Macular Choroidal Thickness in Keratoconus: Systematic Review and Meta-Analysis of Current Evidence
Abstract
1. Introduction
2. Materials and Methods
3. Results
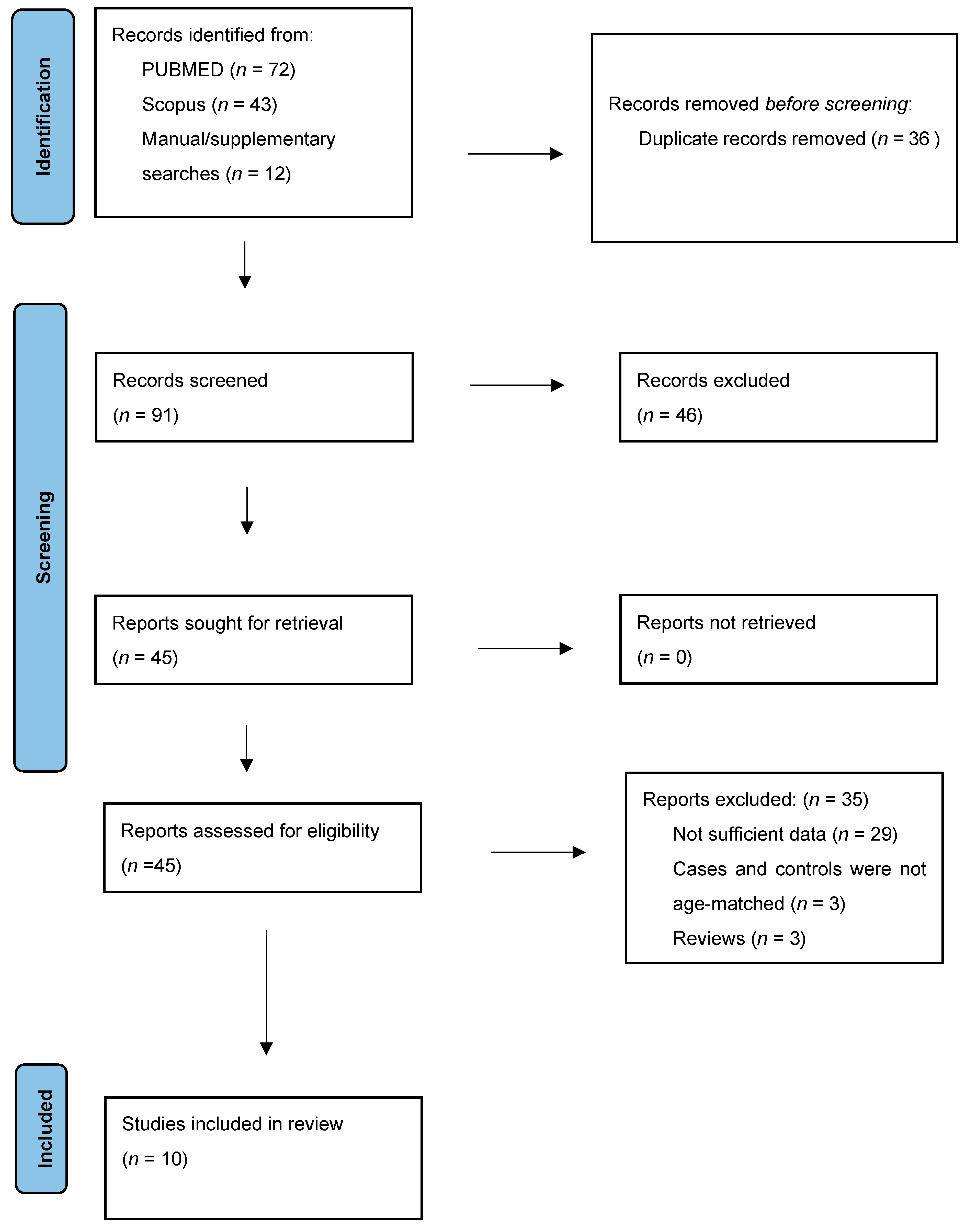
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment of the Included Studies
3.4. Meta-Analysis of Subfoveal Choroidal Thickness
3.5. Certainty of Evidence
3.6. Descriptive Evidence of Keratoconus Severity and Choroidal Thickness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KC | Keratoconus |
| OCT | Optical coherence tomography |
References
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Bui, A.D.; Truong, A.; Pasricha, N.D.; Indaram, M. Keratoconus Diagnosis and Treatment: Recent Advances and Future Directions. Clin. Ophthalmol. 2023, 17, 2705–2718. [Google Scholar] [CrossRef]
- Davidson, A.E.; Hayes, S.; Hardcastle, A.J.; Tuft, S.J. The pathogenesis of keratoconus. Eye 2014, 28, 189–195. [Google Scholar] [CrossRef]
- Song, M.; Fang, Q.Y.; Seth, I.; Baird, P.N.; Daniell, M.D.; Sahebjada, S. Non-genetic risk factors for keratoconus. Clin. Exp. Optom. 2023, 106, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Chatziralli, I.P.; Koutsandrea, C.; Siasou, G.; Droutsas, D. Assessment of the macula in keratoconus: An optical coherence tomography and multifocal electroretinography study. Ophthalmologica 2013, 229, 203–207. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yu, H.G. Keratoconus associated with choroidal neovascularization: A case report. J. Med. Case Rep. 2010, 4, 58. [Google Scholar] [CrossRef]
- Hashemi, H.; Heirani, M.; Ambrósio, R., Jr.; Hafezi, F.; Naroo, S.A.; Khorrami-Nejad, M. The link between Keratoconus and posterior segment parameters: An updated, comprehensive review. Ocul. Surf. 2022, 23, 116–122. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Shetty, R.; D’Souza, S.; Khamar, P.; Ghosh, A.; Nuijts, R.M.M.A.; Sethu, S. Biochemical Markers and Alterations in Keratoconus. Asia Pac. J. Ophthalmol. 2020, 9, 533–540. [Google Scholar] [CrossRef]
- van Velthoven, M.E.; Faber, D.J.; Verbraak, F.D.; van Leeuwen, T.G.; de Smet, M.D. Recent developments in optical coherence tomography for imaging the retina. Prog. Retin. Eye Res. 2007, 26, 57–77. [Google Scholar] [CrossRef]
- Laviers, H.; Zambarakji, H. Enhanced depth imaging-OCT of the choroid: A review of the current literature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1871–1883. [Google Scholar] [CrossRef]
- Tan, K.A.; Gupta, P.; Agarwal, A.; Chhablani, J.; Cheng, C.Y.; Keane, P.A.; Agrawal, R. State of science: Choroidal thickness and systemic health. Surv. Ophthalmol. 2016, 61, 566–581. [Google Scholar] [CrossRef]
- Steiner, M.; Esteban-Ortega, M.D.M.; Muñoz-Fernández, S. Choroidal and retinal thickness in systemic autoimmune and inflammatory diseases: A review. Surv. Ophthalmol. 2019, 64, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, S.; Qiu, Z.; He, M.; Wang, L.; Li, Y.; Huang, W. Choroidal Thickness in Diabetes and Diabetic Retinopathy: A Swept Source OCT Study. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, K.A.; Kazantzis, D.; Vrachatis, D.A.; Giotaki, S.G.; Papaconstantinou, E.; Kanakis, M.; Avramides, D.; Deftereos, S.; Chatziralli, I.; Georgalas, I. Choroidal thickness in patients with systemic arterial hypertension: A systematic review and meta-analysis. Ther. Adv. Ophthalmol. 2022, 14, 25158414221132825. [Google Scholar] [CrossRef]
