Clinical and Prognostic Impact of Hemodynamic Gain Index and Heart Hemodynamic Reserve in Heart Failure with Reduced and Mildly Reduced Ejection Fraction: A Multicenter Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Cardiopulmonary Exercise Test: HGI and HHR
2.3. Study Objectives
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Population
3.2. Functional Characterization of HGI and HHR
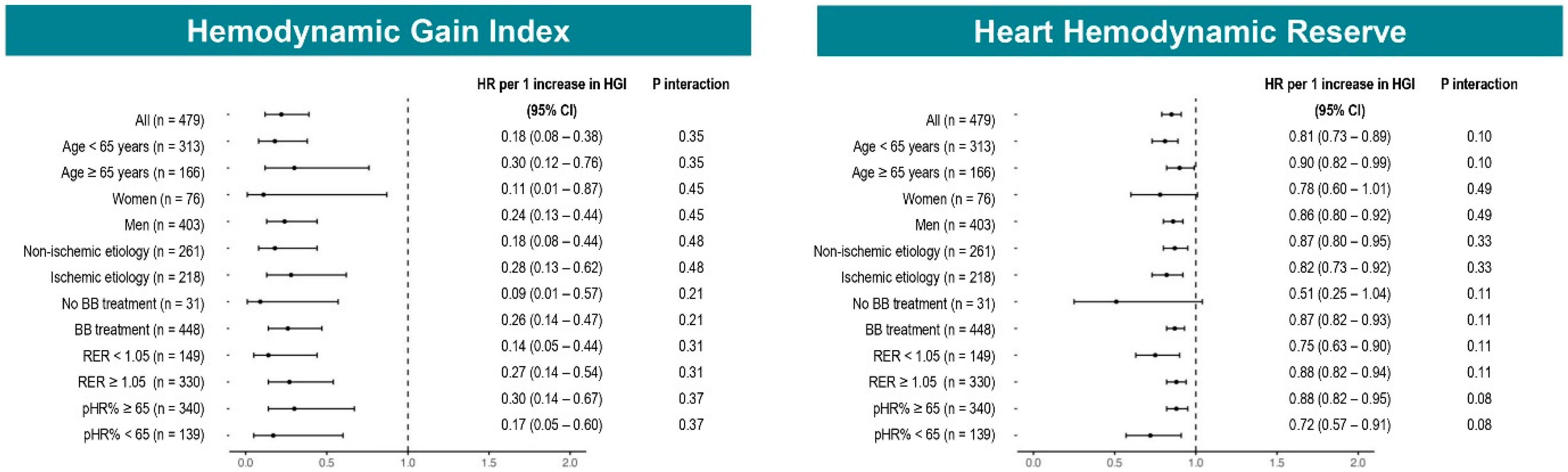
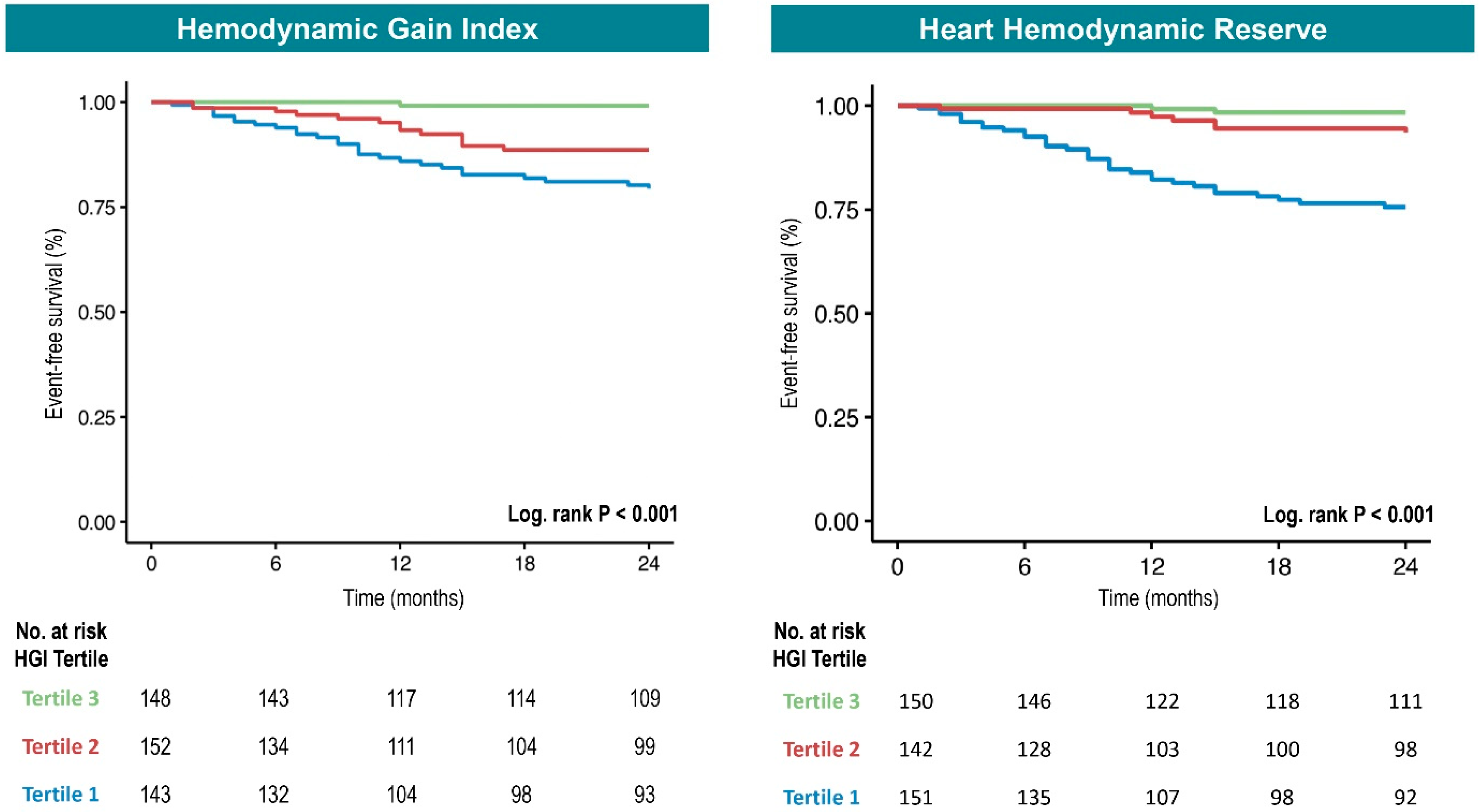
3.3. Prognostic Relevance of HGI and HHR
3.4. Prognostic Accuracy of HGI and HHR
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savarese, G.; Stolfo, D.; Sinagra, G.; Lund, L.H. Heart failure with mid-range or mildly reduced ejection fraction. Nat. Rev. Cardiol. 2022, 19, 100–116. [Google Scholar] [CrossRef]
- Lala, A.; Shah, K.B.; Lanfear, D.E.; Thibodeau, J.T.; Palardy, M.; Ambardekar, A.V.; McNamara, D.M.; Taddei-Peters, W.C.; Baldwin, J.T.; Jeffries, N.; et al. Predictive Value of Cardiopulmonary Exercise Testing Parameters in Ambulatory Advanced Heart Failure. JACC Heart Fail. 2021, 9, 226–236. [Google Scholar] [CrossRef]
- Magrì, D.; Gallo, G.; Parati, G.; Cicoira, M.; Senni, M. Risk stratification in heart failure with mild reduced ejection fraction. Eur. J. Prev. Cardiol. 2020, 27 (Suppl. S2), 59–64. [Google Scholar] [CrossRef]
- Magrì, D.; Piepoli, M.; Corrà, U.; Gallo, G.; Maruotti, A.; Vignati, C.; Salvioni, E.; Mapelli, M.; Paolillo, S.; Perrone Filardi, P.; et al. Cardiovascular Death Risk in Recovered Mid-Range Ejection Fraction Heart Failure: Insights From Cardiopulmonary Exercise Test. J. Card. Fail. 2020, 26, 932–943. [Google Scholar] [CrossRef]
- Myhre, P.L.; Kleiven, Ø.; Berge, K.; Grundtvig, M.; Gullestad, L.; Ørn, S. Changes in 6-min walk test is an independent predictor of death in chronic heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2024, 26, 2608–2615. [Google Scholar] [CrossRef]
- Fiori, E.; Magrì, D.; Iacovoni, A. Letter regarding the article ‘Changes in 6-min walk test is an independent predictor of death in chronic heart failure with reduced ejection fraction’. Eur. J. Heart Fail. 2024, 26, 2299. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, M.; Salvioni, E.; Bonomi, A.; Gugliandolo, P.; De Martino, F.; Vignati, C.; Berna, G.; Agostoni, P. How Patients With Heart Failure Perform Daily Life Activities: An Innate Energy-Saving Strategy. Circ. Heart Fail. 2020, 13, e007503. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Dickstein, K.; Vicenzi, M.; Arena, R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: A comparative analysis on clinical and prognostic insights. Circ. Heart Fail. 2009, 2, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Chaikijurajai, T.; Finet, J.E.; Engelman, T.; Wu, Y.; Martens, P.; Van Iterson, E.; Morales-Oyarvide, V.; Grodin, J.L.; Tang, W.H.W. Prognostic Value of Hemodynamic Gain Index in Patients With Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2024, 12, 261–271. [Google Scholar] [CrossRef]
- Vainshelboim, B.; Kokkinos, P.; Myers, J. Prognostic Value and Clinical Usefulness of the Hemodynamic Gain Index in Men. Am. J. Cardiol. 2019, 124, 644–649. [Google Scholar] [CrossRef]
- Morales-Oyarvide, V.; Richards, D.; Hendren, N.S.; Michelis, K.; Chaikijurajai, T.; MacNamara, J.P.; Sarma, S.; Farr, M.A.; Drazner, M.H.; Tang, W.H.W. Hemodynamic Gain Index and Exercise Capacity in Heart Failure With Preserved Ejection Fraction. Am. J. Cardiol. 2023, 190, 17–24. [Google Scholar] [CrossRef]
- Chaikijurajai, T.; Finet, J.E.; Wu, Y.; Harb, S.C.; Grodin, J.L.; Jaber, W.A.; Tang, W.H.W. Risk stratification with the Haemodynamic Gain Index and peak rate-pressure product in patients with chronic heart failure undergoing treadmill exercise testing. Eur. J. Prev. Cardiol. 2025, 32, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Magrì, D.; Piepoli, M.; Gallo, G.; Fiori, E.; Correale, M.; Attanasio, A.; Beltrami, M.; Lauretti, A.; Palazzuoli, A.; Agostoni, P. Chronotropic incompetence across heart failure categories. Eur. J. Prev. Cardiol. 2025, 32, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the Italian Working Group on Cardiac Rehabilitation Prevention; Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology; Piepoli, M.F.; Corrà, U.; Agostoni, P.G.; Belardinelli, R.; Cohen-Solal, A.; Hambrecht, R.; Vanhees, L. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: Recommendations for performance and interpretation. Part I: Definition of cardiopulmonary exercise testing parameters for appropriate use in chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 150–164. [Google Scholar] [CrossRef]
- Katritsis, D.; Camm, A.J. Chronotropic incompetence: A proposal for definition and diagnosis. Br. Heart J. 1993, 70, 400–402. [Google Scholar] [CrossRef]
- Astrand, I. Aerobic work capacity in men and women with special reference to age. Acta Physiol. Scand. Suppl. 1960, 49, 1–92. [Google Scholar]
