-Gerlach, B.; Kopatz, J.; Neumann, H. Siglec functions of microglia. Glycobiology 2014, 24, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Virtanen, H.; Silvola, J.M.U.; Autio, A.; Li, X.G.; Liljenbäck, H.; Hellberg, S.; Siitonen, R.; Ståhle, M.; Käkelä, M.; Airaksinen, A.J.; et al. Comparison of 68Ga-DOTA-Siglec-9 and 18F-Fluorodeoxyribose-Siglec-9: Inflammation Imaging and Radiation Dosimetry. Contrast Media Mol. Imaging 2017, 2017, 7645070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aalto, K.; Autio, A.; Kiss, E.A.; Elima, K.; Nymalm, Y.; Veres, T.Z.; Marttila-Ichihara, F.; Elovaara, H.; Saanijoki, T.; Crocker, P.R.; et al. Siglec-9 is a novel leukocyte ligand for vascular adhesion protein-1 and can be used in PET imaging of inflammation and cancer. Blood 2011, 118, 3725–3733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viitanen, R.; Moisio, O.; Lankinen, P.; Li, X.G.; Koivumäki, M.; Suilamo, S.; Tolvanen, T.; Taimen, K.; Mali, M.; Kohonen, I.; et al. First-in-Humans Study of 68Ga-DOTA-Siglec-9, a PET Ligand Targeting Vascular Adhesion Protein 1. J. Nucl. Med. 2021, 62, 577–583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensen, S.B.; Käkelä, M.; Jødal, L.; Moisio, O.; Alstrup, A.K.O.; Jalkanen, S.; Roivainen, A. Exploring the radiosynthesis and in vitro characteristics of [68 Ga]Ga-DOTA-Siglec-9. J. Labelled Comp. Radiopharm. 2017, 60, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Autio, A.; Ahtinen, H.; Helariutta, K.; Liljenbäck, H.; Jalkanen, S.; Roivainen, A.; Airaksinen, A.J. Translating the concept of peptide labeling with 5-deoxy-5-[18F]fluororibose into preclinical practice: 18F-labeling of Siglec-9 peptide for PET imaging of inflammation. Chem. Commun. 2013, 49, 3682–3684. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Buteau, J.; Roduit, R.; Susini, S.; Prentki, M. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 1999, 42, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jun, H.S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unterluggauer, H.; Hampel, B.; Zwerschke, W.; Jansen-Dürr, P. Senescence-associated cell death of human endothelial cells: The role of oxidative stress. Exp. Gerontol. 2003, 38, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Oeseburg, H.; de Boer, R.A.; Buikema, H.; van der Harst, P.; van Gilst, W.H.; Silljé, H.H. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arter. Thromb Vasc Biol. 2010, 30, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hagberg, C.E.; Silva Cascales, H.; Lang, S.; Hyvönen, M.T.; Salehzadeh, F.; Chen, P.; Alexandersson, I.; Terezaki, E.; Harms, M.J.; et al. Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat. Med. 2021, 27, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456, Erratum in EBioMedicine 2020, 52, 102595. https://doi.org/10.1016/j.ebiom.2019.12.004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reich, N.; Hölscher, C. The neuroprotective effects of glucagon-like peptide 1 in Alzheimer’s and Parkinson’s disease: An in-depth review. Front. Neurosci. 2022, 16, 970925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Helmstädter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kröller-Schön, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide in Mice with Experimental Arterial Hypertension. Arter. Thromb. Vasc. Biol. 2020, 40, 145–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drucker, D.J. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016, 24, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Fandiño, J.; Toba, L.; González-Matías, L.C.; Diz-Chaves, Y.; Mallo, F. GLP-1 receptor agonist ameliorates experimental lung fibrosis. Sci. Rep. 2020, 10, 18091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kreiner, F.F.; von Scholten, B.J.; Kurtzhals, P.; Gough, S.C.L. Glucagon-like peptide-1 receptor agonists to expand the healthy lifespan: Current and future potentials. Aging Cell 2023, 22, e13818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skurikhin, E.G.; Pershina, O.V.; Pakhomova, A.V.; Pan, E.S.; Krupin, V.A.; Ermakova, N.N.; Vaizova, O.E.; Pozdeeva, A.S.; Zhukova, M.A.; Skurikhina, V.E.; et al. Endothelial Progenitor Cells as Pathogenetic and Diagnostic Factors, and Potential Targets for GLP-1 in Combination with Metabolic Syndrome and Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2019, 20, 1105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McClean, P.L.; Parthsarathy, V.; Faivre, E.; Hölscher, C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 6587–6594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McClean, P.L.; Hölscher, C. Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology 2014, 86, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gejl, M.; Brock, B.; Egefjord, L.; Vang, K.; Rungby, J.; Gjedde, A. Blood-Brain Glucose Transfer in Alzheimer’s disease: Effect of GLP-1 Analog Treatment. Sci. Rep. 2017, 7, 17490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cukierman-Yaffe, T.; Gerstein, H.C.; Colhoun, H.M.; Diaz, R.; García-Pérez, L.E.; Lakshmanan, M.; Bethel, A.; Xavier, D.; Probstfield, J.; Riddle, M.C.; et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: An exploratory analysis of the REWIND trial. Lancet Neurol. 2020, 19, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Ell, P.; Soderlund, T.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; et al. Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Investig. 2013, 123, 2730–2736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, L.; Chen, L.; Li, D.; Xu, H.; Chen, J.; Min, X.; He, M.; Wu, T.; Zhong, J.; Yang, H.; et al. Effect of GLP-1/GLP-1R on the Polarization of Macrophages in the Occurrence and Development of Atherosclerosis. Mediat. Inflamm. 2021, 2021, 5568159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Somm, E.; Montandon, S.A.; Loizides-Mangold, U.; Gaïa, N.; Lazarevic, V.; De Vito, C.; Perroud, E.; Bochaton-Piallat, M.L.; Dibner, C.; Schrenzel, J.; et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl. Res. 2021, 227, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.M.; Cercueil, J.P.; Loffroy, R.; Denimal, D.; Bouillet, B.; Fourmont, C.; Chevallier, O.; Duvillard, L.; Vergès, B. Effect of Liraglutide Therapy on Liver Fat Content in Patients with Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J. Clin. Endocrinol. Metab. 2017, 102, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Mathijs, I.; Xavier, C.; Peleman, C.; Caveliers, V.; Brom, M.; Gotthardt, M.; Herrera, P.L.; Lahoutte, T.; Bouwens, L. A standardized method for in vivo mouse pancreas imaging and semiquantitative β cell mass measurement by dual isotope SPECT. Mol. Imaging Biol. 2015, 17, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, O.; Laughlin, M.; Brom, M.; Nuutila, P.; Roden, M.; Hwa, A.; Bonadonna, R.; Gotthardt, M. In vivo imaging of beta cells with radiotracers: State of the art, prospects and recommendations for development and use. Diabetologia 2016, 59, 1340–1349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Migliari, S.; Sammartano, A.; Scarlattei, M.; Baldari, G.; Janota, B.; Bonadonna, R.C.; Ruffini, L. Feasibility of a Scale-down Production of [68Ga]Ga-NODAGA-Exendin-4 in a Hospital Based Radiopharmacy. Curr. Radiopharm. 2022, 15, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Boss, M.; Buitinga, M.; Jansen, T.J.P.; Brom, M.; Visser, E.P.; Gotthardt, M. PET-Based Human Dosimetry of 68Ga-NODAGA-Exendin-4, a Tracer for β-Cell Imaging. J. Nucl. Med. 2020, 61, 112–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mikkola, K.; Yim, C.B.; Fagerholm, V.; Ishizu, T.; Elomaa, V.V.; Rajander, J.; Jurttila, J.; Saanijoki, T.; Tolvanen, T.; Tirri, M.; et al. 64Cu- and 68Ga-labelled [Nle(14),Lys(40)(Ahx-NODAGA)NH2]-exendin-4 for pancreatic beta cell imaging in rats. Mol. Imaging Biol. 2014, 16, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.J.P.; Brom, M.; Boss, M.; Buitinga, M.; Tack, C.J.; van Meijel, L.A.; de Galan, B.E.; Gotthardt, M. Importance of beta cell mass for glycaemic control in people with type 1 diabetes. Diabetologia 2023, 66, 