- Kazantzis, D.; Machairoudia, G.; Theodossiadis, P.; Chatziralli, I. Subfoveal choroidal thickness changes in patients with pseudoexfoliation syndrome (PEX) compared to healthy controls: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2024, 47, 104095. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quailty of Nonrandomised Studies in Meta-Analyses. 2009. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11 July 2025).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Akkaya, S. Macular and Peripapillary Choroidal Thickness in Patients with Keratoconus. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 664–673. [Google Scholar] [CrossRef]
- Yilmaz, I.; Saracoglu Yilmaz, B.; Guleryuz, N.B.; Perente, I.; Ozkaya, A.; Taskapili, M. Assessment of the macula and choroid in pediatric keratoconus patients. Saudi J. Ophthalmol. 2018, 32, 126–129. [Google Scholar] [CrossRef]
- Pinheiro-Costa, J.; Viana Pinto, J.; Perestrelo, S.; Beato, J.N.; Torrão, L.; Brandão, E.; Carneiro, Â.; Madeira, M.D.; Falcão-Reis, F. Increased Choroidal Thickness in Keratoconus Patients: Perspectives in the Disease Pathophysiology. J. Ophthalmol. 2019, 2019, 2453931. [Google Scholar] [CrossRef]
- Bilgin, B.; Karadag, A.S. Choroidal thickness in keratoconus. Int. Ophthalmol. 2020, 40, 135–140. [Google Scholar] [CrossRef]
- Fahmy, R.M.; AlGhamdi, M.S.; Mostafa, A.M. The Correlation between Choroidal Thickness and Keratoconus Severity among Saudi Population (Albaha). J. Ophthal. Opto. 2021, 3, 100009. [Google Scholar]
- Dogan, B.; Bozdogan, Y.C.; Gedik, B.; Erol, M.K.; Bulut, M.; Duman, F. Optic disc and retinal vessel densities assessment by optical coherence tomography angiography in patients with keratoconus. Photodiagnosis Photodyn. Ther. 2023, 41, 103218. [Google Scholar] [CrossRef]
- Aksoy Aydemir, G.; Ozkoyuncu Kocabas, D.; Aydemir, E.; Bayat, A.H.; Cınar, S.S.; Karadağ, A.S. Alterations in the choroidal thickness and retinal vascular caliber in keratoconus. Int. Ophthalmol. 2023, 43, 95–103. [Google Scholar] [CrossRef]
- Pierro, L.; Bianco, L.; Bertuzzi, F.; Arrigo, A.; Saladino, A.; Distefano, A.; Berni, A.; Knutsson, K.A.; Rama, P.; Bandello, F. New Findings in Early-Stage Keratoconus: Lamina Cribrosa Curvature, Retinal Nerve Fiber Layer Thickness, and Vascular Perfusion. Am. J. Ophthalmol. 2023, 246, 122–129. [Google Scholar] [CrossRef]
- Burguera-Giménez, N.; Díez-Ajenjo, M.A.; Burguera, N.; Briceno-Lopez, C.; Peris-Martínez, C. Subfoveal and Parafoveal Choroidal Thickening in Patients with Keratoconus Using the ETDRS Grid on Swept-Source OCT. Ophthalmol. Ther. 2024, 13, 509–527. [Google Scholar] [CrossRef]