- Magrì, D.; Piepoli, M.; Gallo, G.; Corrà, U.; Metra, M.; Paolillo, S.; Filardi, P.P.; Maruotti, A.; Salvioni, E.; Mapelli, M. Old and new equations for maximal heart rate prediction in patients with heart failure and reduced ejection fraction on beta-blockers treatment: Results from the MECKI score data set. Eur. J. Prev. Cardiol. 2022, 29, 1680–1688. [Google Scholar] [CrossRef]
- Magrì, D.; Corrà, U.; Di Lenarda, A.; Cattadori, G.; Maruotti, A.; Iorio, A.; Mezzani, A.; Giannuzzi, P.; Mantegazza, V.; Gondoni, E. Cardiovascular mortality and chronotropic incompetence in systolic heart failure: The importance of a reappraisal of current cut-off criteria. Eur. J. Heart Fail. 2014, 16, 201–209. [Google Scholar] [CrossRef]
- Magrì, D.; Gallo, G.; Piepoli, M.; Salvioni, E.; Mapelli, M.; Vignati, C.; Fiori, E.; Muthukkattil, M.L.; Corrà, U.; Metra, M. What about chronotropic incompetence in heart failure with mildly reduced ejection fraction? Clinical and prognostic implications from the Metabolic Exercise combined with Cardiac and Kidney Indexes score dataset. Eur. J. Prev. Cardiol. 2024, 31, 263–271. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Nadruz, W.; West, E.; Sengeløv, M.; Santos, M.; Groarke, J.D.; Forman, D.E.; Claggett, B.; Skali, H.; Shah, A.M. Prognostic Value of Cardiopulmonary Exercise Testing in Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e006000. [Google Scholar] [CrossRef]
- Agostoni, P.; Paolillo, S.; Mapelli, M.; Gentile, P.; Salvioni, E.; Veglia, F.; Bonomi, A.; Corrà, U.; Lagioia, R.; Limongelli, G. Multiparametric prognostic scores in chronic heart failure with reduced ejection fraction: A long-term comparison. Eur. J. Heart Fail. 2018, 20, 700–710. [Google Scholar] [CrossRef]
- Forman, D.E.; Myers, J.; Lavie, C.J.; Guazzi, M.; Celli, B.; Arena, R. Cardiopulmonary exercise testing: Relevant but underused. Postgrad. Med. 2010, 122, 68–86. [Google Scholar] [CrossRef]
- Buber, J.; Glikson, M.; Eldar, M.; Luria, D. Exercise Heart Rate Acceleration Patterns during Atrial Fibrillation and Sinus Rhythm. Ann. Noninvasive Electrocardiol. 2011, 16, 357–364. [Google Scholar] [CrossRef]
