Maresin-1 and S-Equol as Emerging Metabolic Biomarkers in Gestational Diabetes-Associated Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Storage of Biological Samples
2.2. The Manufacturer Information and Analysis of Biological Samples via ELISA
- TMAO: Human TMAO ELISA Kit (Shanghai Sunredbio Technology Co., Ltd., Shanghai, China, Cat. No: 201-12-7378); sensitivity: 0.043 ng/mL, detection range: 0.05–10 ng/mL
- Indoxyl Sulfate (IS): Human IS ELISA Kit (Shanghai Sunredbio Technology Co., Ltd., Shanghai, China, Cat. No: 201-12-7596); sensitivity: 1.854 µg/mL, detection range: 2–600 µg/mL
- Maresin-1 (MaR-1): Human MaR-1 ELISA Kit (Shanghai Sunredbio Technology Co., Ltd., Shanghai, China, Cat. No: 201-12-7339); sensitivity: 28.625 ng/L, detection range: 30–9000 ng/L
- S-Equol: Human S-Equol ELISA Kit (Shanghai Sunredbio Technology Co., Ltd., Shanghai, China, Cat. No: 201-12-8142); sensitivity: 0.247 ng/mL, detection range: 0.25–70 ng/mL
- High-Sensitivity CRP (hs-CRP): Human hs-CRP ELISA Kit (Shanghai Sunredbio Technology Co., Ltd., Shanghai, China, Cat. No: 201-12-1806); sensitivity: 0.112 mg/L, detection range: 0.15–40 mg/L
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| CRP | C-reactive protein |
| DHA | Docosahexaenoic acid |
| DM | Diabetes mellitus |
| EPA | Eicosapentaenoic acid |
| FUBAP | Firat university scientific research projects unit |
| GDM | Gestational diabetes mellitus |
| Hs-CRP | High-sensitivity c-reactive protein |
| IS | Indoxyl sulfate |
| MaR-1 | Maresin-1 |
| RAAS | Renin–angiotensin–aldosterone system |
| SPM | Specialized pro-resolving mediators |
| TMA | Trimethylamine |
| TMAO | Trimethylamine n-oxide |
| Th1 | T-helper 1 cell |
References
- Mittal, R.; Prasad, K.; Lemos, J.R.N.; Arevalo, G.; Hirani, K. Unveiling Gestational Diabetes: An Overview of Pathophysiology and Management. Int. J. Mol. Sci. 2025, 26, 2320. [Google Scholar] [CrossRef]
- Pedersen, J. Diabetes and Pregnancy: Blood Sugar of Newborn Infants. Ph.D. Thesis, University of Copenhagen, København, Denmark, 1952. [Google Scholar]
- Habibi, N.; Mousa, A.; Tay, C.T.; Khomami, M.B.; Patten, R.K.; Andraweera, P.H.; Wassie, M.; Vandersluys, J.; Aflatounian, A.; Bianco-Miotto, T.; et al. Maternal metabolic factors and the association with gestational diabetes: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2022, 38, e3532. [Google Scholar] [CrossRef]
- Skajaa, G.O.; Fuglsang, J.; Knorr, S.; Møller, N.; Ovesen, P.; Kampmann, U. Changes in insulin sensitivity and insulin secretion during pregnancy and post partum in women with gestational diabetes. BMJ Open Diabetes Res. Care. 2020, 8, e001728. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Huston Presley, L.P.; Locascio, J.J.; Catalano, P.M. Augmented insulin secretory response in early pregnancy. Diabetologia 2019, 62, 1445–1452. [Google Scholar] [CrossRef]