- Feo, A.; Vinciguerra, R.; Antropoli, A.; Barone, G.; Criscuolo, D.; Vinciguerra, P.; Romano, V.; Romano, M.R. Pachychoroid pigment epitheliopathy in keratoconic eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2025, 263, 87–95. [Google Scholar] [CrossRef]
- Gutierrez-Bonet, R.; Ruiz-Medrano, J.; Biarnés, M.; Rasheed, M.A.; Vupparaboina, K.K.; Chhablani, J.; Ruiz-Moreno, J.M. Analysis of Choroidal Vascularity Index in Keratoconus Patients Using Swept-Source Optical Coherence Tomography-Based Binarization Techniques. J. Ophthalmol. 2020, 2020, 1682463. [Google Scholar] [CrossRef]
- Hashemian, M.N.; Ghafarian, S.; Riazi-Esfahani, H.; Khalili Pour, E. Evaluation of Choroidal Vascularity Index in Keratoconus Patients: Does Choroidal Vascularity Change in Keratoconus? J. Curr. Ophthalmol. 2023, 35, 36–41. [Google Scholar] [CrossRef]
- Abbasi Mehrabadi, A.; Sadeghi, J.; Shoeibi, N.; Heravian Shandiz, J.; Motamed Shariati, M.; Derakhshan, A.; Yazdani, N. Macular choroidal thickness in keratoconus. Clin. Exp. Optom. 2024, 108, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Sánchez, A.; De-Hita-Cantalejo, C.; Sánchez-González, M.C.; Bautista-Llamas, M.J.; Sánchez-González, J.M.; Gargallo-Martínez, B. Choroidal thickness assessment in keratoconus patients treated with cross-linking compared to healthy population. Int. Ophthalmol. 2023, 43, 1185–1192. [Google Scholar] [CrossRef]
- Meek, K.M.; Tuft, S.J.; Huang, Y.; Gill, P.S.; Hayes, S.; Newton, R.H.; Bron, A.J. Changes in collagen orientation and distribution in keratoconus corneas. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Chaerkady, R.; Shao, H.; Scott, S.G.; Pandey, A.; Jun, A.S.; Chakravarti, S. The keratoconus corneal proteome: Loss of epithelial integrity and stromal degeneration. J. Proteom. 2013, 87, 122–131. [Google Scholar] [CrossRef]
- Cheng, E.L.; Maruyama, I.; SundarRaj, N.; Sugar, J.; Feder, R.S.; Yue, B.Y. Expression of type XII collagen and hemidesmosome-associated proteins in keratoconus corneas. Curr. Eye Res. 2001, 22, 333–340. [Google Scholar] [CrossRef]
- Akhtar, S.; Bron, A.J.; Salvi, S.M.; Hawksworth, N.R.; Tuft, S.J.; Meek, K.M. Ultrastructural analysis of collagen fibrils and proteoglycans in keratoconus. Acta Ophthalmol. 2008, 86, 764–772. [Google Scholar] [CrossRef]
- Ahuja, P.; Dadachanji, Z.; Shetty, R.; Nagarajan, S.A.; Khamar, P.; Sethu, S.; D’Souza, S. Relevance of IgE, allergy and eye rubbing in the pathogenesis and management of Keratoconus. Indian J. Ophthalmol. 2020, 68, 2067–2074. [Google Scholar] [CrossRef]
- Wisse, R.P.; Kuiper, J.J.; Gans, R.; Imhof, S.; Radstake, T.R.; Van der Lelij, A. Cytokine Expression in Keratoconus and its Corneal Microenvironment: A Systematic Review. Ocul. Surf. 2015, 13, 272–283. [Google Scholar] [CrossRef]
- Asanad, S.; Bayomi, M.; Brown, D.; Buzzard, J.; Lai, E.; Ling, C.; Miglani, T.; Mohammed, T.; Tsai, J.; Uddin, O.; et al. Ehlers-Danlos syndromes and their manifestations in the visual system. Front. Med. 2022, 9, 996458. [Google Scholar] [CrossRef] [PubMed]
