The Predictive Power of Barotrauma from the Macklin Effect in the ARDS Population: A Comparison of COVID-19 and Non-COVID-19 ARDS—Could the Macklin Effect Serve as a Helpful Tool for Evaluating Transfer to ARDS Reference Centers?
Abstract
1. Introduction
2. Methods
- Age >18 years;
- The need for invasive ventilation or non-invasive ventilation;
- The availability of at least a chest CT scan.
- The genesis of post-procedure pneumothorax or pneumomediastinum (central venous line insertion, tracheostomy, and thoracic drainage);
- Evidence of pneumothorax or pneumomediastinum at the first available chest CT scan.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Madotto, F.; Pham, T.; Bellani, G.; Bos, L.D.; Simonis, F.D.; Fan, E.; Artigas, A.; Brochard, L.; Schultz, M.J.; Laffey, J.G.; et al. Resolved versus Confirmed ARDS after 24 h: Insights from the LUNG SAFE Study. Intensive Care Med. 2018, 44, 564–577. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-Induced Lung Injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, D.H.L.; Abu Hilal, M.; Bnà, C.; Prezioso, C.; Cavallo, E.; Nencini, N.; Crisci, S.; Fusina, F.; Natalini, G. Pneumomediastinum and Subcutaneous Emphysema in COVID-19: Barotrauma or Lung Frailty? ERJ Open Res. 2020, 6, 00385–02020. [Google Scholar] [CrossRef] [PubMed]
- Belletti, A.; Todaro, G.; Valsecchi, G.; Losiggio, R.; Palumbo, D.; Landoni, G.; Zangrillo, A. Barotrauma in Coronavirus Disease 2019 Patients Undergoing Invasive Mechanical Ventilation: A Systematic Literature Review. Crit. Care Med. 2022, 50, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Agerstrand, C.; Beitler, J.R.; Karagiannidis, C.; Madahar, P.; Yip, N.H.; Pesenti, A.; Slutsky, A.S.; Brochard, L.; Brodie, D. Risks and Benefits of Ultra–Lung-Protective Invasive Mechanical Ventilation Strategies with a Focus on Extracorporeal Support. Am. J. Respir. Crit. Care Med. 2022, 205, 873–882. [Google Scholar] [CrossRef]
- Macklin, C.C. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: Clinical implications. Arch. Intern. Med. 1939, 64, 913–926. [Google Scholar] [CrossRef]
- Belletti, A.; Palumbo, D.; Zangrillo, A.; Fominskiy, E.V.; Franchini, S.; Dell’Acqua, A.; Marinosci, A.; Monti, G.; Vitali, G.; Colombo, S.; et al. Predictors of Pneumothorax/Pneumomediastinum in Mechanically Ventilated COVID-19 Patients. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3642–3651. [Google Scholar] [CrossRef]