367–375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jansen, T.J.P.; Buitinga, M.; Boss, M.; Nijhoff, M.F.; Brom, M.; de Galan, B.E.; van der Graaf, M.; van Koeverden, S.; Vantyghem, M.C.; Beron, A.; et al. Monitoring β-Cell Survival After Intrahepatic Islet Transplantation Using Dynamic Exendin PET Imaging: A Proof-of-Concept Study in Individuals with Type 1 Diabetes. Diabetes 2023, 72, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, O.; Velikyan, I.; Haack, T.; Bossart, M.; Laitinen, I.; Larsen, P.J.; Berglund, J.E.; Antoni, G.; Johansson, L.; Pierrou, S.; et al. Glucagonlike Peptide-1 Receptor Imaging in Individuals with Type 2 Diabetes. J. Nucl. Med. 2022, 63, 794–800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carlsson, P.O.; Hu, X.; Scholz, H.; Ingvast, S.; Lundgren, T.; Scholz, T.; Eriksson, O.; Liss, P.; Yu, D.; Deuse, T.; et al. Survival of Transplanted Allogeneic Beta Cells with No Immunosuppression. N. Engl. J. Med. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ståhle, M.; Kytö, V.; Kiugel, M.; Liljenbäck, H.; Metsälä, O.; Käkelä, M.; Li, X.G.; Oikonen, V.; Saukko, P.; Nuutila, P.; et al. Glucagon-like peptide-1 receptor expression after myocardial infarction: Imaging study using 68Ga-NODAGA-exendin-4 positron emission tomography. J. Nucl. Cardiol. 2020, 27, 2386–2397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Liu, Y.; Xu, Y.; Sheng, J.; Pan, D.; Wang, X.; Yan, J.; Yang, R.; Yang, M. Age-related change of GLP-1R expression in rats can be detected by [18F]AlF-NOTA-MAL-Cys39-exendin-4. Brain Res. 2018, 1698, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira-Gonzalez, S.; Lu, W.Y.; Raven, A.; Dwyer, B.; Man, T.Y.; O’Duibhir, E.; Lewis, P.J.S.; Campana, L.; Kendall, T.J.; Bird, T.G.; et al. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat. Commun. 2018, 9, 1020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lo, A.; Wang, L.S.; Scholler, J.; Monslow, J.; Avery, D.; Newick, K.; O’Brien, S.; Evans, R.A.; Bajor, D.J.; Clendenin, C.; et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015, 75, 2800–2810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, S.; Hara, T.; Miura, Y.; Ishii, H. Fibroblast activation protein constitutes a novel target of chimeric antigen receptor T-cell therapy in solid tumors. Cancer Sci. 2024, 115, 3532–3542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duperret, E.K.; Trautz, A.; Ammons, D.; Perales-Puchalt, A.; Wise, M.C.; Yan, J.; Reed, C.; Weiner, D.B. Alteration of the Tumor Stroma Using a Consensus DNA Vaccine Targeting Fibroblast Activation Protein (FAP) Synergizes with Antitumor Vaccine Therapy in Mice. Clin. Cancer Res. 2018, 24, 1190–1201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acharya, P.S.; Zukas, A.; Chandan, V.; Katzenstein, A.L.; Puré, E. Fibroblast activation protein: A serine protease expressed at the remodeling interface in idiopathic pulmonary fibrosis. Hum. Pathol. 2006, 37, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Luo, Q.; Wang, X.; Fang, Q.; Fu, Z.; Li, J.; Lai, Y.; Chen, X.; Xu, X.; Peng, X.; et al. Comprehensive Analysis of Fibroblast Activation Protein Expression in Interstitial Lung Diseases. Am. J. Respir. Crit. Care Med. 2023, 207, 160–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernandez-Gonzalez, F.; Prats, N.; Ramponi, V.; López-Domínguez, J.A.; Meyer, K.; Aguilera, M.; Muñoz Martín, M.I.; Martínez, D.; Agusti, A.; Faner, R.; et al. Human senescent fibroblasts trigger progressive lung fibrosis in mice. Aging 2023, 15, 6641–6657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, A.T.; Kim, Y.O.; Yan, X.Z.; Abe, H.; Aslam, M.; Park, K.S.; Zhao, X.Y.; Jia, J.D.; Klein, T.; You, H.; et al. Fibroblast Activation Protein Activates Macrophages and Promotes Parenchymal Liver Inflammation and Fibrosis. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 841–867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Croft, A.P.; Campos, J.; Jansen, K.; Turner, J.D.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.J.; Kemble, S.; et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019, 570, 246–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rurik, J.G.; Tombácz, I.; Yadegari, A.; Méndez Fernández, P.O.; Shewale, S.V.; Li, L.