- Sorenson, R.L.; Brelje, T.C. Adaptation of islets of Langerhans to pregnancy: Beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm. Metab. Res. 1997, 29, 301–307. [Google Scholar] [CrossRef]
- Association, A.D. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- Brial, F.; Le Lay, A.; Dumas, M.E.; Gauguier, D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell Mol. Life Sci. 2018, 75, 3977–3990. [Google Scholar] [CrossRef] [PubMed]
- Battson, M.L.; Lee, D.M.; Weir, T.L.; Gentile, C.L. The gut microbiota as a novel regulator of cardiovascular function and disease. J. Nutr. Biochem 2018, 56, 1–15. [Google Scholar] [CrossRef]
- Papandreou, C.; Moré, M.; Bellamine, A. Trimethylamine N-Oxide in Relation to Cardiometabolic Health-Cause or Effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, Y.; Yuan, S.; Cai, X.; He, Y.; Chen, J.; Wu, Q.; He, D.; Fang, A.; Bo, Y.; et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: An umbrella review and updated meta-analysis. Am. J. Clin. Nutr. 2022, 116, 230–243. [Google Scholar] [CrossRef]
- Arslan, S.; Kaya, M.K.; Aydin, S.; Aydin, S. Trimethylamine N-oxide, S-equol, and indoxyl sulfate inflammatory microbiota players in ocular Behçet’s disease. Turk. J. Biochem. 2025, 50, 73–79. [Google Scholar] [CrossRef]
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. 2018, 132, 509–522. [Google Scholar] [CrossRef]
- Aoki, K.; Teshima, Y.; Kondo, H.; Saito, S.; Fukui, A.; Fukunaga, N.; Nawata, T.; Shimada, T.; Takahashi, N.; Shibata, H. Role of indoxyl sulfate as a predisposing factor for atrial fibrillation in renal dysfunction. J. Am. Heart Assoc. 2015, 4, e002023. [Google Scholar] [CrossRef]
- Atoh, K.; Itoh, H.; Haneda, M. Serum indoxyl sulfate levels in patients with diabetic nephropathy: Relation to renal function. Diabetes Res. Clin. Pract. 2009, 83, 220–226. [Google Scholar] [CrossRef]
- Lu, C.L.; Zheng, C.M.; Lu, K.C.; Liao, M.T.; Wu, K.L.; Ma, M.C. Indoxyl-Sulfate-Induced Redox Imbalance in Chronic Kidney Disease. Antioxidants 2021, 10, 936. [Google Scholar] [CrossRef]
- Chern, Y.B.; Tsai, J.P.; Liu, C.H.; Lin, Y.L.; Wang, C.H.; Hsu, B.G. Serum Indoxyl Sulfate as a Potential Biomarker of Peripheral Arterial Stiffness in Patients with Non-Dialysis Chronic Kidney Disease Stages 3 to 5. Toxins 2025, 17, 283. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Martínez, M.S.; Chávez, M.; Toledo, A.; Añez, R.; Torres, Y.; Apruzzese, V.; Silva, C.; Rojas, J.; Bermúdez, V. C-reactive protein: Clinical and epidemiological perspectives. Cardiol. Res. Pract. 2014, 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Yun, J.M.; Adamson, G.; Galvez, J.; Jialal, I. C-reactive protein impairs the endothelial glycocalyx resulting in endothelial dysfunction. Cardiovasc. Res. 2009, 84, 479–484. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandris, C.; Lauro, R.; Presta, I.; Sesti, G. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby impairing the insulin signalling pathway that promotes glucose transport. Diabetologia 2007, 50, 840–849. [Google Scholar] [CrossRef]