- Paternoster, G.; Belmonte, G.; Scarano, E.; Rotondo, P.; Palumbo, D.; Belletti, A.; Corradi, F.; Bertini, P.; Landoni, G.; Guarracino, F.; et al. Macklin Effect on Baseline Chest CT Scan Accurately Predicts Barotrauma in COVID-19 Patients. Respir. Med. 2022, 197, 106853. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Belletti, A.; Pallanch, O.; Bonizzoni, M.A.; Guidi, L.; Cobelli, F.D.; Landoni, G.; Zangrillo, A.; Bonis, M.D.; Palumbo, D. Clinical Use of Macklin-like Radiological Sign (Macklin Effect): A Systematic Review. Respir. Med. 2023, 210, 107178. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, V.; Liou, C.; D’souza, B.; Salvatore, M.M.; Leb, J.; Belletti, A.; Palumbo, D.; Landoni, G.; Capaccione, K.M. The Macklin Effect Closely Correlates with Pneumomediastinum in Acutely Ill Intubated Patients with COVID-19 Infection. Clin. Imaging 2023, 97, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Casadiego Monachello, F.J.; de la Torre Terron, M.C.; Mendez Barraza, J.A.; Casals Vila, S. Macklin Effect as an Early Radiological Predictor of Barotrauma in ARDS COVID-19 Patients in Invasive Mechanical Ventilation. Med. Intensiv. 2023, 47, 235–236. [Google Scholar] [CrossRef]
- Anzueto, A.; Frutos–Vivar, F.; Esteban, A.; Alía, I.; Brochard, L.; Stewart, T.; Benito, S.; Tobin, M.J.; Elizalde, J.; Palizas, F.; et al. Incidence, Risk Factors and Outcome of Barotrauma in Mechanically Ventilated Patients. Intensive Care Med. 2004, 30, 612–619. [Google Scholar] [CrossRef]
- Schnapp, L.M.; Chin, D.P.; Szaflarski, N.; Matthay, M.A. Frequency and Importance of Barotrauma in 100 Patients with Acute Lung Injury. Crit. Care Med. 1995, 23, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.B.; Barbas, C.S.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Munoz, C.; Oliveira, R.; et al. Effect of a Protective-Ventilation Strategy on Mortality in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef]
- Serck, N.; Piagnerelli, M.; Augy, J.L.; Annoni, F.; Ottavy, G.; Courcelle, R.; Carbutti, G.; Lejeune, F.; Vinsonneau, C.; Sauneuf, B.; et al. Barotrauma in COVID-19 Acute Respiratory Distress Syndrome: Retrospective Analysis of the COVADIS Prospective Multicenter Observational Database. BMC Anesthesiol. 2023, 23, 138. [Google Scholar] [CrossRef]
