A Systematic Review of Optic Disc Drusen in the Modern Imaging Era: Structure–Function Correlates, Diagnostic Performance, and NAION Co-Occurrence
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Risk of Bias Assessment and Synthesis
3. Results
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamann, S.; Malmqvist, L.; Costello, F. Optic disc drusen: Understanding an old problem from a new perspective. Acta Ophthalmol. 2018, 96, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, L.; Bursztyn, L.; Costello, F.; Digre, K.; Fraser, J.A.; Fraser, C.; Katz, B.; Lawlor, M.; Petzold, A.; Sibony, P.; et al. The Optic Disc Drusen Studies Consortium Recommendations for Diagnosis of Optic Disc Drusen Using Optical Coherence Tomography. J. Neuroophthalmol. 2018, 38, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ghassibi, M.P.; Chien, J.L.; Abumasmah, R.K.; Liebmann, J.M.; Ritch, R.; Park, S.C. Optic Nerve Head Drusen Prevalence and Associated Factors in Clinically Normal Subjects Measured Using Optical Coherence Tomography. Ophthalmology 2017, 124, 320–325. [Google Scholar] [CrossRef] [PubMed]
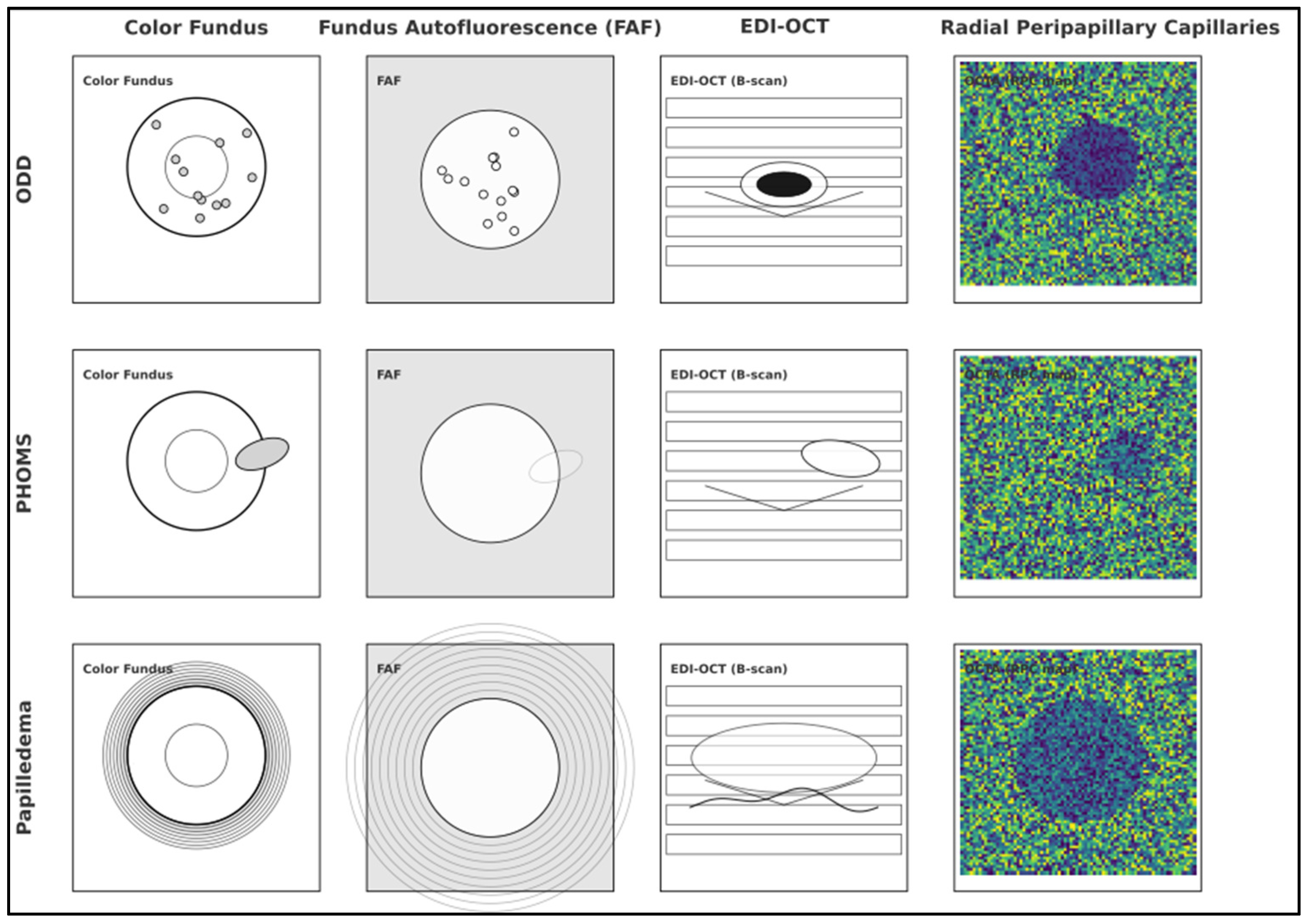
- Xie, X.; Liu, T.; Wang, W.; Tian, G.; Wang, J.; Guan, J.; Chen, M.; Wang, X.; Zhou, Q. Clinical and Multi-Mode Imaging Features of Eyes with Peripapillary Hyperreflective Ovoid Mass-Like Structures. Front. Med. 2022, 9, 796667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heath Jeffery, R.C.; Chen, F.K. Peripapillary hyperreflective ovoid mass-like structures: Multimodal imaging—A review. Clin. Exp. Ophthalmol. 2023, 51, 67–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casado, A.; Rebolleda, G.; Guerrero, L.; Leal, M.; Contreras, I.; Oblanca, N.; Muñoz-Negrete, F.J. Measurement of retinal nerve fiber layer and macular ganglion cell-inner plexiform layer with spectral-domain optical coherence tomography in patients with optic nerve head drusen. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Skaat, A.; Muylaert, S.; Mogil, R.S.; Furlanetto, R.L.; Netto, C.F.; Banik, R.; Liebmann, J.M.; Ritch, R.; Park, S.C. Relationship Between Optic Nerve Head Drusen Volume and Structural and Functional Optic Nerve Damage. J. Glaucoma 2017, 26, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Gaier, E.D.; Rizzo, J.F., 3rd; Miller, J.B.; Cestari, D.M. Focal Capillary Dropout Associated with Optic Disc Drusen Using Optical Coherence Tomographic Angiography. J. Neuroophthalmol. 