High-Flow Nasal Oxygen Therapy in Preventing Post-Extubation Hypoxaemia and Postoperative Pulmonary Complications: A Systematic Review and Meta-Analysis †
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Data Sources and Searches
2.3. Inclusion and Exclusion Criteria
2.4. Definition
2.5. Study Selection
2.6. Data Extraction
2.7. Risk of Bias Assessment and Quality of Evidence
2.8. Statistical Analysis
3. Results
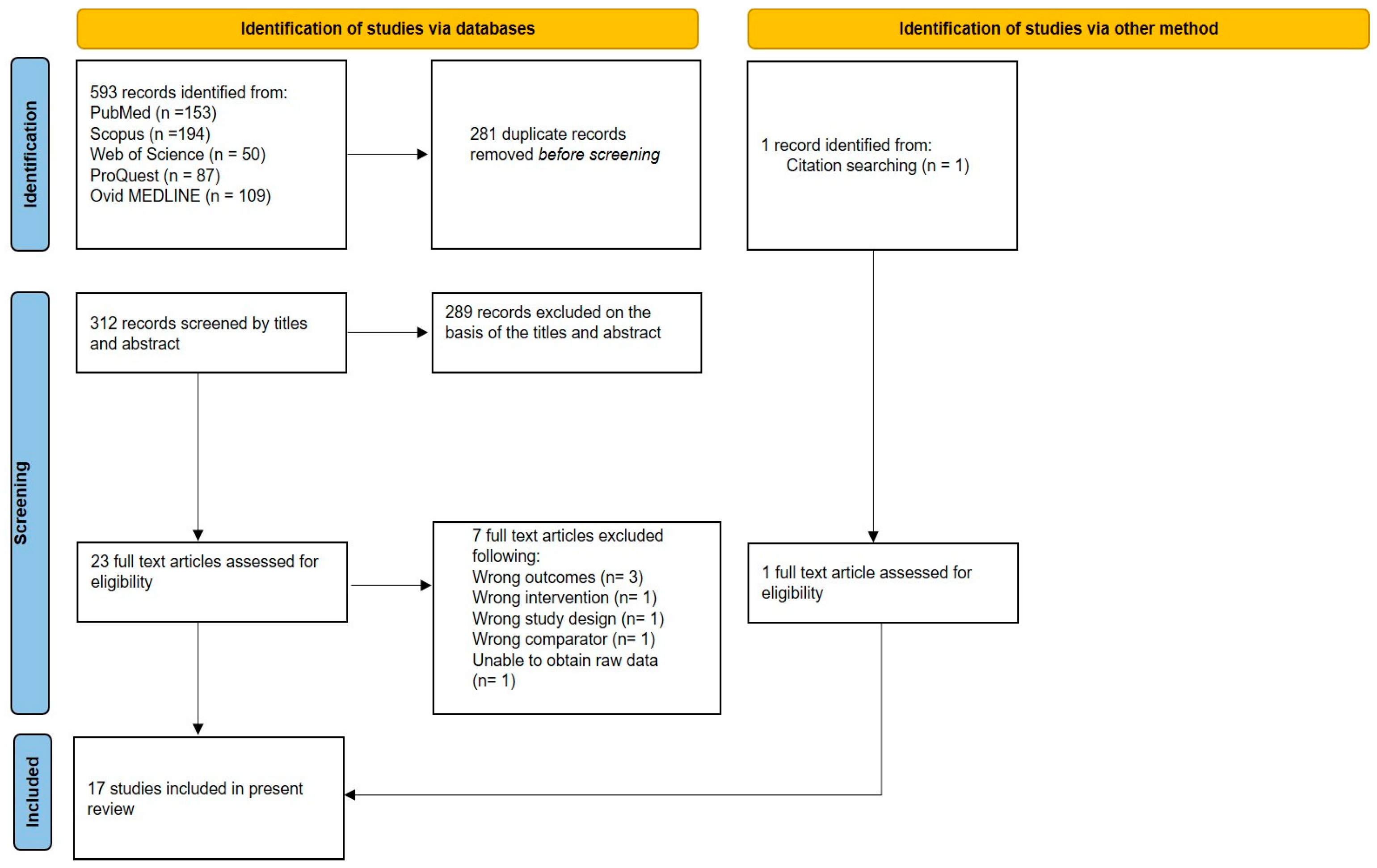
3.1. Trial Selection
3.2. Trial Characteristics