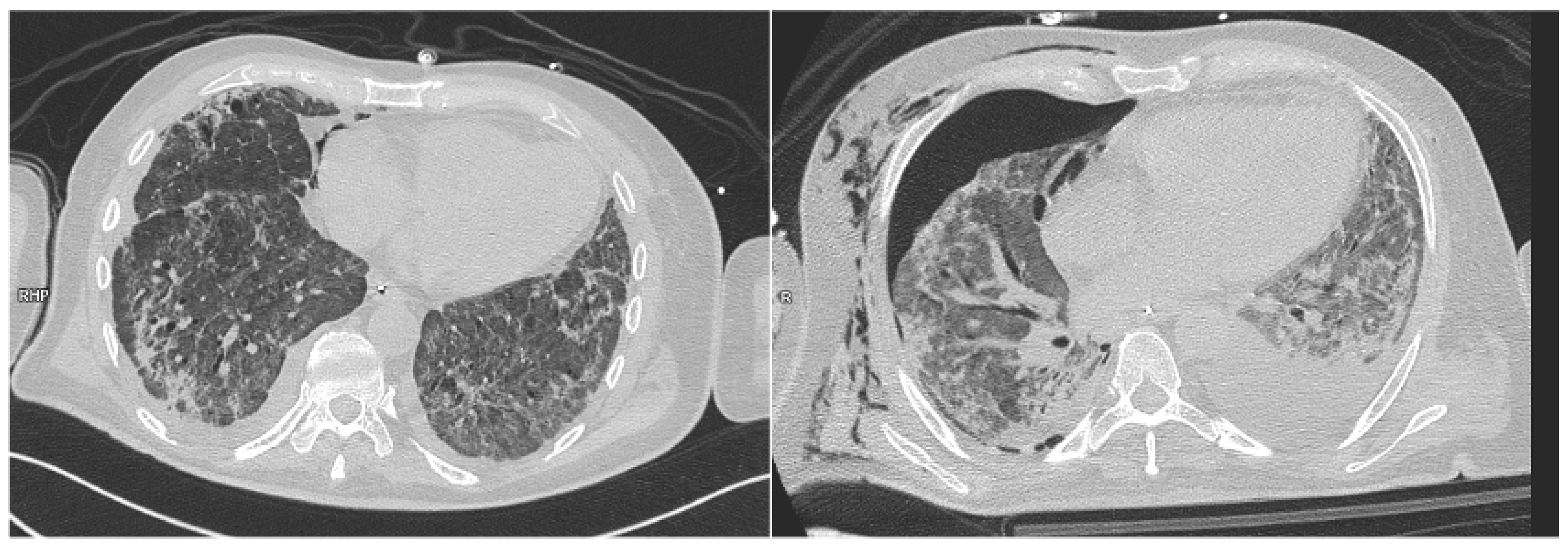

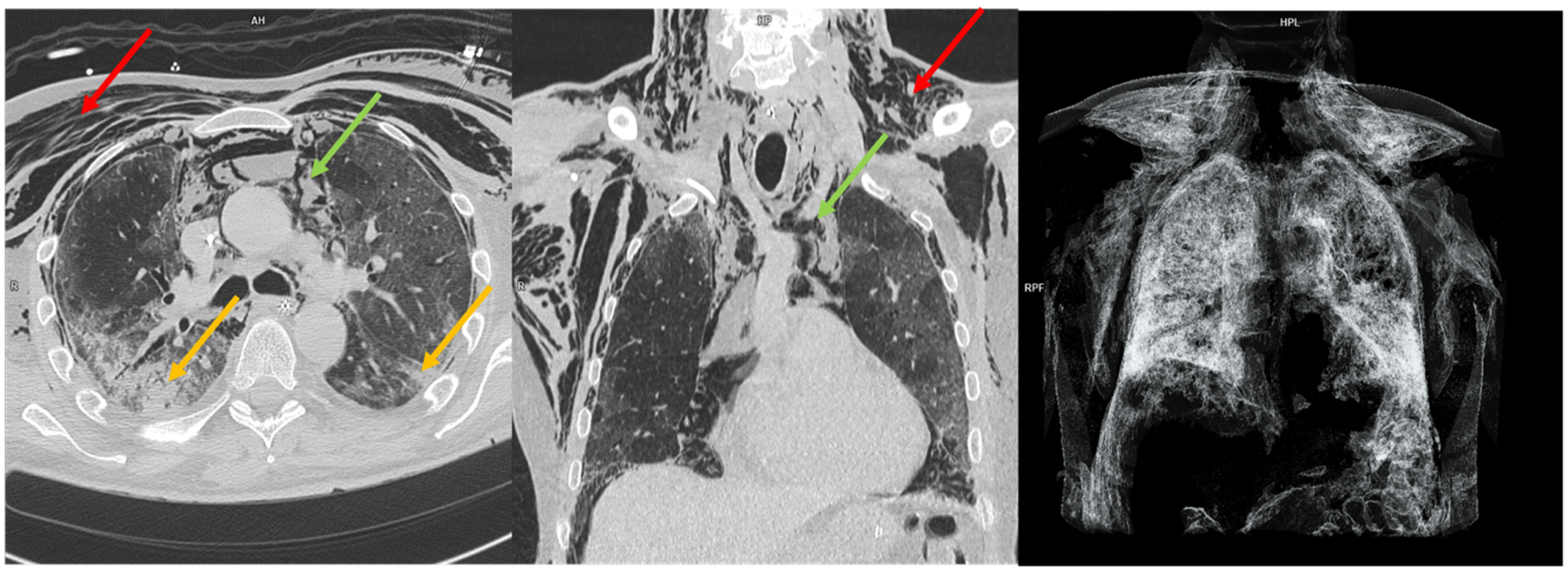
| Characteristics | N (%) or Mean (±SD) |
|---|---|
| Total patients | 225 (100%) |
| Males | 159 (71%) |
| Females | 66 (29%) |
| Age (years) | 61 (±13.3) |
| Horowitz index at ICU admission | 104 (±41.9) |
| Time from symptoms’ onset to ICU admission (days) | 10 (±9) |
| Days of NIV before invasive ventilation | 6 (±5.7) |
| Days of NIV before ECMO | 10 (±6.9) |
| Days from intubation to ECMO | 5 (±4) |
| Days of ventilation before barotrauma | 17 (±20.1) |
| Number of subjects with barotrauma | 65 (28.8%) |
| ICU mortality | 102 (45.3%) |
| In-hospital mortality | 108 (48%) |
| Aetiology | |
| COVID-19 | 139 (62%) |
| Non COVID-19 | 86 (38%) |
| H1N1 | 38 (16%) |
| Bacterial | 26 (12%) |
| Unknown | 8 (4%) |
| Septic shock | 4 (2%) |
| HELLP syndrome | 2 (1%) |
| ANCA vasculitis | 1 (0.5%) |
| Drug overdose | 1 (0.5%) |
| Pancreatitis | 1 (0.5%) |
| Endocarditis | 1 (0.5%) |
| Antisynthetase syndrome | 2 (1%) |
| Acute Myeloid Leukemia | 1 (0.5%) |
| Dermatomyositis | 1 (0.5%) |
| Macklin Group | Non-Macklin Group | p-Value | |
|---|---|---|---|
| Number of subjects | 44 | 181 | |
| Males | 34 (77%) | 125 (69%) | 0.283 |
| COVID-19 | 32 (73%) | 107 (59%) | 0.095 |
| Horowitz index at ICU admission | 98 (±21.7) | 106 (±45.3) | 0.888 |
| Barotrauma | 29 (66%) | 36 (20%) | <0.001 |
| Length of NIV before invasive mechanical ventilation | 5.7 days (±5.1) | 6.3 days (±5.8) | 0.764 |
| Length of mechanical ventilation before cannulation in ECMO recipients | 6 days (±4.3) | 5 days (±3.8) | 0.471 |
| Length of NIV before cannulation in ECMO recipients | 12 days (±7.9) | 9 (±6.5) | 0.158 |
| Length of ventilation before barotrauma genesis | 17 days (±18.1) | 17 (±21.8) | 0.818 |
| ICU mortality | 20 (45.4%) | 82 (45.3%) | 0.985 |
| In-hospital mortality | 24 (47.7%) | 87 (48%) | 0.967 |
| Barotrauma Group | Non-Barotrauma Group | p-Value | |
|---|---|---|---|
| Number of subjects | 65 (29%) | 160 (71%) | / |
| Males | 48 (74%) | 111 (69%) | 0.504 |
| COVID-19 | 42 (64.6%) | 97 (60%) | 0.576 |
| Horowitz index at ICU admission | 101 (±28.6) | 106 (±46.1) | 0.920 |
| Length of NIV before invasive mechanical ventilation | 6.1 (±6) | 6.2 (±6) | 1.000 |