2017, 37, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Tebaldi, S.; Amoroso, F.; Arvanitis, D.; Breve, M.; Cennamo, G. Optical Coherence Tomography Angiography in Optic Nerve Drusen. Ophthalmic Res. 2018, 59, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Abri Aghdam, K.; Ashraf Khorasani, M.; Soltan Sanjari, M.; Habibi, A.; Shenazandi, H.; Kazemi, P.; Ghasemi Falavarjani, K. Optical coherence tomography angiography features of optic nerve head drusen and nonarteritic anterior ischemic optic neuropathy. Can. J. Ophthalmol. 2019, 54, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.; Loshusan, B.; Armstrong, J.J.; Fraser, J.A.; Hamann, S.; Bursztyn, L.L.C.D. A Comparison of Diagnostic Accuracy of Imaging Modalities to Detect Optic Disc Drusen: The Age of Enhanced Depth Imaging Optical Coherence Tomography. Am. J. Ophthalmol. 2023, 248, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, Y. Advances in origin, evolution, and pathogenesis of optic disc drusen: A narrative review. Indian. J. Ophthalmol. 2025, 73, 637–647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, K.M.; Woo, S.J.; Hwang, J.M. Factors associated with visual field defects of optic disc drusen. PLoS ONE 2018, 13, e0196001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Traber, G.L.; Weber, K.P.; Sabah, M.; Keane, P.A.; Plant, G.T. Enhanced Depth Imaging Optical Coherence Tomography of Optic Nerve Head Drusen: A Comparison of Cases with and without Visual Field Loss. Ophthalmology 2017, 124, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Costello, F.; Rothenbuehler, S.P.; Sibony, P.A.; Hamann, S.; Optic Disc Drusen Studies Consortium. Diagnosing Optic Disc Drusen in the Modern Imaging Era: A Practical Approach. Neuroophthalmology 2020, 45, 1–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiu, H.H.; Yang, F.P.; VandenHoven, C.; Wan, M.J. Utility of spectral domain OCT in differentiating optic disc drusen from papilledema in children. Can. J. Ophthalmol. 2021, 56, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Kwok, S.H.W.; Mak, A.C.Y.; Lai, F.H.P.; Ng, D.S.C.; Chen, L.J.; Iu, L.P.; Young, A.L.; Brelen, M. Fundus Autofluorescence and Optical Coherence Tomography Characteristics in Different Stages of Central Serous Chorioretinopathy. J. Ophthalmol. 2021, 2021, 6649064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajagopal, R.; Mitchell, E.; Sylvester, C.; Lope, L.A.; Nischal, K.K. Detection of Optic Disc Drusen in Children Using Ultrasound through the Lens and Avoiding the Lens—Point of Care Ultrasound Technique of Evaluation Revisited. J. Clin. Med. 2019, 8, 1449. [Google Scholar] [CrossRef]