- Mir, M.M.; Jeelani, M.; Alharthi, M.H.; Rizvi, S.F.; Sohail, S.K.; Wani, J.I.; Sabah, Z.U.; BinAfif, W.F.; Nandi, P.; Alshahrani, A.M.; et al. Unraveling the Mystery of Insulin Resistance: From Principle Mechanistic Insights and Consequences to Therapeutic Interventions. Int. J. Mol. Sci. 2025, 26, 2770. [Google Scholar] [CrossRef]
- Lowe, G.D.; Woodward, M.; Rumley, A.; Morrison, C.E.; Nieuwenhuizen, W. Associations of plasma fibrinogen assays, C-reactive protein and interleukin-6 with previous myocardial infarction. J. Thromb. Haemost. 2003, 1, 2312–2316. [Google Scholar] [CrossRef][Green Version]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediators Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef]
- Bedi, G.N.; Acharya, S.; Kumar, S.; Mapari, S.A. Salivary High-Sensitivity C-Reactive Protein and Its Clinical Relevance in Modern Medicine: A Comprehensive Review. Cureus 2024, 16, e58165. [Google Scholar] [CrossRef]
- Rothkrantz-Kos, S.; Bekers, O.; Gubbels, A.; Drent, M.; Schmitz, M.P.; van Dieijen-Visser, M.P. Evaluation of two new high-sensitivity methods for C-reactive protein. Ann. Clin. Biochem. 2003, 40, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Zhu, M.; Vlasenko, N.A.; Deng, B.; Haeggström, J.Z.; Petasis, N.A.; Serhan, C.N. The novel 13S, 14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013, 27, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wang, C.W.; Arnardottir, H.H.; Li, Y.; Cheng, C.Y.; Dalli, J.; Serhan, C.N. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS ONE 2014, 9, e102362. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Invest. 2019, 129, 5294–5311. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Yu, S.H.; Fretwurst, T.; Larsson, L.; Sugai, J.V.; Oh, J.; Lehner, K.; Jin, Q.; Giannobile, W.V. Maresin 1 Promotes Wound Healing and Socket Bone Regeneration for Alveolar Ridge Preservation. J. Dent. Res. 2020, 99, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Su, W.; Zhao, Y.; Wei, Z.; Hua, Y.; Xue, P.; Zhu, X.; Chen, Y.; Chen, G. Maresin 1 promotes nerve regeneration and alleviates neuropathic pain after nerve injury. J. Neuroinflammation. 2022, 19, 32. [Google Scholar]
- Marrian, G.F.; Haslewood, G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932, 26, 1227–1232. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Hod, R.; Maniam, S.; Mohd Nor, N.H. A Systematic Review of the Effects of Equol (Soy Metabolite) on Breast Cancer. Molecules 2021, 26, 1105. [Google Scholar] [CrossRef]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef]