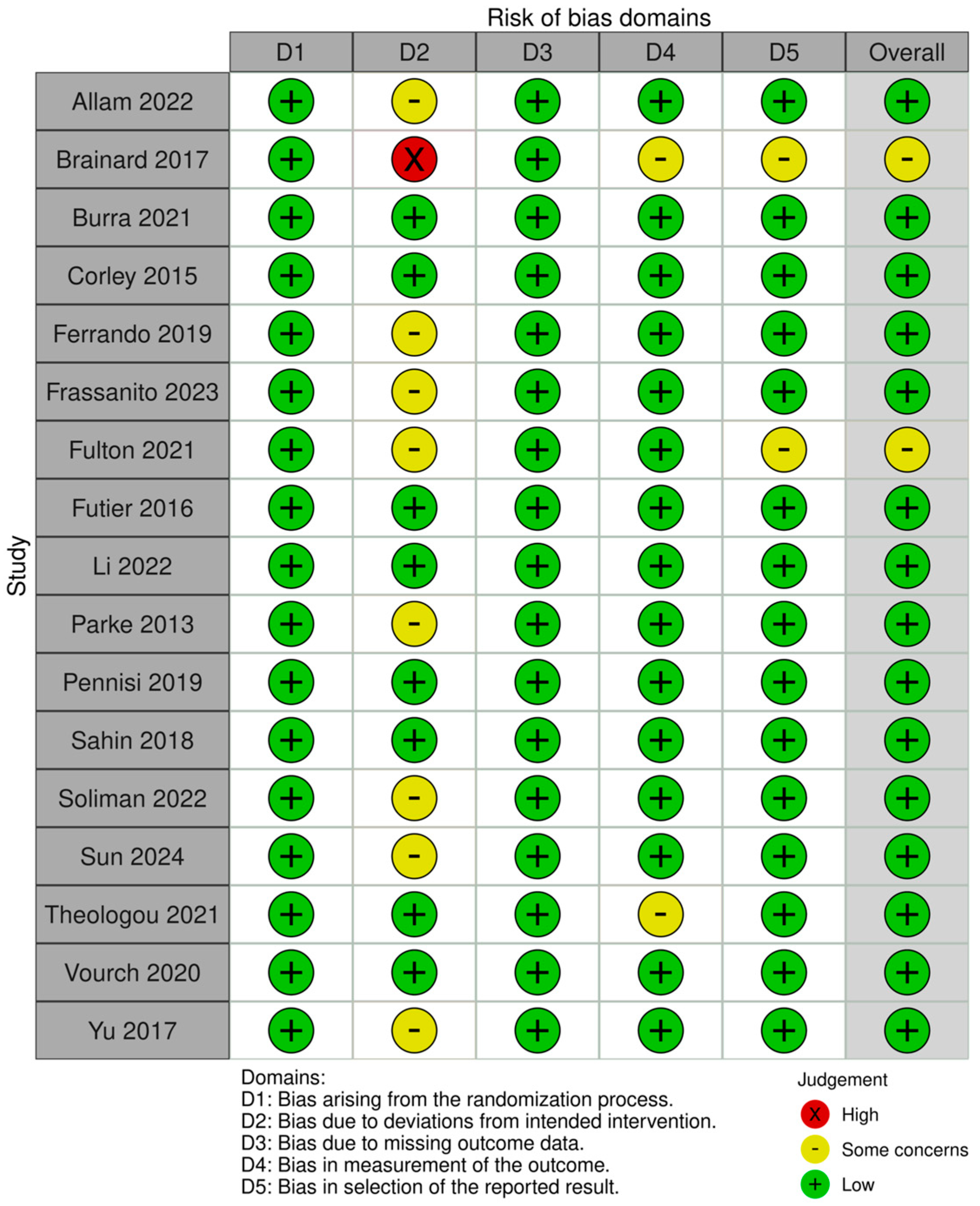
3.3. Risk of Bias Assessment
3.4. Outcomes
3.4.1. Primary Outcomes
3.4.2. Secondary Outcomes
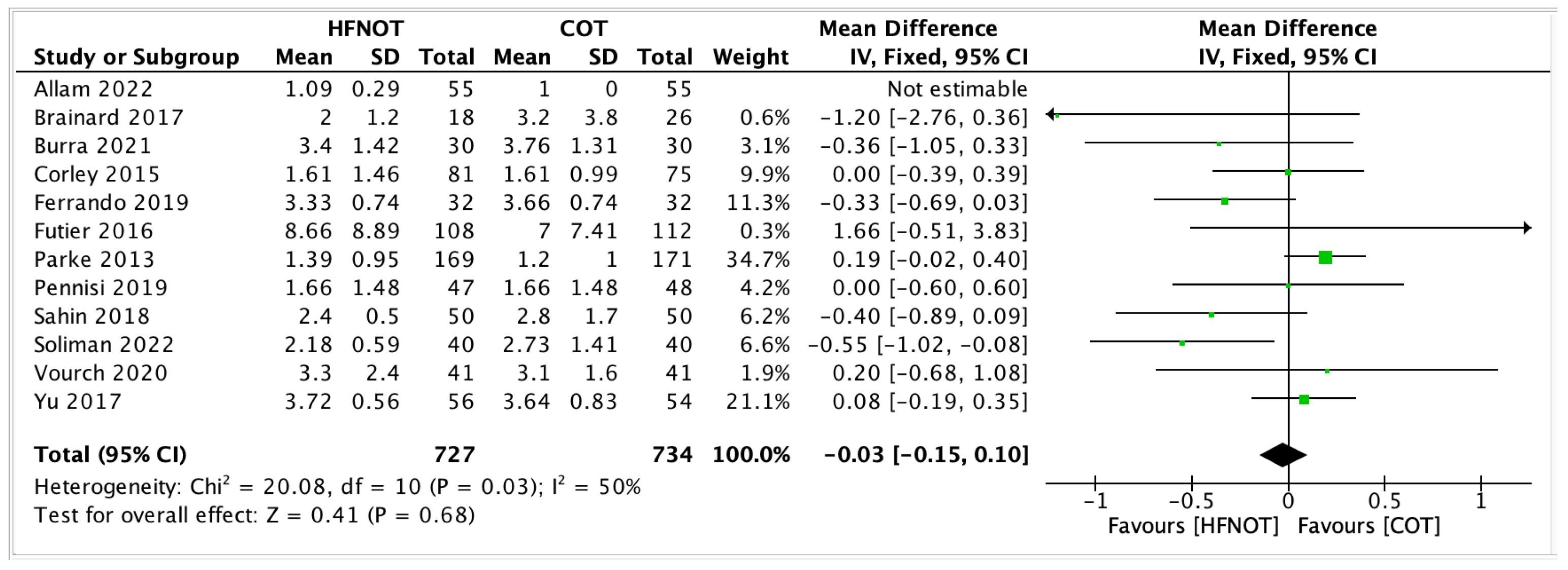
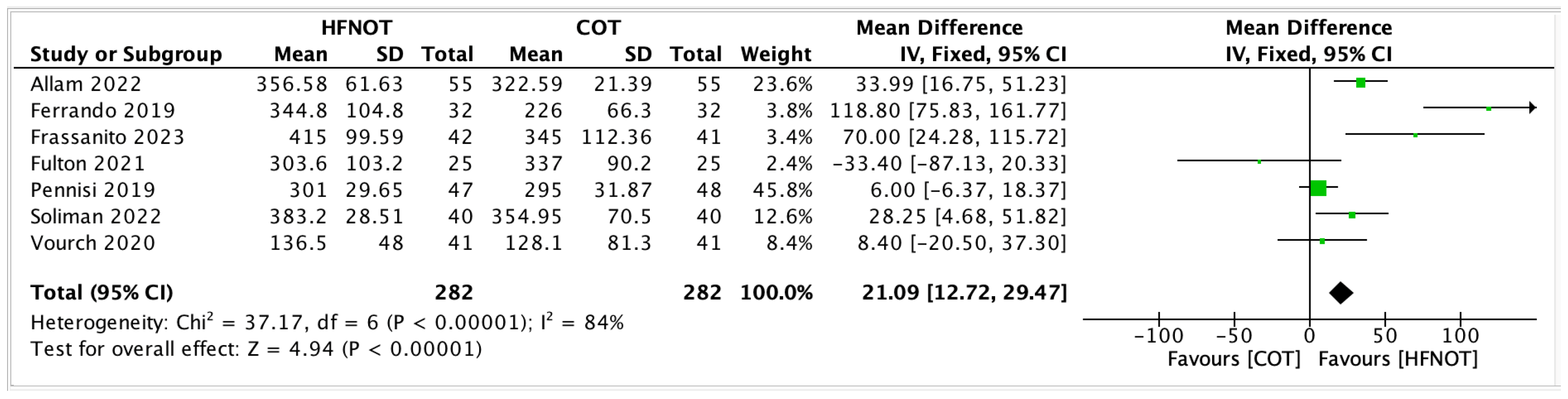
- ICU Length of Stay
- 2
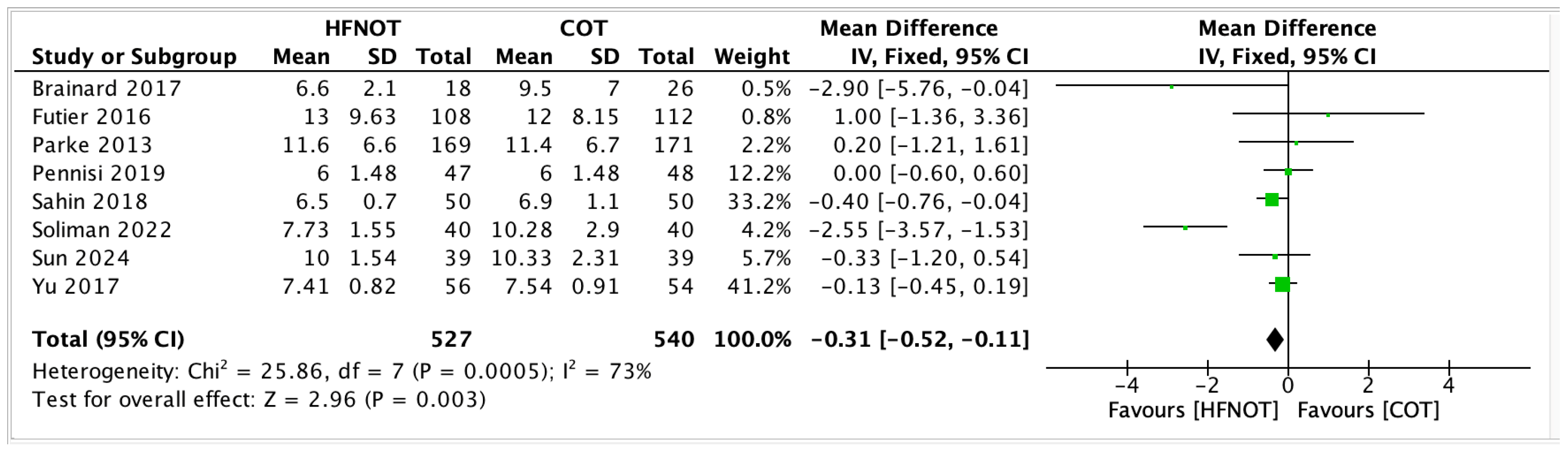
- Hospital Length of Stay
- 3
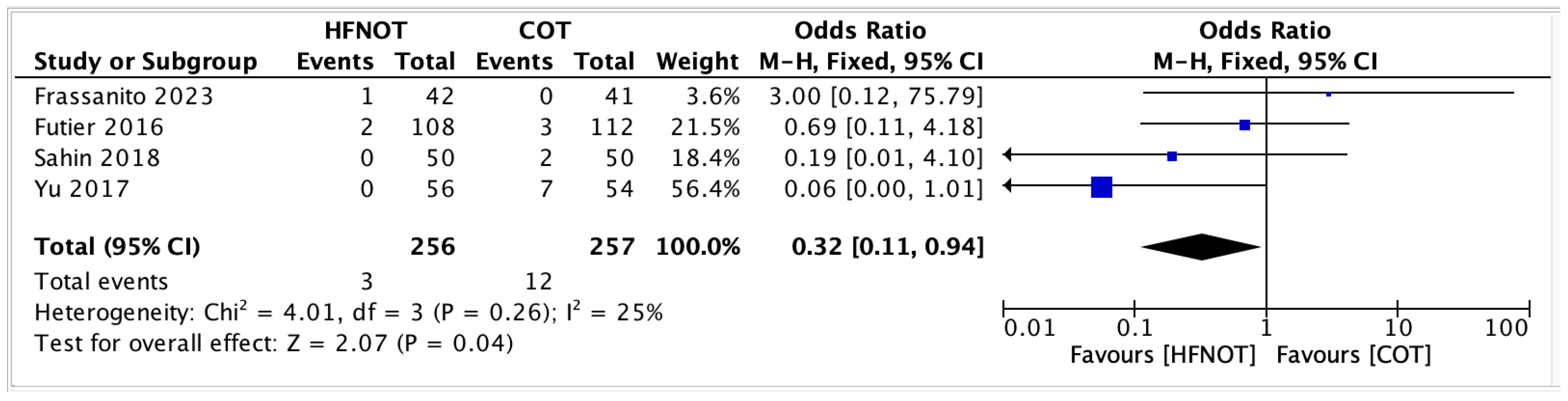
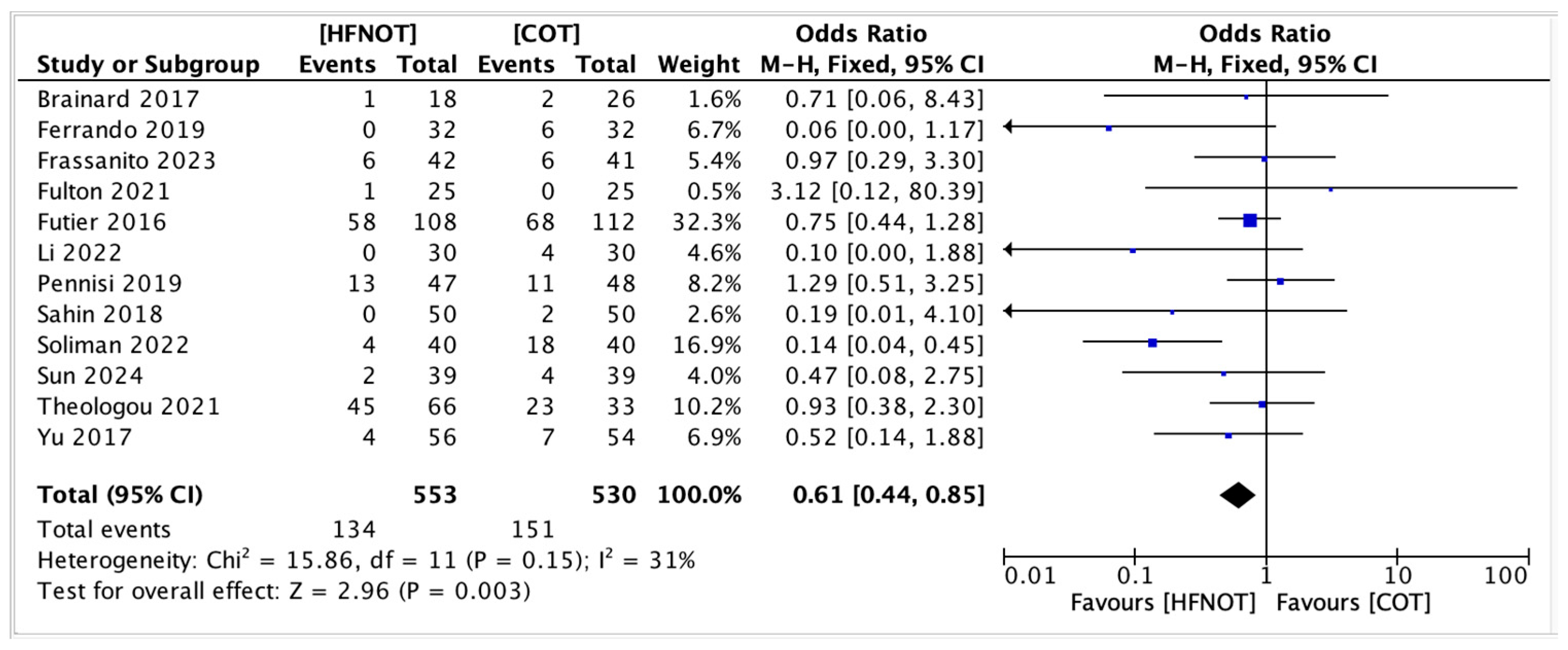
- Mortality rateFour trials reported on postoperative mortality in patients receiving HFNOT (n = 256) versus COT (n = 257). The pooled analysis showed a significantly lower odds of mortality in the HFNOT group (OR 0.32, 95% CI 0.11 to 0.94; p = 0.04) (Figure 7).A total of 3 deaths occurred in the HFNOT group compared to 12 deaths in the COT group. Heterogeneity was low (I2 = 25%, χ2 = 4.01; p = 0.26), indicating consistency across studies.
- 4
- Escalation of respiratory supportEscalation of respiratory support was mainly performed under the discretion of individual intensivists/primary physicians unless a therapy algorithm was in place. Table 3 summarizes escalation events from initial oxygen therapy to more advanced support modalities—including escalation to high-flow nasal oxygen therapy (HFNOT), non-invasive ventilation (NIV), and reintubation—across included studies. Total escalation refers to the combined number of patients requiring any of these interventions. Overall, escalation events were more frequent in the COT group compared to HFNOT, particularly for transitions to NIV or reintubation (Table 3).