- Rueløkke, L.L.; Malmqvist, L.; Wegener, M.; Hamann, S. Optic Disc Drusen Associated Anterior Ischemic Optic Neuropathy: Prevalence of Comorbidities and Vascular Risk Factors. J. Neuroophthalmol. 2020, 40, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Montorio, D.; Giunta, P.; Tranfa, F. Optical coherence tomography angiography in nonarteritic anterior ischemic optic neuropathy due to optic nerve head drusen. Neurol. Sci. 2020, 41, 3349–3351. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, Y.; Zhou, X.; Chu, Z.; Stell, L.; Shariati, M.A.; Wang, R.K.; Liao, Y.J. Topographic Quadrant Analysis of Peripapillary Superficial Microvasculature in Optic Disc Drusen. Front. Neurol. 2021, 12, 666359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Estrela, T.; Jammal, A.A.; El-Dairi, M.; Medeiros, F.A. Rates of Visual Field Change in Eyes With Optic Disc Drusen. J. Neuroophthalmol. 2023, 43, 353–358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kulkarni, K.M.; Pasol, J.; Rosa, P.R.; Lam, B.L. Differentiating mild papilledema and buried optic nerve head drusen using spectral domain optical coherence tomography. Ophthalmology 2014, 121, 959–963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosa, N.; De Bernardo, M.; Abbinante, G.; Vecchio, G.; Cione, F.; Capasso, L. Optic Nerve Drusen Evaluation: A Comparison between Ultrasound and OCT. J. Clin. Med. 2022, 11, 3715. [Google Scholar] [CrossRef]
- Fraser, J.A.; Rueløkke, L.L.; Malmqvist, L.; Hamann, S. Prevalence of Optic Disc Drusen in Young Patients with Nonarteritic Anterior Ischemic Optic Neuropathy: A 10-Year Retrospective Study. J. Neuroophthalmol. 2021, 41, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Mukriyani, H.; Malmqvist, L.; Subhi, Y.; Hamann, S. Prevalence of optic disc drusen: A systematic review, meta-analysis and forecasting study. Acta Ophthalmol. 2024, 102, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lykkebirk, L.; Wessel Lindberg, A.S.; Karlesand, I.; Heiberg, M.; Malmqvist, L.; Hamann, S. Peripapillary Vessel Density in Relation to Optic Disc Drusen: A Multimodal Optical Coherence Tomography Study. J. Neuroophthalmol. 