- Setchell, K.D.; Zhao, X.; Jha, P.; Heubi, J.E.; Brown, N.M. The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am. J. Clin. Nutr. 2009, 90, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.; Shoelson, S. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Carson, A.P.; Reynolds, K.; Fonseca, V.A.; Muntner, P. Comparison of A1C and Fasting Glucose Criteria to Diagnose Diabetes Among U.S. Adults. Diabetes Care. 2010, 33, 95–97. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. 1), S181–S206. [Google Scholar] [CrossRef]
- Basturk, B.; Koc Ozerson, Z.; Yuksel, A. Evaluation of the Effect of Macronutrients Combination on Blood Sugar Levels in Healthy Individuals. Iran J. Public Health. 2021, 50, 280–287. [Google Scholar] [CrossRef]
- Aydin, S.; Emre, E.; Ugur, K.; Aydin, M.A.; Sahin, İ.; Cinar, V.; Akbulut, T. An overview of ELISA: A review and update on best laboratory practices for quantifying peptides and proteins in biological fluids. J. Int. Med. Res. 2025, 53, 3000605251315913. [Google Scholar] [CrossRef]
- Xiong, X.; Saunders, L.D.; Wang, F.L.; Demianczuk, N.N. Gestational diabetes mellitus: Prevalence, risk factors, maternal and infant outcomes. Int. J. Gynecol. Obstet. 2001, 75, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Iman, A.E.H.; Huniadi, A.; Sandor, M.; Zaha, I.A.; Rotar, I.; Iuhas, C. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Romania: Maternal and Fetal Outcomes. Medicina 2025, 61, 194. [Google Scholar] [CrossRef]
- Roy, S.; Yuzefpolskaya, M.; Nandakumar, R.; Colombo, P.C.; Demmer, R.T. Plasma trimethylamine-N-oxide and impaired glucose regulation: Results from the oral infections, glucose intolerance and insulin resistance study (Origins). PLoS ONE 2020, 15, e0227482. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef]
- Burton, K.J.; Krüger, R.; Scherz, V.; Münger, L.H.; Picone, G.; Vionnet, N.; Bertelli, C.; Greub, G.; Capozzi, F.; Vergères, G. Trimethylamine-N-Oxide Postprandial Response in Plasma and Urine Is Lower After Fermented Compared to Non-Fermented Dairy Consumption in Healthy Adults. Nutrients 2020, 12, 234. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, J.; Wang, Z.; Xu, S.; Zhang, Q.; Duan, Z.; Tan, R.; Qi, X.; Guo, J.; Chang, X.; et al. Associations of Diet with Urinary Trimethylamine-N-Oxide (TMAO) and Its Precursors among Free-Living 10-Year-Old Children: Data from SMBCS. Nutrients 2022, 14, 3419. [Google Scholar] [CrossRef] [PubMed]
- Kymberleigh, A.; Romano, K.A.; Vivas, E.J.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-Oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metabol. 2019, 30, 1141–1151. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Xu, J.; Xue, C.; Xue, Y.; Wang, Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J. Biosci. Bioeng. 2014, 118, 476–481. [Google Scholar] [CrossRef]
- Parkinson, A. Biotransformation of Xenobiotics. In Casarett and Doull’s Toxicology, 5th ed.; Klaasen, C.D., Ed.; McGraw-Hill: New York, NY, USA, 1996; pp. 113–186. [Google Scholar]