3.5. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BiPAP | Bilevel positive airway pressure |
| BMI | Body mass index |
| χ2 | Chi-squared |
| CI | Confidence interval |
| COPD | Chronic obstructive pulmonary disorder |
| COT | Conventional oxygen therapy |
| CPAP | Continuous positive airway pressure |
| EPCO | European perioperative clinical outcome |
| FiO2 | Fraction of inspired oxygen |
| HFNOT | High-flow nasal oxygen therapy |
| I2 | I-squared |
| IQRs | Interquartile ranges |
| ICU | Intensive care unit |
| MDs | Mean differences |
| NIV | Non-invasive ventilation |
| ORs | Odds ratios |
| RCTs | Randomized controlled trials |
| PEEP | Positive end-expiratory pressure |
| PF | PaO2/FiO2 ratio |
| PPCs | Postoperative pulmonary complications |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-analyses |
| SpO2 | Oxygen saturation |
References
- Rock, P.; Rich, P.B. Postoperative Pulmonary Complications. Curr. Opin. Anaesthesiol. 2003, 16, 123–131. [Google Scholar] [CrossRef]
- Tokics, L.; Hedenstierna, G.; Strandberg, Å.; Brismar, B.; Lundquist, H. Lung Collapse and Gas Exchange during General Anesthesia. Anesthesiology 1987, 66, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Arozullah, A.M.; Khuri, S.F.; Henderson, W.G.; Daley, J. Development and Validation of a Multifactorial Risk Index for Predicting Postoperative Pneumonia after Major Noncardiac Surgery. Ann. Intern. Med. 2001, 135, 847–857. [Google Scholar] [CrossRef]
- Canet, J.; Gallart, L. Postoperative Respiratory Failure. Curr. Opin. Crit. Care 2014, 20, 56–62. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative Pulmonary Complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Khuri, S.F.; Henderson, W.G.; DePalma, R.G.; Mosca, C.; Healey, N.A.; Kumbhani, D.J. Determinants of Long-Term Survival After Major Surgery and the Adverse Effect of Postoperative Complications. Ann. Surg. 2005, 242, 326–343. [Google Scholar] [CrossRef]
- Smetana, G.W.; Lawrence, V.A.; Cornell, J.E. Preoperative Pulmonary Risk Stratification for Noncardiothoracic Surgery: Systematic Review for the American College of Physicians. Ann. Intern. Med. 2006, 144, 581–595. [Google Scholar] [CrossRef]
- Nafiu, O.O.; Ramachandran, S.K.; Ackwerh, R.; Tremper, K.K.; Campbell, D.A.; Stanley, J.C. Factors Associated with and Consequences of Unplanned Post-Operative Intubation in Elderly Vascular and General Surgery Patients. Eur. J. Anaesthesiol. 2011, 28, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.K.; Nafiu, O.O.; Ghaferi, A.; Tremper, K.K.; Shanks, A.; Kheterpal, S. Independent Predictors and Outcomes of Unanticipated Early Postoperative Tracheal Intubation after Nonemergent, Noncardiac Surgery. Anesthesiology 2011, 115, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ireland, C.J.; Chapman, T.M.; Mathew, S.F.; Herbison, G.P.; Zacharias, M. Continuous Positive Airway Pressure (CPAP) during the Postoperative Period for Prevention of Postoperative Morbidity and Mortality Following Major Abdominal Surgery. Cochrane Database Syst. Rev. 2014, 2014, CD008930. [Google Scholar] [CrossRef]
- Cammarota, G.; Simonte, R.; De Robertis, E. Comfort During Non-Invasive Ventilation. Front. Med. 2022, 9, 874250. [Google Scholar] [CrossRef]
- Helviz, Y.; Einav, S. A Systematic Review of the High-Flow Nasal Cannula for Adult Patients. Crit. Care 2018, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, L.; Pan, K.; Zhou, J.; Huang, X. High-Flow Nasal Cannula Therapy for Adult Patients. J. Int. Med. Res. 2016, 44, 1200–1211. [Google Scholar] [CrossRef]
- Lee, C.C.; Perez, O.; Farooqi, F.I.; Akella, T.; Shaharyar, S.; Elizee, M. Use of High-Flow Nasal Cannula in Obese Patients Receiving Colonoscopy under Intravenous Propofol Sedation: A Case Series. Respir. Med. Case Rep. 2018, 23, 118–121. [Google Scholar] [CrossRef]
- Gaunt, K.A.; Spilman, S.K.; Halub, M.E.; Jackson, J.A.; Lamb, K.D.; Sahr, S.M. High-Flow Nasal Cannula in a Mixed Adult ICU. Respir. Care 2015, 60, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for Definitions and Use of Outcome Measures for Clinical Effectiveness Research in Perioperative Medicine. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Cochrane: London, UK, 2022. [Google Scholar]
- Abbas, A.; Hefnawy, M.T.; Negida, A. Meta-Analysis Accelerator: A Comprehensive Tool for Statistical Data Conversion in Systematic Reviews with Meta-Analysis. BMC Med. Res. Methodol. 2024, 24, 243. [Google Scholar] [CrossRef]