2023, 43, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya Cakir, G.; Solmaz, B.; Cakir, I.; Pasaoglu, I.B.; Taskapili, M. Optical coherence tomography angiography findings in optic disc drusen and idiopathic intracranial hypertension. Eur. J. Ophthalmol. 2024, 34, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Pilat, A.V.; Proudlock, F.A.; Kumar, P.; Gottlob, I. Short-term progression of optic disc and macular changes in optic nerve head drusen. Eye 2023, 37, 1496–1502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, Y.; Zhou, X.; Chu, Z.; Stell, L.; Shariati, M.A.; Wang, R.K.; Liao, Y.J. Vision Loss in Optic Disc Drusen Correlates with Increased Macular Vessel Diameter and Flux and Reduced Peripapillary Vascular Density. Am. J. Ophthalmol. 2020, 218, 214–224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamann, S.; Malmqvist, L.; Wegener, M.; Fard, M.A.; Biousse, V.; Bursztyn, L.; Citirak, G.; Costello, F.; Crum, A.V.; Digre, K.; et al. Young Adults with Anterior Ischemic Optic Neuropathy: A Multicenter Optic Disc Drusen Study. Am. J. Ophthalmol. 2020, 217, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, R.G.; Lykkebirk, L.; Jørgensen, M.; Malmqvist, L.; Hamann, S. Optic Nerve Head Anatomy and Vascular Risk Factors in Patients with Optic Disc Drusen Associated Anterior Ischemic Optic Neuropathy. Am. J. Ophthalmol. 2022, 242, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Rothenbuehler, S.P.; Malmqvist, L.; Belmouhand, M.; Bjerager, J.; Maloca, P.M.; Larsen, M.; Hamann, S. Comparison of Spectral-Domain OCT versus Swept-Source OCT for the Detection of Deep Optic Disc Drusen. Diagnostics 2022, 12, 2515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sim, P.Y.; Soomro, H.; Karampelas, M.; Barampouti, F. Enhanced Depth Imaging Optical Coherence Tomography of Optic Nerve Head Drusen in Children. J. Neuroophthalmol. 2020, 40, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, I.; Cruz, C.A.; Pistilli, M.; Kohli, A.A.; Liu, G.T.; Shindler, K.S.; Avery, R.A.; Garvin, M.K.; Wang, J.K.; Ross, A.; et al. Utility of Spectral-Domain Optical Coherence Tomography in Differentiating Papilledema from Pseudopapilledema: A Prospective Longitudinal Study. J. Neuroophthalmol. 2021, 41, e509–e515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Study | Endpoint (Units) | ODD Result | Comparator Result | Effect/p | N (Eyes) | Imaging Method (OCT/OCTA) |