- Wakabayashi, I.; Marumo, M. Evidence for Indoxyl Sulfate as an Inducer of Oxidative Stress in Patients with Diabetes. In Vivo 2022, 36, 1790–1794. [Google Scholar] [CrossRef]
- Patney, N.L.; Saxena, S.K.; Mehrotra, M.P.; Khanna, H.K.; Kumar, A. Urinary indican in diabetes mellitus. J. Indian Med. Assoc. 1977, 68, 94–97. [Google Scholar] [PubMed]
- Leipold, H.; Worda, C.; Gruber, C.J.; Prikoszovich, T.; Wagner, O.; Kautzky-Willer, A. Gestational diabetes mellitus is associated with increased C-reactive protein concentrations in the third but not second trimester. Eur. J. Clin. Invest. 2005, 35, 752–757. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Schulze, F.; Wehner, J.; Kratschmar, D.V.; Makshana, V.; Meier, D.T.; Häuselmann, S.P.; Dalmas, E.; Thienel, C.; Dror, E.; Wiedemann, S.J.; et al. Inhibition of IL-1beta improves Glycaemia in a Mouse Model for Gestational Diabetes. Sci. Rep. 2020, 10, 3035. [Google Scholar] [CrossRef]
- Clària, J.; Nguyen, B.T.; Madenci, A.L.; Ozaki, C.K.; Serhan, C.N. Diversity of lipid mediators in human adipose tissue depots. Am. J. Physiol. Cell Physiol. 2013, 304, 1141–1149. [Google Scholar] [CrossRef]
- León, I.C.; Quesada-Vázquez, S.; Sáinz, N.; Guruceaga, E.; Escoté, X.; Moreno-Aliaga, M.J. Effects of Maresin 1 (MaR1) on Colonic Inflammation and Gut Dysbiosis in Diet-Induced Obese Mice. Microorganisms 2020, 8, 1156. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, L.; González-Muniesa, P.; Sáinz, N.; Escoté, X.; Martínez, J.A.; Arbones-Mainar, J.M.; Moreno-Aliaga, M.J. Maresin 1 regulates insulin signaling in human adipocytes as well as in adipose tissue and muscle of lean and obese mice. J. Physiol. Biochem. 2021, 77, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lu, Y.; Tian, H.; Alapure, B.V.; Wang, Q.; Bunnell, B.A.; Laborde, J.M. Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem. Biol. 2014, 21, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Harada, N.; Adachi, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-Equol enantioselectively activates cAMP-protein kinase A signaling and reduces alloxan-induced cell death in INS-1 pancreatic β-cells. J. Nutr. Sci. Vitaminol. 2014, 60, 291–296. [Google Scholar] [CrossRef]
- Horiuchi, H.; Usami, A.; Shirai, R.; Harada, N.; Ikushiro, S.; Sakaki, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-Equol Activates cAMP Signaling at the Plasma Membrane of INS-1 Pancreatic β-Cells and Protects against Streptozotocin-Induced Hyperglycemia by Increasing β-Cell Function in Male Mice. J. Nutr. 2017, 147, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. 2013, 78, 365–372. [Google Scholar] [CrossRef]
- Li, Y.; Hansotia, T.; Yusta, B.; Ris, F.; Halban, P.A.; Drucker, D.J. Glucagon-like peptide-1 receptor signaling modulates β cell apoptosis. J. Biol. Chem. 2003, 278, 471–478. [Google Scholar] [CrossRef]
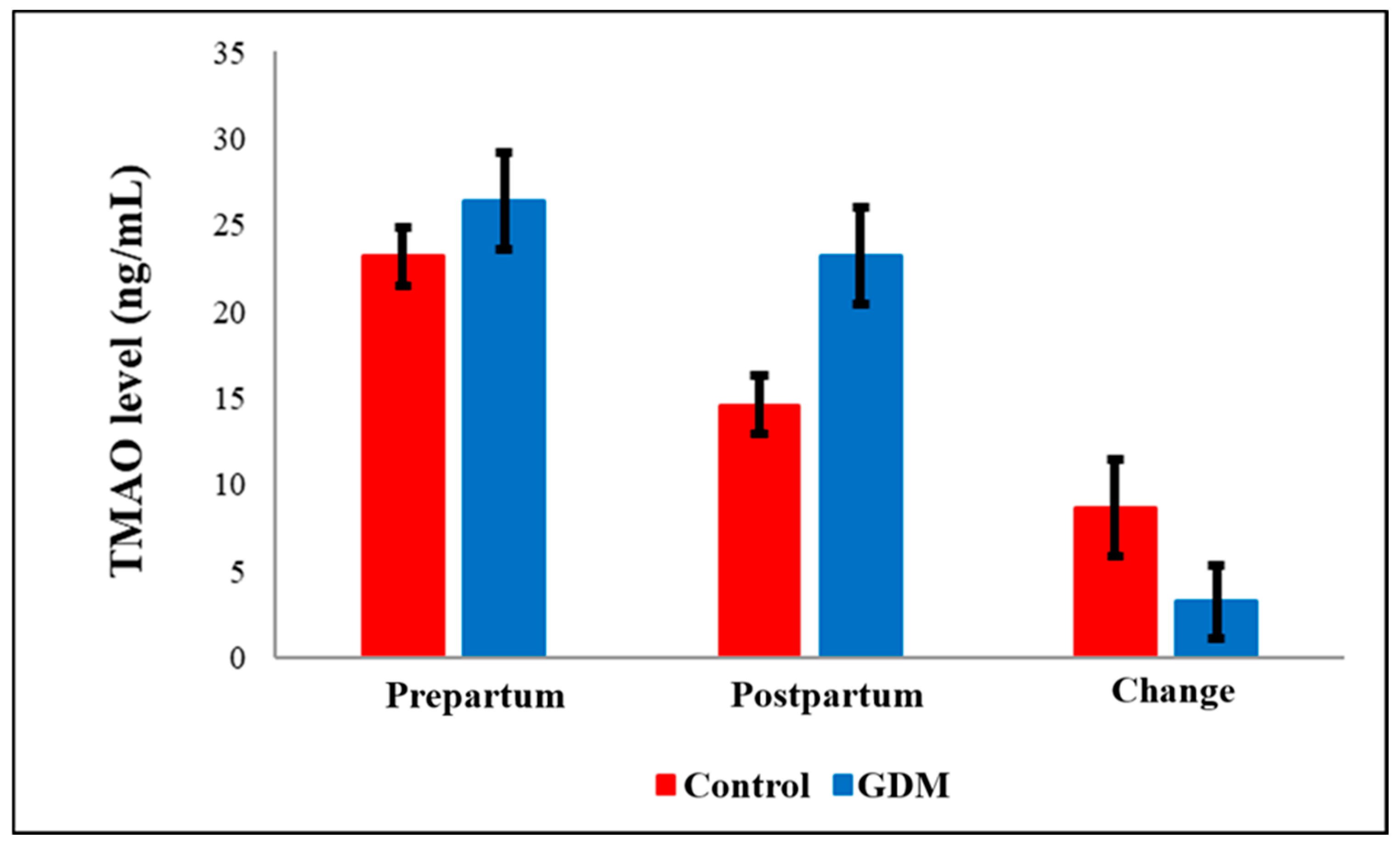




