Uncommon Cause of Pulmonary Hypertension: Imaging Diagnosis of Cardiac Myxoma Embolism
Abstract
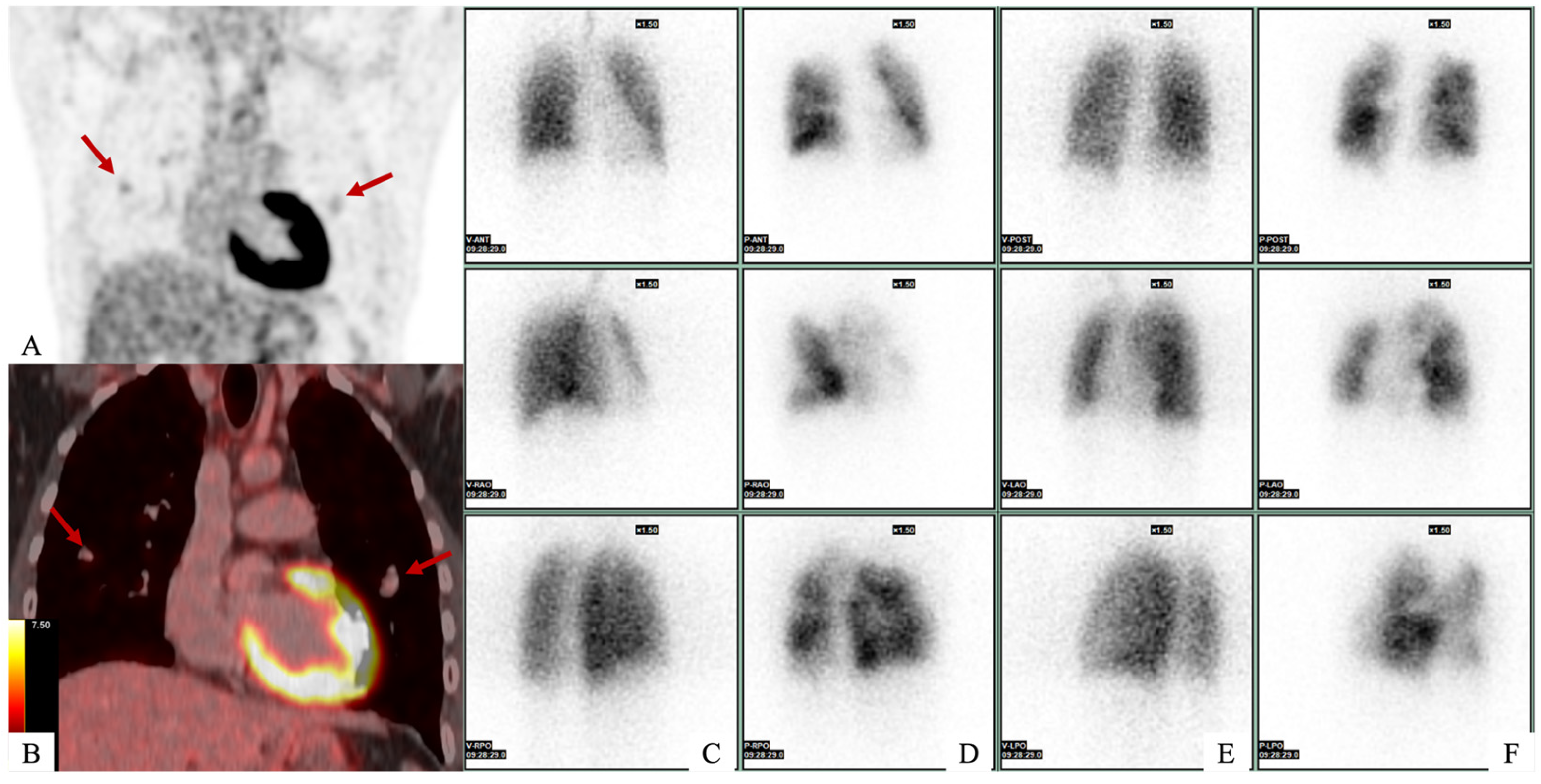
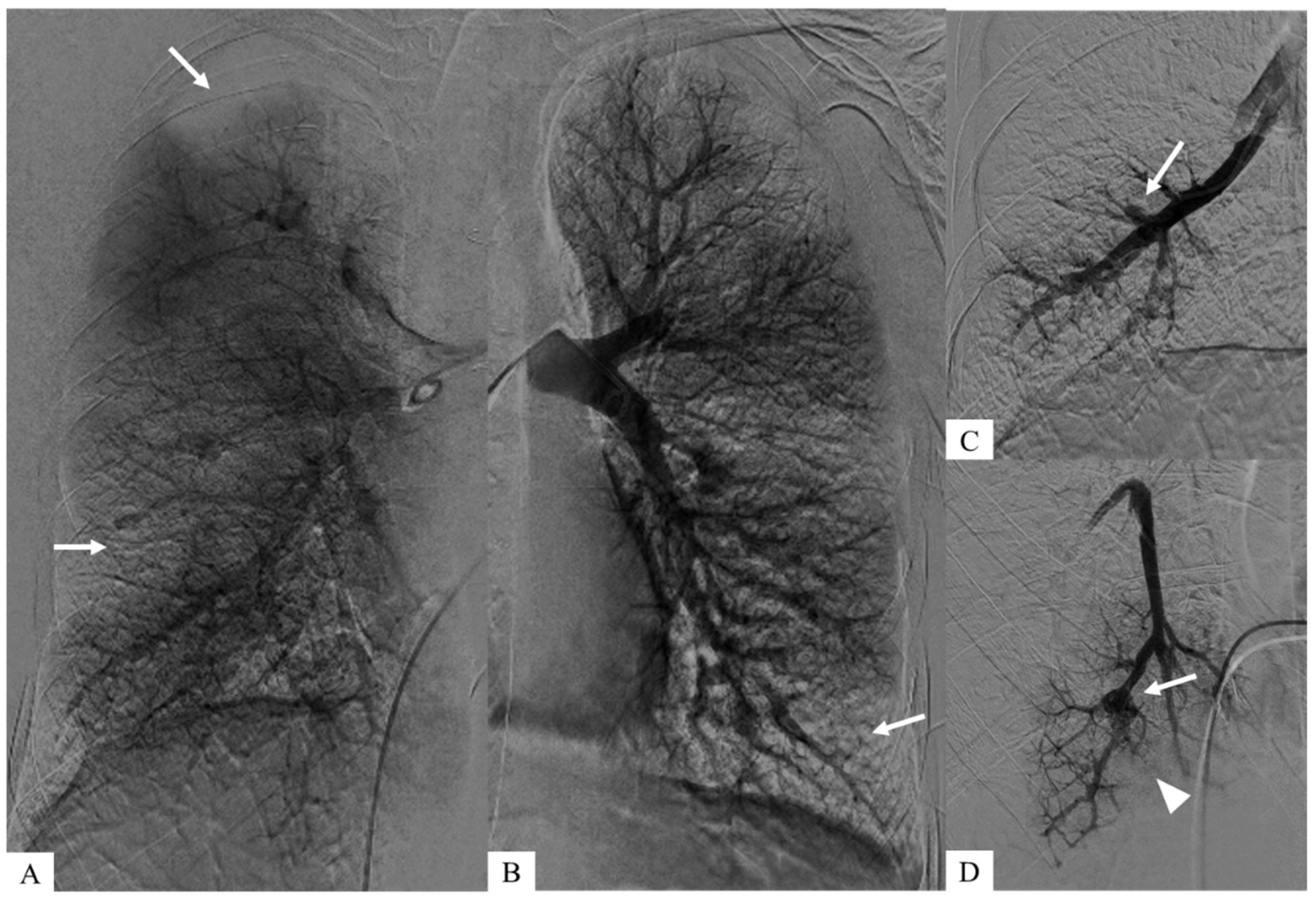
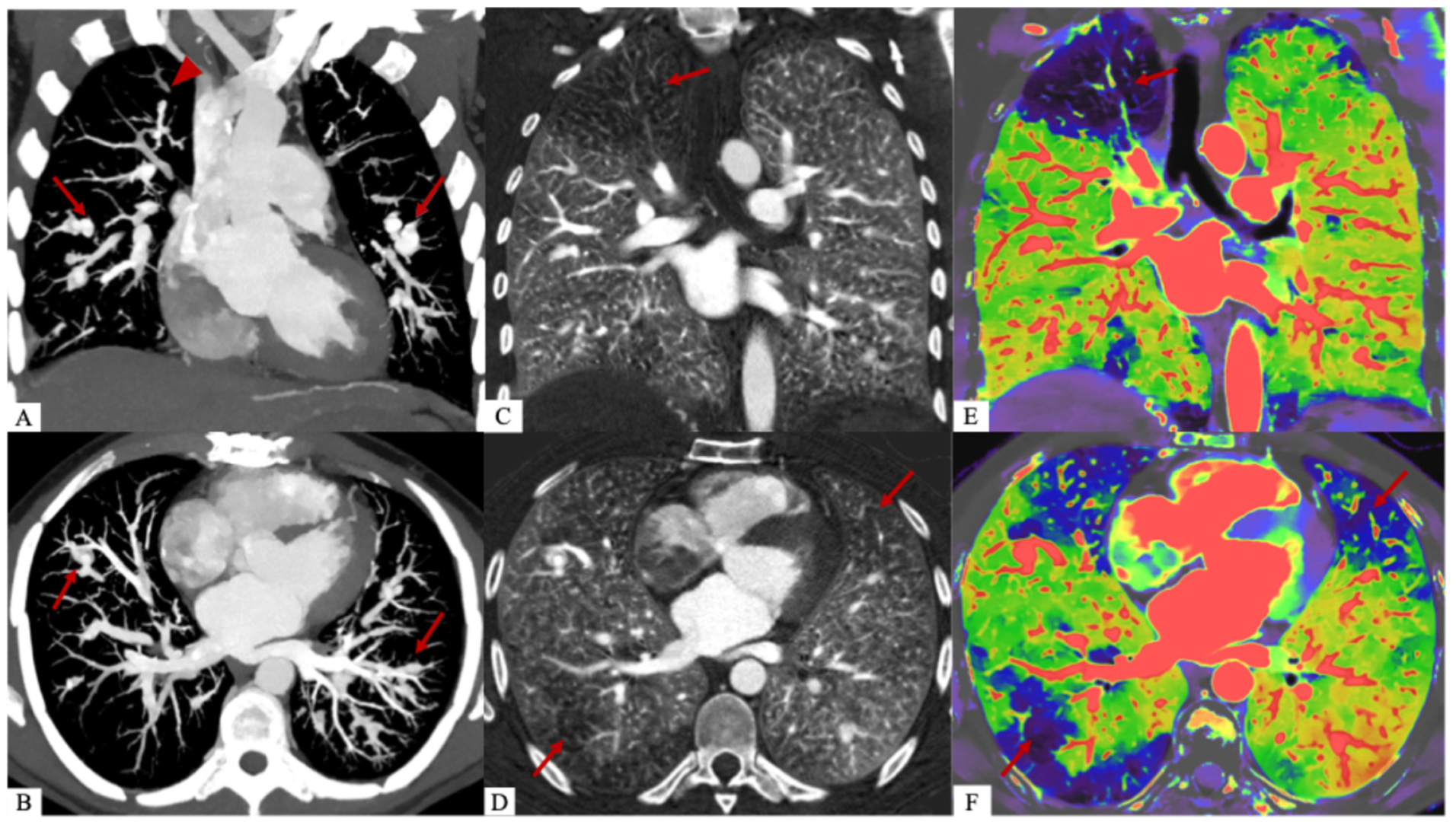
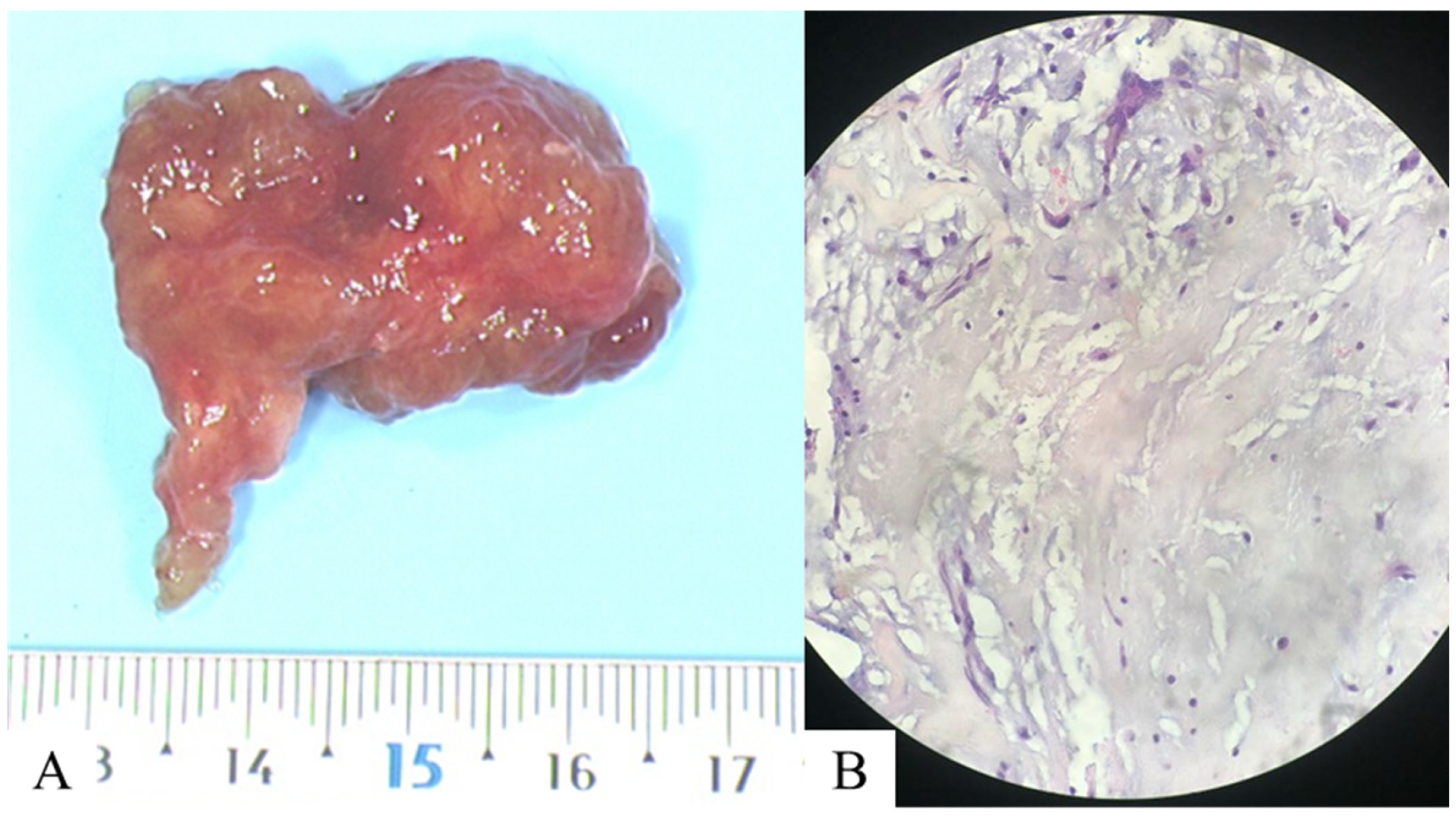
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed Tomography |
| FDG-PET scan | Fluorodeoxyglucose-Positron Emission Tomography |
| DECT | Dual-Energy CT |
| MIP | Maximum Intensity Projection |
| CTEPH | Chronic Tromboembolic Pulmonary Hypertension |
References
- Nasser, S.B.; Doeblin, P.; Doltra, A.; Schnackenburg, B.; Kelle, S. Cardiac Myxomas Show Elevated Native T1, T2 Relaxation Time and ECV on Parametric CMR. Front. Cardiovasc. Med. 2020, 7, 602137. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wang, D.; He, Y.; Zhang, R.; Zhou, Y.; Ying, K. Pulmonary embolism as the initial manifestation of right atrial myxoma: A case report and review of the literature. Medicine 2019, 98, e18386. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.; Mackinnon, J. Pulmonary hypertension due to myxoma of the right atrium; with special reference to the behavior of emboli of myxoma in the lung. Am. Heart J. 1964, 68, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.A.; Zumbihl, L.; Turquier, S.; Boccalini, S.; Mornex, J.-F.; Douek, P.; Cottin, V.; Boussel, L. Lung Dual-Energy CT Perfusion Blood Volume as a Marker of Severity in Chronic Thromboembolic Pulmonary Hypertension. Diagnostics 2023, 13, 769. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-F.; Chang, Y.-P.; Chai, J.-W. Investigating Patients with Pulmonary Hypertension Under Detector-Based Spectral Computed Tomography. Diagnostics 2025, 15, 1069. [Google Scholar] [CrossRef] [PubMed]
- Perrella, A.; Bagnacci, G.; Di Meglio, N.; Di Martino, V.; Mazzei, M.A. Thoracic Diseases: Technique and Applications of Dual-Energy CT. Diagnostics 2023, 13, 2440. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Bodega, F.; Bergamaschi, L.; Armillotta, M.; Pizzi, C. Multimodality Imaging in the Diagnostic Work-up of Patients with Cardiac Masses. JACC Cardiooncology 2024, 6, 847–862. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braillon, A.; Patural, P.; Laville, D.; Perros, F.; Turquier, S.; Cottin, V.; L’Huillier, R.; Si-Mohamed, S. Uncommon Cause of Pulmonary Hypertension: Imaging Diagnosis of Cardiac Myxoma Embolism. Diagnostics 2025, 15, 2420. https://doi.org/10.3390/diagnostics15192420
Braillon A, Patural P, Laville D, Perros F, Turquier S, Cottin V, L’Huillier R, Si-Mohamed S. Uncommon Cause of Pulmonary Hypertension: Imaging Diagnosis of Cardiac Myxoma Embolism. Diagnostics. 2025; 15(19):2420. https://doi.org/10.3390/diagnostics15192420
Chicago/Turabian StyleBraillon, Alexandra, Paul Patural, David Laville, Frédéric Perros, Ségolène Turquier, Vincent Cottin, Romain L’Huillier, and Salim Si-Mohamed. 2025. "Uncommon Cause of Pulmonary Hypertension: Imaging Diagnosis of Cardiac Myxoma Embolism" Diagnostics 15, no. 19: 2420. https://doi.org/10.3390/diagnostics15192420
APA StyleBraillon, A., Patural, P., Laville, D., Perros, F., Turquier, S., Cottin, V., L’Huillier, R., & Si-Mohamed, S. (2025). Uncommon Cause of Pulmonary Hypertension: Imaging Diagnosis of Cardiac Myxoma Embolism. Diagnostics, 15(19), 2420. https://doi.org/10.3390/diagnostics15192420