- Magrì, D.; Agostoni, P.; Corrà, U.; Passino, C.; Scrutinio, D.; Perrone-Filardi, P.; Correale, M.; Cattadori, G.; Metra, M.; Girola, D.; et al. Deceptive meaning of oxygen uptake measured at the anaerobic threshold in patients with systolic heart failure and atrial fibrillation. Eur. J. Prev. Cardiol. 2015, 22, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Mcmeekin, J.D.; Lautner, D.; Hanson, S.; Gulamhusein, S.S. Importance of Heart Rate Response During Exercise in Patients Using Atrioventricular Synchronous and Ventricular Pacemakers. Pacing Clin. Electrophysiol. 1990, 13, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Altaie, S.; Khalife, W. The prognosis of mid-range ejection fraction heart failure: A systematic review and meta-analysis. ESC Heart Fail. 2018, 5, 1008–1016. [Google Scholar] [CrossRef]
- Bassareo, P.P.; Crisafulli, A. Gender Differences in Hemodynamic Regulation and Cardiovascular Adaptations to Dynamic Exercise. Curr. Cardiol. Rev. 2020, 16, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Grilli, G.; Salvioni, E.; Moscucci, F.; Bonomi, A.; Sinagra, G.; Schaeffer, M.; Campodonico, J.; Mapelli, M.; Rossi, M.; Carriere, C. A matter of sex-persistent predictive value of MECKI score prognostic power in men and women with heart failure and reduced ejection fraction: A multicenter study. Front. Cardiovasc. Med. 2024, 11, 1390544. [Google Scholar] [CrossRef]


| General Data | n: 479 |
|---|---|
| Age (years) | 59.7 (12) |
| Female, n (%) | 76 (15.9) |
| BMI (kg/m2) | 26.9 (4.2) |
| Ischemic etiology, n (%) | 218 (45.5) |
| NYHA class, n (%) | |
| I | 116 (24) |
| II | 251 (52.5) |
| III | 112 (23.5) |
| HF medications | |
| ACEi, n (%) | 161 (34.9) |
| ARB, n (%) | 33 (9.3) |
| ARNi, n (%) | 250 (52.4) |
| Beta-blocker, n (%) | 448 (93.9) |
| MRA, n (%) | 381 (79.7) |
| SGLT2i, n (%) | 113 (31.7) |
| Diuretics, n (%) | 338 (71) |
| ICD, n (%) | 186 (39.1) |
| CRT-D, n (%) | 129 (27.1) |
| Laboratory variables | |
| Hb, g/dL | 14.2 (13.1–15.3) |
| Na+, mEq/L | 140 (139–142) |
| Creatinine, mg/dL | 1.1 (0.9–1.3) |
| eGFR (MDRD), mL/min/m2 | 53.6 (42.2–67.8) |
| BNP, pg/mL | 219 (97.5–478) |
| NT-proBNP, pg/mL | 918 (362–1914) |
| Echographic variables | |
| LVEF, % | 32.0 (7.9) |
| HFrEF, n (%) | 393 (82) |
| HFmrEF, n (%) | 86 (18) |
| sPAP, mmHg | 34.4 (12.2) |
| Exercise variables | |
| Baseline SBP, mmHg | 109.1 (15.1) |
| Baseline DBP, mmHg | 69.5 (9.2) |
| Baseline HR, bpm | 68.8 (12.4) |
| AT identified, n (%) | 313 (87.7) |
| AT VO2, mL/min | 954.4 (286.8) |
| Workload peak, Watts | 100.4 (39.7) |
| RER | 1.1 (0.1) |
| Peak SBP, mmHg | 139.2 (26.9) |
| Peak DBP, mmHg | 80.4 (12.2) |
| Peak HR, mmHg | 115.9 (23.6) |
| pHR% | 0.70 (0.1) |
| pVO2, mL/min | 1347.7 (443.8) |