- Allam, A.A.N.N.A.; Elsersi, M.H.S.A.; Elkady, G.A.M.A.; Hafez, A.F.; Algendy, M.A.A. A Comparative Study between High-Flow Nasal Oxygen Therapy and Venturi Mask Oxygen Therapy for Postoperative Laparoscopic Bariatric Surgery Patients with Atelectasis: A Randomized Clinical Trial. Ain-Shams J. Anesthesiol. 2022, 14, 38. [Google Scholar] [CrossRef]
- Brainard, J.; Scott, B.K.; Sullivan, B.L.; Fernandez-Bustamante, A.; Piccoli, J.R.; Gebbink, M.G.; Bartels, K. Heated Humidified High-Flow Nasal Cannula Oxygen after Thoracic Surgery—A Randomized Prospective Clinical Pilot Trial. J. Crit. Care 2017, 40, 225–228. [Google Scholar] [CrossRef]
- Burra, V.; Putta, G.; Prasad, S.R.; Manjunatha, N. A Prospective Study on Use of Thrive (Transnasal Humidified Rapid Insufflation Ventilatory Exchange) versus Conventional Nasal Oxygenation Following Extubation of Adult Cardiac Surgical Patients. Ann. Card. Anaesth. 2021, 24, 353–357. [Google Scholar] [CrossRef]
- Corley, A.; Bull, T.; Spooner, A.J.; Barnett, A.G.; Fraser, J.F. Direct Extubation onto High-Flow Nasal Cannulae Post-Cardiac Surgery versus Standard Treatment in Patients with a BMI ≥ 30: A Randomised Controlled Trial. Intensive Care Med. 2015, 41, 887–894. [Google Scholar] [CrossRef]
- Ferrando, C.; Puig, J.; Serralta, F.; Carrizo, J.; Pozo, N.; Arocas, B.; Gutierrez, A.; Villar, J.; Belda, F.J.; Soro, M. High-Flow Nasal Cannula Oxygenation Reduces Postoperative Hypoxemia in Morbidly Obese Patients: A Randomized Controlled Trial. Minerva Anestesiol. 2019, 85, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Frassanito, L.; Grieco, D.L.; Zanfini, B.A.; Catarci, S.; Rosà, T.; Settanni, D.; Fedele, C.; Scambia, G.; Draisci, G.; Antonelli, M. Effect of a Pre-Emptive 2-Hour Session of High-Flow Nasal Oxygen on Postoperative Oxygenation after Major Gynaecologic Surgery: A Randomised Clinical Trial. Br. J. Anaesth. 2023, 131, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.; Millar, J.E.; Merza, M.; Johnston, H.; Corley, A.; Faulke, D.; Rapchuk, I.L.; Tarpey, J.; Fanning, J.P.; Lockie, P.; et al. Prophylactic Postoperative High Flow Nasal Oxygen Versus Conventional Oxygen Therapy in Obese Patients Undergoing Bariatric Surgery (OXYBAR Study): A Pilot Randomised Controlled Trial. Obes. Surg. 2021, 31, 4799–4807. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Paugam-Burtz, C.; Godet, T.; Khoy-Ear, L.; Rozencwajg, S.; Delay, J.-M.; Verzilli, D.; Dupuis, J.; Chanques, G.; Bazin, J.-E.; et al. Effect of Early Postextubation High-Flow Nasal Cannula vs Conventional Oxygen Therapy on Hypoxaemia in Patients after Major Abdominal Surgery: A French Multicentre Randomised Controlled Trial (OPERA). Intensive Care Med. 2016, 42, 1888–1898. [Google Scholar] [CrossRef]
- Li, X.-N.; Zhou, C.-C.; Lin, Z.-Q.; Jia, B.; Li, X.-Y.; Zhao, G.-F.; Ye, F. High-Flow Nasal Cannula Oxygen Therapy during Anesthesia Recovery for Older Orthopedic Surgery Patients: A Prospective Randomized Controlled Trial. World J. Clin. Cases 2022, 10, 8615–8624. [Google Scholar] [CrossRef]
- Parke, R.; McGuinness, S.; Dixon, R.; Jull, A. Open-Label, Phase II Study of Routine High-Flow Nasal Oxygen Therapy in Cardiac Surgical Patients. Br. J. Anaesth. 2013, 111, 925–931. [Google Scholar] [CrossRef]
- Pennisi, M.A.; Bello, G.; Congedo, M.T.; Montini, L.; Nachira, D.; Ferretti, G.M.; Meacci, E.; Gualtieri, E.; De Pascale, G.; Grieco, D.L.; et al. Early Nasal High-Flow versus Venturi Mask Oxygen Therapy after Lung Resection: A Randomized Trial. Crit. Care 2019, 23, 68. [Google Scholar] [CrossRef]
- Sahin, M.; El, H.; Akkoç, I. Comparison of Mask Oxygen Therapy and High-Flow Oxygen Therapy after Cardiopulmonary Bypass in Obese Patients. Can. Respir. J. 2018, 2018, 1039635. [Google Scholar] [CrossRef]
- Soliman, H.A.Z.; Fikry, D.M.; El-Attar, A.M.; El Hadidy, M.S. High Flow Nasal Cannula Effect on Pulmonary Complications after Major Elective Upper Abdominal Surgeries: A Randomized Control Study. Egypt. J. Anaesth. 2022, 38, 656–664. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Wei, P.; Ruan, W.-Q.; Guo, J.; Yin, Z.-Y.; Li, X.; Song, J.-G. Randomized Controlled Trial Investigating the Impact of High-Flow Nasal Cannula Oxygen Therapy on Patients Undergoing Robotic-Assisted Laparoscopic Rectal Cancer Surgery, with a Post-Extubation Atelectasis as a Complication. J. Multidiscip. Heal. 2024, 17, 379–389. [Google Scholar] [CrossRef]