| Parameters | Control (n = 22) | GDM (n = 22) | p |
|---|---|---|---|
| Age (year) | 30.7 ± 4.6 | 33.1 ± 5.2 | 0.113 * |
| Fetus weight (g) | 1285.2 ± 121.9 | 1052.5 ± 210.3 | <0.001 * |
| Birth week | 37.5 ± 1.2 | 35.2 ± 2.8 | 0.005 ** |
| Birth weight (g) | 3555.7 ± 320.3 | 2945.0 ± 667.3 | 0.001 ** |
| Pre-pregnancy BMI (kg/m2) | 23.8 ± 2.7 | 24.3 ± 2.9 | 0.372 * |
| Maternal BMI (kg/m2) | 30.0 ± 3.5 | 31.0 ± 3.1 | 0.306 * |
| Weigh gain during pregnancy (kg) | 12.9 ± 1.1 | 13.7 ± 1.7 | 0.47 |
| Parity | 2 | 2 | …… |
| Family history of diabetes | None | None | …… |
| Parameters | Age | Fetus Weight | Birth Week | Birth Weight | Maternal BMI | Pre TMAO | Pre IS | Pre Hs-CRP | Pre MaR-1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fetus weight | r | −0.037 | ||||||||
| p | 0.871 | |||||||||
| Birth week | r | 0.163 | −0.417 | |||||||
| p | 0.470 | 0.054 | ||||||||
| Birth weight | r | 0.262 | 0.395 | −0.230 | ||||||
| p | 0.240 | 0.069 | 0.304 | |||||||
| Maternal BMI | r | −0.128 | −0.123 | −0.282 | −0.035 | |||||
| p | 0.572 | 0.586 | 0.203 | 0.877 | ||||||
| Pre TMAO | r | −0.140 | 0.064 | 0.111 | −0.121 | −0.019 | ||||
| p | 0.534 | 0.776 | 0.624 | 0.592 | 0.934 | |||||
| Pre IS | r | −0.015 | 0.118 | −0.339 | −0.028 | 0.340 | 0.256 | |||
| p | 0.946 | 0.602 | 0.123 | 0.902 | 0.122 | 0.249 | ||||
| Pre Hs-CRP | r | −0.037 | −0.368 | 0.191 | 0.019 | 0.081 | 0.049 | −0.250 | ||
| p | 0.869 | 0.092 | 0.395 | 0.934 | 0.721 | 0.828 | 0.262 | |||
| Pre MaR-1 | r | −0.003 | −0.031 | 0.158 | −0.133 | −0.163 | 0.201 | 0.188 | −0.003 | |
| p | 0.988 | 0.893 | 0.483 | 0.556 | 0.469 | 0.371 | 0.402 | 0.990 | ||
| Pre S-Equol | r | 0.119 | 0.034 | 0.021 | −0.075 | −0.086 | 0.154 | 0.239 | 0.025 | 0.874 |
| p | 0.598 | 0.880 | 0.925 | 0.741 | 0.702 | 0.493 | 0.283 | 0.911 | 0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yavuzkir, S.; Cinar, D.K.; Cinar, A.; Bildirici, F.; Aydin, S. Maresin-1 and S-Equol as Emerging Metabolic Biomarkers in Gestational Diabetes-Associated Inflammation. Diagnostics 2025, 15, 2439. https://doi.org/10.3390/diagnostics15192439
Yavuzkir S, Cinar DK, Cinar A, Bildirici F, Aydin S. Maresin-1 and S-Equol as Emerging Metabolic Biomarkers in Gestational Diabetes-Associated Inflammation. Diagnostics. 2025; 15(19):2439. https://doi.org/10.3390/diagnostics15192439
Chicago/Turabian StyleYavuzkir, Seyda, Derya Kardas Cinar, Ahmet Cinar, Furkan Bildirici, and Suleyman Aydin. 2025. "Maresin-1 and S-Equol as Emerging Metabolic Biomarkers in Gestational Diabetes-Associated Inflammation" Diagnostics 15, no. 19: 2439. https://doi.org/10.3390/diagnostics15192439
APA StyleYavuzkir, S., Cinar, D. K., Cinar, A., Bildirici, F., & Aydin, S. (2025). Maresin-1 and S-Equol as Emerging Metabolic Biomarkers in Gestational Diabetes-Associated Inflammation. Diagnostics, 15(19), 2439. https://doi.org/10.3390/diagnostics15192439