- Mathan, J.J.; Gokul, A.; Simkin, S.K.; Meyer, J.J.; McGhee, C.N.J. Keratoconus in Down syndrome: Prevalence, risk factors, severity and corneal tomographic characteristics. Clin. Exp. Ophthalmol. 2024, 52, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Csincsik, L.; Nelson, R.; Walpert, M.J.; Peto, T.; Holland, A.; Lengyel, I. Increased choroidal thickness in adults with Down syndrome. Alzheimers Dement. 2021, 13, e12170. [Google Scholar] [CrossRef] [PubMed]
- Eandi, C.M.; Del Priore, L.V.; Bertelli, E.; Ober, M.D.; Yannuzzi, L.A. Central serous chorioretinopathy in patients with keratoconus. Retina 2008, 28, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, Y.; Kawaguchi, K.; Nouchi, T.; Yasuno, Y. Choroidal thickness in healthy Japanese subjects. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2173–2176. [Google Scholar] [CrossRef]
- Tan, C.S.; Ouyang, Y.; Ruiz, H.; Sadda, S.R. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 261–266. [Google Scholar] [CrossRef] [PubMed]


| Author/Ref. | Year | Region | Study Design | No of Eyes of Patients/Controls | Age of Patients/Controls (Years) | Thinnest Corneal Thickness (μm) | Kmax (D) | OCT Device |
|---|---|---|---|---|---|---|---|---|
| Akkaya [20] | 2018 | Turkey | Prospective cross-sectional | 45/56 | 24.5 ± 7.2/22.5 ± 7.4 | na | na | Spectralis OCT, Heidelberg Engineering |
| Yilmaz [21] | 2018 | Turkey | Cross-sectional | 50/50 | 12.4 ± 1.9/12.0 ± 2.1 | 456 ± 57 | 57.45 ± 11.16 | Spectralis OCT, Heidelberg Engineering |
| Pinheiro-Costa [22] | 2019 | Portugal | Case–control | 74/39 | 23.01 ± 4.68/22.40 ± 5.77 | 456.66 ± 51.91 | 56.49 ± 7.83 | Spectralis OCT, Heidelberg Engineering |
| Bilgin [23] | 2020 | Turkey | Cross-sectional | 80/80 | 18.9 ± 6.9/19 ± 5.6 | 449.7 ± 3.2 | na | Spectralis OCT, Heidelberg Engineering |
| Fahmy [24] | 2021 | Saudi Arabia | Case–control | 78/80 | 29.60 ± 7.405 and 27.55 ± 7.207 for males and females/22.625 ± 4.87 and 23.0 ± 5.416 in controls | na | na | Spectralis OCT, Heidelberg Engineering |
| Dogan [25] | 2023 | Turkey | Case–control | 32/24 | 28.50 ± 11.48 /30.63 ± 8.17 | 464.65 ± 42.41 | 54.93 ± 3.92 | OCT Optovue |
| Aydemir [26] | 2023 | Turkey | Prospective cross-sectional | 72/113 | 24.44 ± 6.35/21.55 ± 5.30 | na | 49.82 ± 4.47 | Spectralis OCT, Heidelberg Engineering |
| Pierro [27] | 2023 | Italy | Observational case–control | 32/32 | 26.92 ± 9.6/26.66 ± 1.8 | 495 ± 32 | 49.77 ± 4 | Topcon Triton OCT |
| Burguera-Giménez [28] | 2024 | Spain | Prospective cross-sectional | 62/45 | 30 ± 12/ 32 ± 9.1 | 483 ± 39.42 | 48.40 ± 4.73 | DRI-1 OCT, Topcon Medical |
| Feo [29] | 2025 | Italy | Retrospective cross-sectional | 56/62 | 35.2 ± 13.2/34.6 ± 15.0 | 466 ± 47 | 50.7 ± 4.8 | Spectralis OCT, Heidelberg Engineering |
| Study (Year)/Ref. | Selection | Comparability | Exposure | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Definition | Representativeness of the Cases | Selection of Controls | Definition of Controls | On Age | On Other Risk Factors | Assessment of Exposure | Same Method of Assessment in Cases and Controls | Non-Response Rate | ||