- Theologou, S.; Ischaki, E.; Zakynthinos, S.G.; Charitos, C.; Michopanou, N.; Patsatzis, S.; Mentzelopoulos, S.D. High Flow Oxygen Therapy at Two Initial Flow Settings versus Conventional Oxygen Therapy in Cardiac Surgery Patients with Postextubation Hypoxemia: A Single-Center, Unblinded, Randomized, Controlled Trial. J. Clin. Med. 2021, 10, 2079. [Google Scholar] [CrossRef]
- Vourc’h, M.; Nicolet, J.; Volteau, C.; Caubert, L.; Chabbert, C.; Lepoivre, T.; Senage, T.; Roussel, J.-C.; Rozec, B. High-Flow Therapy by Nasal Cannulae Versus High-Flow Face Mask in Severe Hypoxemia After Cardiac Surgery: A Single-Center Randomized Controlled Study—The HEART FLOW Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 157–165. [Google Scholar] [CrossRef]
- Yu, Y.; Qian, X.; Liu, C.; Zhu, C. Effect of High-Flow Nasal Cannula versus Conventional Oxygen Therapy for Patients with Thoracoscopic Lobectomy after Extubation. Can. Respir. J. 2017, 2017, 7894631. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chang, W.; Meng, S.-S.; Zhang, X.; Xie, J.; Xu, J.-Y.; Qiu, H.; Yang, Y.; Guo, F. Effect of High-Flow Nasal Cannula Oxygen Therapy Compared with Conventional Oxygen Therapy in Postoperative Patients: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e027523. [Google Scholar] [CrossRef]
- Zochios, V.; Klein, A.A.; Jones, N.; Kriz, T. Effect of High-Flow Nasal Oxygen on Pulmonary Complications and Outcomes After Adult Cardiothoracic Surgery: A Qualitative Review. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Wu, Q.; Xie, L.; Song, J.; Wu, X.; Hao, S.; Zhong, M.; Li, S. High Flow Nasal Cannula versus Conventional Oxygen Therapy in Postoperative Patients at High Risk for Pulmonary Complications: A Systematic Review and Meta-analysis. Int. J. Clin. Pract. 2021, 75, e13828. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, D.; Ni, Y.; Liang, Z. High-Flow Nasal Cannula vs Conventional Oxygen Therapy for Postcardiothoracic Surgery. Respir. Care 2020, 65, 1730–1737. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, H.; Zhang, R.; Wei, J. High-Flow Nasal Cannula Oxygen Therapy vs Conventional Oxygen Therapy in Cardiac Surgical Patients: A Meta-Analysis. J. Crit. Care 2017, 38, 123–128. [Google Scholar] [CrossRef]
- Wu, X.; Cao, W.; Zhang, B.; Wang, S. Effect of High-Flow Nasal Cannula Oxygen Therapy vs Conventional Oxygen Therapy on Adult Postcardiothoracic Operation. Medicine 2018, 97, e12783. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Study ID, Location | Inclusion Criteria | Clinical Setting | Target SpO2 | Number of Patients Total (H/C) |
|---|---|---|---|---|
| Allam [19], Egypt | Age 18–60 BMI > 40 Preop ASA III Postop atelectasis Laparoscopic sleeve gastrectomy | Bariatric surgery | N/A | 110 (55/55) |
| Brainard [20], United States | Age > 18 Postop thoracic surgery | Thoracic surgery | 90 | 44 (18/26) |
| Burra [21], India | Age 18–65 BMI < 30 Preop ASA < III Elective cardiac surgery | Cardiac surgery | N/A | 60 (30/30) |
| Corley [22], Australia | Age > 18 BMI > 30 Cardiac Surgery on cardiopulmonary bypass | Cardiac Surgery | 95 | 155 (81/75) |
| Ferrando [23], Spain | Age > 18 BMI > 35 ASA II–III Laparoscopic bariatric surgery | Bariatric surgery | N/A | 64 (32/32) |
| Frassanito [24], Italy | Age > 18 Female BMI < 35 Laparoscopic gynae surgery > 2 h | Gynaecological Surgery | 94 | 83 (42/41) |
| Fulton [25], Australia | Age > 18 BMI > 30 Laparoscopic bariatric surgery | Bariatric surgery | 95 | 50 (25/25) |
| Futier [26], France | BMI < 35 Surgery > 2 H Intermediate–High-risk PPC | Abdominal Surgery | 95 | 220 (108/112) |
| Li [27], China | Age > 65 ASA I–III | Orthopedic surgery | 90 | 60 (30/30) |
| Parke [28], New Zealand | Age > 18 Surgery with full median sternotomy | Cardiac surgery | 93 | 340 (169/171) |
| Pennisi [29], Italy | BMI < 35 Elective thoracotomic pulmonary lobar resection | Thoracic surgery | 92 | 95 (47/48) |
| Sahin [30], Turkey | Age > 18 BMI > 30 | Cardiac surgery | 93 | 100 (50/50) |
| Soliman [31], Egypt | Age 50–70 BMI < 35 ASA I–III Major elective upper abdomen procedures | Upper abdominal surgery | 94 | 80 (40/40) |
| Sun [32], China | BMI > 18 and < 30 Robotic-assisted laparoscopic rectal cancer surgery | Colorectal surgery | 95 | 78 (39/39) |
| Theologou [33], France | Age > 18 Spontaneous Breathing Trial PF ratio < 200 Elective or urgent cardiac surgery | Cardiac surgery | 92 | 99 (66/33) |
| Vourc’h [34], France | SpO2 < 96% after extubation with Venturi mask FiO2 0.5 | Cardiac surgery | 96 | 82 (41/41) |