| Length of mechanical ventilation before cannulation in ECMO recipients | 6 (±4.5) | 5 (±3.6) | 0.080 |
| Length of NIV before cannulation in ECMO recipients | 12 (±6.4) | 9 (±7) | 0.020 |
| Length of ventilation before barotrauma genesis | 13 (±15.2) | 19 (±22.4) | 0.271 |
| ICU mortality | 34 (52.3%) | 68 (42.5%) | 0.180 |
| In-hospital mortality | 35 (53.8%) | 73 (45.6%) | 0.253 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marabotti, A.; Pelagatti, F.; Frezzetti, G.; Albanesi, M.; Galluzzo, A.; Valletta, A.; Sorrentino, L.A.; Cardoni, A.; Cianchi, G.; Ciapetti, M.; et al. The Predictive Power of Barotrauma from the Macklin Effect in the ARDS Population: A Comparison of COVID-19 and Non-COVID-19 ARDS—Could the Macklin Effect Serve as a Helpful Tool for Evaluating Transfer to ARDS Reference Centers? Diagnostics 2025, 15, 2514. https://doi.org/10.3390/diagnostics15192514
Marabotti A, Pelagatti F, Frezzetti G, Albanesi M, Galluzzo A, Valletta A, Sorrentino LA, Cardoni A, Cianchi G, Ciapetti M, et al. The Predictive Power of Barotrauma from the Macklin Effect in the ARDS Population: A Comparison of COVID-19 and Non-COVID-19 ARDS—Could the Macklin Effect Serve as a Helpful Tool for Evaluating Transfer to ARDS Reference Centers? Diagnostics. 2025; 15(19):2514. https://doi.org/10.3390/diagnostics15192514
Chicago/Turabian StyleMarabotti, Alberto, Filippo Pelagatti, Gianluca Frezzetti, Marco Albanesi, Antonio Galluzzo, Alessandra Valletta, Laura Arianna Sorrentino, Andrea Cardoni, Giovanni Cianchi, Marco Ciapetti, and et al. 2025. "The Predictive Power of Barotrauma from the Macklin Effect in the ARDS Population: A Comparison of COVID-19 and Non-COVID-19 ARDS—Could the Macklin Effect Serve as a Helpful Tool for Evaluating Transfer to ARDS Reference Centers?" Diagnostics 15, no. 19: 2514. https://doi.org/10.3390/diagnostics15192514
APA StyleMarabotti, A., Pelagatti, F., Frezzetti, G., Albanesi, M., Galluzzo, A., Valletta, A., Sorrentino, L. A., Cardoni, A., Cianchi, G., Ciapetti, M., Lazzeri, C., Peris, A., & Bonizzoli, M. (2025). The Predictive Power of Barotrauma from the Macklin Effect in the ARDS Population: A Comparison of COVID-19 and Non-COVID-19 ARDS—Could the Macklin Effect Serve as a Helpful Tool for Evaluating Transfer to ARDS Reference Centers? Diagnostics, 15(19), 2514. https://doi.org/10.3390/diagnostics15192514