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; et al. CAR T cells produced in vivo to treat cardiac injury. Science 2022, 375, 91–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spreckelmeyer, S.; Balzer, M.; Poetzsch, S.; Brenner, W. Fully-automated production of [68Ga]Ga-FAPI-46 for clinical application. EJNMMI Radiopharm. Chem. 2020, 5, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Migliari, S.; Scarlattei, M.; Baldari, G.; Ruffini, L. Automated Synthesis Method to Produce the PET Tracer [68Ga]Ga-FAPI-46 for Clinical Applications: Development, Optimization and Validation. J. Biotechnol. Biomed. 2023, 6, 336–346. [Google Scholar] [CrossRef]
- Lavis, P.; Pingitore, J.; Doumont, G.; Garabet, A.; Van Simaeys, G.; Lacroix, S.; Passon, N.; Van Heymbeek, C.; De Maeseneire, C.; Allard, J.; et al. Usefulness of FAPα assessment in bronchoalveolar lavage as a marker of fibrogenesis: Results of a preclinical study and first report in patients with idiopathic pulmonary fibrosis. Respir. Res. 2023, 24, 254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergmann, C.; Distler, J.H.W.; Treutlein, C.; Tascilar, K.; Müller, A.T.; Atzinger, A.; Matei, A.E.; Knitza, J.; Györfi, A.H.; Lück, A.; et al. 68Ga-FAPI-04 PET-CT for molecular assessment of fibroblast activation and risk evaluation in systemic sclerosis-associated interstitial lung disease: A single-centre, pilot study. Lancet Rheumatol. 2021, 3, e185–e194. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrans, Z.T.; Massey, C.F.; Bernau, K.; Ferreira, C.A.; Jeffery, J.J.; Schulte, J.J.; Moore, M.; Valla, F.; Batterton, J.M.; Drake, C.R.; et al. [68 Ga]Ga-FAPI-46 PET for non-invasive detection of pulmonary fibrosis disease activity. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3705–3716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, Y.; Pan, Q.; Zhou, Z.; Li, M.; Wei, Y.; Jiang, X.; Yang, H.; Li, F. 68Ga-FAPI PET/CT for Rheumatoid Arthritis: A Prospective Study. Radiology 2023, 307, e222052. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, L.M.; Sisk, A.E., Jr.; Czernin, J.; Such, B.M.; Calais, J.; Hotta, M. [68Ga]Ga-FAPI-46 PET for Visualization of Postinfarction Renal Fibrosis. J. Nucl. Med. 2023, 64, 1660–1661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Quan, W.; Zhu, T.; Feng, S.; Huang, X.; Meng, H.; Du, R.; Zhu, Z.; Qu, X.; Li, P.; et al. [68Ga]Ga-DOTA-FAPI-04 PET/MR in patients with acute myocardial infarction: Potential role of predicting left ventricular remodeling. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 839–848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Röhrich, M.; Leitz, D.; Glatting, F.M.; Wefers, A.K.; Weinheimer, O.; Flechsig, P.; Kahn, N.; Mall, M.A.; Giesel, F.L.; Kratochwil, C.; et al. Fibroblast Activation Protein-Specific PET/CT Imaging in Fibrotic Interstitial Lung Diseases and Lung Cancer: A Translational Exploratory Study. J. Nucl. Med. 2022, 63, 127–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Yang, X.; Liu, H.; Luo, W.; Liu, H.; Lv, T.; Wang, J.; Qin, J.; Ou, S.; Chen, Y. Value of [68Ga]Ga-FAPI-04 imaging in the diagnosis of renal fibrosis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Conen, P.; Pennetta, F.; Dendl, K.; Hertel, F.; Vogg, A.; Haberkorn, U.; Giesel, F.L.; Mottaghy, F.M. [68 Ga]Ga-FAPI uptake correlates with the state of chronic kidney disease. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3365–3372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heckmann, M.B.; Reinhardt, F.; Finke, D.; Katus, H.A.; Haberkorn, U.; Leuschner, F.; Lehmann, L.H. Relationship Between Cardiac Fibroblast Activation Protein Activity by Positron Emission Tomography and Cardiovascular Disease. Circ. Cardiovasc. Imaging 2020, 13, e010628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barton, A.K.; Craig, N.J.; Loganath, K.; Joshi, S.; Tsampasian, V.; Mahendran, M.; Lenell, J.; Tzolos, E.; Singh, T.; Whittington, B.; et al. Myocardial Fibroblast Activation After Acute Myocardial Infarction: A Positron Emission Tomography and Magnetic Resonance Study. J. Am. Coll Cardiol. 