|---|---|---|---|---|---|---|
| Yan 2021 [22] | Global RNFL thickness (µm) | 117.54 ± 18.75 | 105.81 ± 14.45 | p = 0.007 (ODD thicker) | 34 ODD/33 controls | SD-OCT (Cirrus); ODD confirmation required EDI-OCT; OCTA: Zeiss AngioPlex |
| Yan 2021 [22] | VF mean deviation (dB) | - | - | Group difference: −1.78 ± 3.87 dB (ODD vs. control), 95% CI −3.20 to −0.36; p = 0.016 | 34/33 | As above |
| Yan 2021 [22] | Peripapillary vessel-area density (fraction)—Inferior | 0.30 ± 0.07 | 0.34 ± 0.06 | p = 0.012 | 34/33 | OCTA: AngioPlex; custom MATLAB R2021b quantification |
| Yan 2021 [22] | Peripapillary vessel-area density—Temporal | 0.44 ± 0.06 | 0.48 ± 0.06 | p = 0.008 | 34/33 | As above |
| Yan 2021 [22] | Peripapillary vessel-area density—Superonasal | 0.44 ± 0.06 | 0.49 ± 0.05 | p = 0.001 | 34/33 | As above |
| Lee 2018 [13] | Average RNFL thickness (µm) | 101 ± 12 | 97 ± 10 | p = 0.02 (ODD thicker) | 40 ODD | SD-OCT; EDI not stated |
| Kulkarni 2014 [24] | RNFL quadrant thickness (µm)—Superior | 137.2 ± 48.2 | 177.6 ± 81.4 (papilledema) | p = 0.25 | 16 buried ODD/12 papilledema (+2 normal eyes) | SD-OCT; 8 eyes imaged with EDI-SD-OCT |
| Kulkarni 2014 [24] | RNFL quadrant—Nasal | 77.2 ± 20.3 | 132.8 ± 84.2 | p = 0.17 | As above | As above |
| Kulkarni 2014 [24] | RNFL quadrant—Inferior | 139.5 ± 28.0 | 205.8 ± 113.5 | p = 0.22 | As above | As above |
| Kulkarni 2014 [24] | RNFL quadrant—Temporal | 77.1 ± 12.6 | 83.1 ± 15.5 | p = 0.42 | As above | As above |
| Study | Endpoint | ODD Result | Comparator | Conclusion | N (Eyes) | Imaging Method |
|---|---|---|---|---|---|---|
| Lee 2018 [13] | Baseline VF categories | Normal 44%; enlarged blind spot 29%; arcuate 6%; nasal step 5%; other 16% | Healthy controls (structure only) | Enlarged blind spot is the single most common defect | 40 | SD-OCT based typing; EDI not stated. |
| Estrela 2023 [23] | MD progression (dB/year) | Mean −0.23 ± 0.26 (median −0.18) | - | Slow but measurable decline over time | 65 | Longitudinal cohort; SAP 24-2. |
| Yan 2021 [22] | Group MD difference (ODD–control) | −1.78 ± 3.87 dB, 95% CI −3.20 to −0.36 | Healthy controls | ODD eyes have worse MD than controls at baseline | 34/33 | OCTA + structural OCT |
| Study | Endpoint | Result | Clinical Interpretation | N (Eyes/Patients) | Modality Details |
|---|---|---|---|---|---|