| Yu [35], China | Age 18–80 Intermediate–High-risk PPCs Thoracoscopic lobectomy for lung tumour | Thoracic surgery | 95 | 110 (56/54) |
| Study ID, Location | Duration of Intervention | Intervention Details (Flow and FiO2) | Control Details | Therapy Algorithm | Outcomes |
|---|---|---|---|---|---|
| Allam [19], Egypt | 24 h | 30 L/min FiO2 60% | VM FiO2 60% | / | ①③ |
| Brainard [20], United States | 48 h | 40 L/min | NP or FM | ②③④ | |
| Burra [21], India | 4 h | 60 L/min | NP 4 L/min | ③⑤ | |
| Corley [22], Australia | 5 days | 35–50 L/min | NP 2–4 L/min or FM 6 L/min | ①③⑤ | |
| Ferrando [23], Spain | 3 h | 60 L/min FiO2 50% | VM 15 L/min FiO2 50% | / | ①②③⑤ |
| Frassanito [24], Italy | 2 h | 60 L/min FiO2 30% | VM 35% | / | ①②⑤ |
| Fulton [25], Australia | 6 h | 50 L/min FiO2 50% | FM 6 L/min | / | ①③④ |
| Futier [26], France | 24 h | 50–60 L/min | NP or FM | ②③④⑤ | |
| Li [27], China | 1 h | 40 L/min FiO2 60% | FM 2 L/min O2 + 2 L/min air | ② | |
| Parke [28], New Zealand | 72 h | 45 L/min | NP or FM 2–4 L/min | ③④ | |
| Pennisi [29], Italy | 48 h | 50 L/min FiO2 40 ± 5% | VM 8 L/min ± 1 | ①②③④⑤ | |
| Sahin [30], Turkey | 48 h | 25–40 L/min FiO2 50% | FM 2–4 L/min | ②③④⑤ | |
| Soliman [31], Egypt | 48 h | 35–60 L/min | FM 6–10 L/min | ①②③④ | |
| Sun [32], China | 30 L/min FiO2 50% | NP 4 L/min | ②③④ | ||
| Theologou [33], France | 48 h | 60 L/min FiO2 60% 40 L/min FiO2 60% | VM 15 L/min FiO2 60% | / | ②③④⑤ |
| Vourc’h [34], France | 48 h | 45 L/min FiO2 100% | HFFM 15 L/min | / | ①③⑤ |
| Yu [35], China | 72 h | 35–60 L/min FiO2 45–100% | NP or FM | ②③④⑤ |
| Study | Group | N | Escalation to HFNOT | Escalation to NIV | Reintubation | Total Escalations (%) | |
|---|---|---|---|---|---|---|---|
| Corley [22] | HFNOT | 81 | 0 | 3 | 0 | 3 | 3.7 |
| COT | 75 | 3 | 1 | 1 | 5 | 6.7 | |
| Fulton [25] | HFNOT | 25 | 0 | 0 | 0 | 0 | 0 |
| COT | 25 | 1 | 0 | 0 | 1 | 4.0 | |
| Futier [26] | HFNOT | 108 | N/A | N/A | 20 | 20 | 18.5 |
| COT | 112 | N/A | N/A | 14 | 14 | 12.5 | |
| Parke [28] | HFNOT | 169 | 7 | 9 | 2 | 18 | 10.7 |
| COT | 171 | 12 | 5 | 0 | 17 | 9.9 | |
| Pennisi [29] | HFNOT | 47 | 0 | 1 | 1 | 2 | 4.3 |
| COT | 48 | 0 | 3 | 1 | 4 | 8.3 | |
| Sahin [30] | HFNOT | 50 | 0 | 6 | 0 | 6 | 12.0 |
| COT | 50 | 0 | 11 | 4 | 15 | 30.0 | |
| Soliman [31] | HFNOT | 40 | 0 | 1 | 0 | 1 | 2.5 |
| COT | 40 | 0 | 3 | 2 | 5 | 12.5 | |
| Vourc’h [34] | HFNOT | 41 | 0 | 13 | 3 | 16 | 39.0 |
| COT | 41 | 0 | 24 | 1 | 25 | 61.0 | |
| Yu [35] | HFNOT | 56 | 0 | 2 | 0 | 2 | 3.6 |
| COT | 54 | 0 | 9 | 5 | 14 | 25.9 | |
| Outcome | No of Studies | HFNOT (n) | COT (n) | Mean Difference (95% CI) | p-Value | I2 |
|---|---|---|---|---|---|---|
| Duration of intervention | ||||||
| Less than 24 h | 4 | 154 | 154 | 45.45 [27.92, 56.97] | <0.00001 | 86 |
| More than 24 h | 3 | 128 | 129 | 10.51 [0.26, 20.75] | 0.04 | 26 |
| Type of surgery | ||||||
| Cardiothoracic | 2 | 88 | 89 | 6.37 [−5.00, 17.75] | 0.27 | 0 |
| Non-cardiothoracic | 5 | 194 | 193 | 38.50 [26.13, 50.88] | <0.00001 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.W.Y.; Izaham, A.; Abd Rahman, R.; Teo, R.; Masri, S.N.N.S.; Md Ralib, A.; Chin, K.-Y. High-Flow Nasal Oxygen Therapy in Preventing Post-Extubation Hypoxaemia and Postoperative Pulmonary Complications: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 2449. https://doi.org/10.3390/diagnostics15192449
Tan JWY, Izaham A, Abd Rahman R, Teo R, Masri SNNS, Md Ralib A, Chin K-Y. High-Flow Nasal Oxygen Therapy in Preventing Post-Extubation Hypoxaemia and Postoperative Pulmonary Complications: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(19):2449. https://doi.org/10.3390/diagnostics15192449
Chicago/Turabian StyleTan, Jamie Wen Yen, Azarinah Izaham, Raha Abd Rahman, Rufinah Teo, Syarifah Noor Nazihah Sayed Masri, Azrina Md Ralib, and Kok-Yong Chin. 2025. "High-Flow Nasal Oxygen Therapy in Preventing Post-Extubation Hypoxaemia and Postoperative Pulmonary Complications: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 19: 2449. https://doi.org/10.3390/diagnostics15192449
APA StyleTan, J. W. Y., Izaham, A., Abd Rahman, R., Teo, R., Masri, S. N. N. S., Md Ralib, A., & Chin, K.-Y. (2025). High-Flow Nasal Oxygen Therapy in Preventing Post-Extubation Hypoxaemia and Postoperative Pulmonary Complications: A Systematic Review and Meta-Analysis. Diagnostics, 15(19), 2449. https://doi.org/10.3390/diagnostics15192449