2025, 85, 578–591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toms, J.; Kogler, J.; Maschauer, S.; Daniel, C.; Schmidkonz, C.; Kuwert, T.; Prante, O. Targeting Fibroblast Activation Protein: Radiosynthesis and Preclinical Evaluation of an 18F-Labeled FAP Inhibitor. J. Nucl. Med. 2020, 61, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, R.; Huang, Y.; Zhong, J.; Yan, Q.; Yang, J.; Hu, K.; Zhong, Y. [18F]AlF-ND-bisFAPI PET imaging of fibroblast activation protein as a biomarker to monitor the progression of liver fibrosis. Hepatol. Commun. 2024, 8, e0407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, I.K.; Noguera-Ortega, E.; Xiao, Z.; Todd, L.; Scholler, J.; Song, D.; Liousia, M.; Lohith, K.; Xu, K.; Edwards, K.J.; et al. Monitoring Therapeutic Response to Anti-FAP CAR T Cells Using [18F]AlF-FAPI-74. Clin Cancer Res. 2022, 28, 5330–5342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duran, I.; Pombo, J.; Sun, B.; Gallage, S.; Kudo, H.; McHugh, D.; Bousset, L.; Barraga Avila, J.E.; Forlano, R.; Manousou, P.; et al. Detection of senescence using machine learning algorithms based on nuclear features. Nat. Commun. 2024, 15, 1041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cen, P.; Cui, C.; Huang, J.; Chen, H.; Wu, F.; Niu, J.; Zhong, Y.; Jin, C.; Zhu, W.H.; Zhang, H.; et al. Cellular senescence imaging and senolysis monitoring in cancer therapy based on a β-galactosidase-activated aggregation-induced emission luminogen. Acta Biomater. 2024, 179, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Nernekli, K.; Mangarova, D.B.; Suryadevara, V.; Hajipour, M.; Tang, J.H.; Wang, J.; Liang, T.; Harris, M.; Ueyama, T.; Lyons, J.K.; et al. MRI detection of senescent cells in porcine knee joints with a β-galactosidase responsive Gd-chelate. npj Imaging 2025, 3, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daldrup-Link, H.E.; Suryadevara, V.; Tanyildizi, Y.; Nernekli, K.; Tang, J.H.; Meade, T.J. Musculoskeletal imaging of senescence. Skeletal. Radiol. 2024, 53, 1879–1887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mansfield, L.; Ramponi, V.; Gupta, K.; Stevenson, T.; Mathew, A.B.; Barinda, A.J.; Herbstein, F.; Morsli, S. Emerging insights in senescence: Pathways from preclinical models to therapeutic innovations. npj Aging 2024, 10, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamat, P.; Macaluso, N.; Li, Y.; Agrawal, A.; Winston, A.; Pan, L.; Stewart, T.; Starich, B.; Milcik, N.; Min, C.; et al. Single-cell morphology encodes functional subtypes of senescence in aging human dermal fibroblasts. Sci. Adv. 2025, 11, eads1875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hughes, B.K.; Wallis, R.; Bishop, C.L. Yearning for machine learning: Applications for the classification and characterisation of senescence. Cell Tissue Res. 2023, 394, 1–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, A.; Migliari, S.; Guercio, A.; Baldari, G.; Graziani, T.; Cervati, V.; Ruffini, L.; Scarlattei, M. Emerging Radioligands as Tools to Track Multi-Organ Senescence. Diagnostics 2025, 15, 2518. https://doi.org/10.3390/diagnostics15192518
Gagliardi A, Migliari S, Guercio A, Baldari G, Graziani T, Cervati V, Ruffini L, Scarlattei M. Emerging Radioligands as Tools to Track Multi-Organ Senescence. Diagnostics. 2025; 15(19):2518. https://doi.org/10.3390/diagnostics15192518
Chicago/Turabian StyleGagliardi, Anna, Silvia Migliari, Alessandra Guercio, Giorgio Baldari, Tiziano Graziani, Veronica Cervati, Livia Ruffini, and Maura Scarlattei. 2025. "Emerging Radioligands as Tools to Track Multi-Organ Senescence" Diagnostics 15, no. 19: 2518. https://doi.org/10.3390/diagnostics15192518
APA StyleGagliardi, A., Migliari, S., Guercio, A., Baldari, G., Graziani, T., Cervati, V., Ruffini, L., & Scarlattei, M. (2025). Emerging Radioligands as Tools to Track Multi-Organ Senescence. Diagnostics, 15(19), 2518. https://doi.org/10.3390/diagnostics15192518