| Fraser 2021 [26] | ODD prevalence in young NAION (37 patients ≤50 y; 74 eyes) | 56.7% of patients and 53.3% of NAION eyes had ODD; 35.9% of ODD were visible on ophthalmoscopy | ODD are common in young NAION; many ODD are not visible without multimodal imaging | 37 patients/74 eyes | Modalities used across cohort included EDI-OCT in 36 patients. |
| Rosa 2022 [25] | ODD detection by modality in eyes with ODD (n = 86) | Ultrasound: 87.2%; OCT: 80.2%; FAF: 62.8% | Ultrasound has the highest single-test yield; OCT is close; FAF lags | 86 ODD eyes (50 patients) + 54 papilledema eyes | ODD group US-positive; SD-OCT performed on ODD eyes. |
| Kulkarni 2014 [24] | Reader accuracy using OCT alone (buried ODD vs. mild papilledema) | Accuracy 50–64%; κ = 0.35 (95% CI 0.19–0.54) | OCT alone is unreliable for this differential; use multimodal approach | 16 buried ODD eyes/12 papilledema eyes (+2 normal) | SD-OCT; 8 eyes also had EDI-SD-OCT. |
| Study (Year) | Objective Finding | Values | Notes/Definition |
|---|---|---|---|
| Yan et al. (2021) [22] | ODD type distribution | Superficial 64.7% (22/34 eyes); buried 35.3% (12/34) | Case–control; OCT/OCTA confirmed. |
| Visual field severity snapshot | Mean MD −4.37 ± 1.00 dB; 68% of ODD eyes better than −5 dB; 93% better than −10 dB | 34 ODD eyes vs. 33 controls; Mann–Whitney p < 0.0001 for MD difference. | |
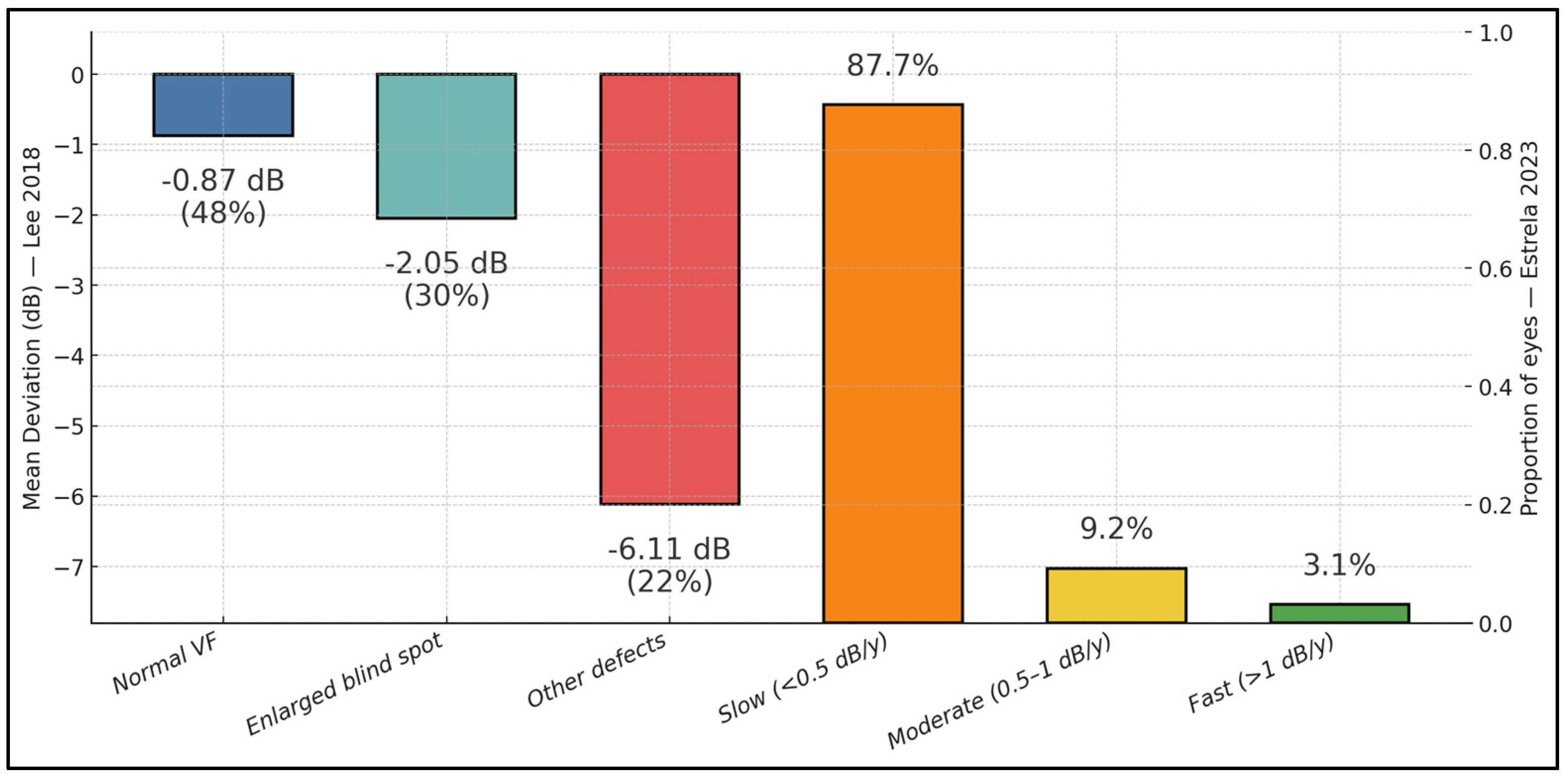
| Lee et al. (2018) [13] | ODD subtype proportion | Type 1 (deep/buried) 82.8% (48/58); Type 2 (superficial) 17.2% (10/58) | SD-OCT classification. |
| Visual-field (VF) pattern distribution | Normal 51.7% (30/58); enlarged blind spot 19.0% (11/58); other localized defects 29.3% (17/58) | Goldmann/SAP categorization. | |
| VF severity by pattern (mean ± SD MD) | Normal −0.58 ± 1.22 dB; enlarged blind spot −3.03 ± 2.46 dB; other defects −7.44 ± 3.70 dB | One-way ANOVA p < 0.001 across groups. | |
| CART-derived risk thresholds | RNFLavg < 85.5 µm → higher odds of “other VF defects” (OR 3.436, 95% CI 1.106–10.676); ODD height > 348 µm → higher odds of enlarged blind spot (OR 3.956, 95% CI 1.250–12.514) | Multivariable logistic regression. | |
| Estrela et al. (2023) [23] | Rate of VF change (SAP MD) | Mean −0.23 ± 0.26 dB/yr (median −0.16; IQR −0.25 to −0.08) | Longitudinal cohort, 65 eyes; SAP 24-2. |
| Progression categories | Slow 87.7% (≤−0.5 dB/yr); moderate 9.2% (−0.5 to −1.0); fast 3.1% (<−1.0) | Category cutoffs prespecified. | |