| Akkaya (2018) [20] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Yilmaz (2018) [21] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Pinheiro-Costa (2019) [22] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Bilgin (2020) [23] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Fahmy (2021) [24] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Dogan (2023) [25] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Aydemir (2023) [26] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Pierro (2023) [27] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Burguera-Giménez (2024) [28] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Feo (2025) [29] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Study (Year)/Ref. | MD | 95% CI | p-Value | I2 |
|---|---|---|---|---|
| Akkaya (2018) [20] | 40.66 | 12.85–68.47 | 0.004 | 95% |
| Yilmaz (2018) [21] | 50.94 | 24.68–77.20 | 0.0001 | 93% |
| Pinheiro-Costa (2019) [22] | 39.85 | 12.58–67.12 | 0.004 | 95% |
| Bilgin (2020) [23] | 45.08 | 14.00–76.16 | 0.004 | 95% |
| Fahmy (2021) [24] | 48.80 | 18.53–79.07 | 0.002 | 95% |
| Dogan (2023) [25] | 45.25 | 16.15–74.34 | 0.002 | 95% |
| Aydemir (2023) [26] | 44.67 | 15.11–74.22 | 0.003 | 95% |
| Pierro (2023) [27] | 46.06 | 17.65–74.49 | 0.001 | 95% |
| Burguera-Giménez (2024) [28] | 45.34 | 16.11–74.57 | 0.002 | 95% |
| Feo (2025) [29] | 32.63 | 11.74–53.51 | 0.002 | 91% |
| Mechanism | Potential Pathologic Contributor | Supporting Evidence |
|---|---|---|
| Collagen dysregulation | Impaired corneal expression in choroid | Abnormal corneal collagen distribution in KC in previous studies [33,34,35] |
| Proteoglycan accumulation | Osmotic choroidal swelling | Increased proteoglycans in keratoconus [36,37] |
| Subclinical Inflammation | Cytokine-mediated vascular remodeling | Elevated cytokine levels in tears of patients with keratoconus [8,38] |
| Systemic associations | Shared connective tissue phenotypes | Increased choroidal thickness in patients with Ehler–Danlos and Down syndrome [39,40,41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazantzis, D.; Machairoudia, G.; Theodossiadis, P.; Chatziralli, I. Macular Choroidal Thickness in Keratoconus: Systematic Review and Meta-Analysis of Current Evidence. Diagnostics 2025, 15, 2394. https://doi.org/10.3390/diagnostics15182394
Kazantzis D, Machairoudia G, Theodossiadis P, Chatziralli I. Macular Choroidal Thickness in Keratoconus: Systematic Review and Meta-Analysis of Current Evidence. Diagnostics. 2025; 15(18):2394. https://doi.org/10.3390/diagnostics15182394
Chicago/Turabian StyleKazantzis, Dimitrios, Genovefa Machairoudia, Panagiotis Theodossiadis, and Irini Chatziralli. 2025. "Macular Choroidal Thickness in Keratoconus: Systematic Review and Meta-Analysis of Current Evidence" Diagnostics 15, no. 18: 2394. https://doi.org/10.3390/diagnostics15182394
APA StyleKazantzis, D., Machairoudia, G., Theodossiadis, P., & Chatziralli, I. (2025). Macular Choroidal Thickness in Keratoconus: Systematic Review and Meta-Analysis of Current Evidence. Diagnostics, 15(18), 2394. https://doi.org/10.3390/diagnostics15182394