| Predictors of faster loss | Per 10 yrs older: −0.06 dB/yr (p = 0.044); per 1 dB lower baseline MD: −0.03 dB/yr (p < 0.001) | Multivariable models; IOP not associated. | |
| Rosa et al. (2022) [25] | OCT vs. ultrasound yield (86 ODD eyes by US) | OCT identified ODD and/or PHOMS in 69/86 eyes → 80.23% relative to US | OCT: ODD only 7 (8.14%), PHOMS only 25 (29.07%), ODD + PHOMS 37 (43.02%); none 17 (19.77%). |
| Kulkarni et al. (2014) [24] | SD-OCT alone to distinguish buried ODD vs. mild papilledema | Reader diagnostic accuracy range 50–64%; inter-reader agreement κ = 0.35 (95% CI 0.19–0.54) | Comparative case series; ultrasound-proven buried ODD vs. IIH papilledema. |
| Fraser et al. (2021) [26] | ODD in young NAION (≤50 yr) | 56.7% of patients (53.3% of affected eyes) had ODD; 95.2% of ODD cases bilateral; only 35.9% visible on ophthalmoscopy | EDI-OCT most sensitive among modalities evaluated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitriu, A.; Dumitriu, B.; Socol, F.G.; Socol, I.D.; Yasar, I.I.; Fizedean, C.; Mavrea, A.; Bondar, A.-C.; Munteanu, M. A Systematic Review of Optic Disc Drusen in the Modern Imaging Era: Structure–Function Correlates, Diagnostic Performance, and NAION Co-Occurrence. Diagnostics 2025, 15, 2414. https://doi.org/10.3390/diagnostics15182414
Dumitriu A, Dumitriu B, Socol FG, Socol ID, Yasar II, Fizedean C, Mavrea A, Bondar A-C, Munteanu M. A Systematic Review of Optic Disc Drusen in the Modern Imaging Era: Structure–Function Correlates, Diagnostic Performance, and NAION Co-Occurrence. Diagnostics. 2025; 15(18):2414. https://doi.org/10.3390/diagnostics15182414
Chicago/Turabian StyleDumitriu, Alina, Bogdan Dumitriu, Flavius George Socol, Ioana Denisa Socol, Ionela Iasmina Yasar, Camelia Fizedean, Adelina Mavrea, Andrei-Cristian Bondar, and Mihnea Munteanu. 2025. "A Systematic Review of Optic Disc Drusen in the Modern Imaging Era: Structure–Function Correlates, Diagnostic Performance, and NAION Co-Occurrence" Diagnostics 15, no. 18: 2414. https://doi.org/10.3390/diagnostics15182414
APA StyleDumitriu, A., Dumitriu, B., Socol, F. G., Socol, I. D., Yasar, I. I., Fizedean, C., Mavrea, A., Bondar, A.-C., & Munteanu, M. (2025). A Systematic Review of Optic Disc Drusen in the Modern Imaging Era: Structure–Function Correlates, Diagnostic Performance, and NAION Co-Occurrence. Diagnostics, 15(18), 2414. https://doi.org/10.3390/diagnostics15182414