| pVO2/Kg, mL/min/Kg | 16.9 (5.2) |
| pVO2% | 60 (20) |
| EOV, n (%) | 34 (9.6) |
| VE peak, L/min | 57.3 (16.7) |
| RF peak, bpm | 36.4 (12.5) |
| VE/VCO2 slope | 34.9 (9.1) |
| CP, mmHg.mL/min/m2 | 2399.9 (992.2) |
| HGI | 1.2 (0.7) |
| HHR | 9.7 (9.5) |
| Variables | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age (years) | 1.01 | 0.98–1.03 | 0.573 |
| Female | 1.65 | 0.70–3.85 | 0.249 |
| BMI (kg/m2) | 0.98 | 0.92–1.04 | 0.495 |
| Ischemic etiology | 1.44 | 1.05–1.98 | 0.025 |
| ACEi | 1.42 | 0.82–2.44 | 0.209 |
| ARB | 0.59 | 0.18–1.95 | 0.387 |
| ARNi | 0.58 | 0.32–1.03 | 0.061 |
| Beta-blocker | 0.31 | 0.15–0.63 | 0.001 |
| MRA | 1.36 | 0.67–2.78 | 0.395 |
| SGLT2i | 4.26 | 1.80–10.1 | <0.001 |
| Diuretics | 6.84 | 2.12–21.9 | 0.001 |
| Hb, g/dL | 0.84 | 0.72–0.98 | 0.026 |
| LVEF, % | 0.91 | 0.88–0.94 | <0.001 |
| sPAP, mmHg | 1.03 | 1.01–1.05 | 0.002 |
| pHR% | 0.02 | 0.002–0.16 | <0.001 |
| pVO2/Kg, mL/min/Kg | 0.89 | 0.85–0.94 | <0.001 |
| pVO2% | 0.02 | 0.006–0.10 | <0.001 |
| EOV, n (%) | 1.97 | 0.68–5.64 | 0.209 |
| VE/VCO2 slope | 1.06 | 1.04–1.08 | <0.001 |
| CP, mmHg.mL/min/m2 | 0.99 | 0.98–0.99 | <0.001 |
| HGI | 0.22 | 0.12–0.39 | <0.001 |
| HHR | 0.85 | 0.79–0.90 | <0.001 |
| Variables | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| HGI | 0.44 | 0.23–0.83 | 0.011 |
| Beta-blocker | 0.32 | 0.15–0.66 | 0.002 |
| Diuretics | 3.56 | 1.09–11.66 | 0.036 |
| LVEF, % | 0.95 | 0.91–0.98 | 0.006 |
| Ischemic etiology | 1.06 | 0.61–1.85 | 0.834 |
| Hb, g/dL | 1.00 | 0.86–1.17 | 0.967 |
| pVO2% | 0.20 | 0.02–1.93 | 0.164 |
| VE/VCO2 slope | 1.03 | 1.00–1.05 | 0.055 |
| Variables | Hazard Ratio | 95% CI | p-Value |
| HHR | 0.91 | 0.85–0.97 | 0.005 |
| Beta-blocker | 0.32 | 0.15–0.67 | 0.002 |
| Diuretics | 3.36 | 1.03–10.98 | 0.045 |
| LVEF, % | 0.95 | 0.91–0.99 | 0.008 |
| Ischemic etiology | 1.00 | 0.58–1.74 | 0.997 |
| Hb, g/dL | 1.01 | 0.87–1.19 | 0.860 |
| pVO2% | 0.21 | 0.021–2.14 | 0.189 |
| VE/VCO2 slope | 1.03 | 1.00–1.06 | 0.048 |
| HGI Tertile | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p | Adjusted Hazard Ratio | 95% CI | p | |
| Composite of CV death, LVAD implantation, and HTX | ||||||
| 1: <0.87 n = 143 | 12.77 | 3.94–41.45 | <0.001 | 5.35 | 1.57–18.22 | 0.007 |
| 2: 0.87–1.43 n = 152 | 5.76 | 1.68–19.77 | 0.005 | 3.47 | 0.98–12.24 | 0.053 |
| 3: >1.43 n = 148 | 1.00 | - | - | 1.00 | - | - |
| HHR Tertile | Univariate Model | Multivariate Model | ||||
| Hazard Ratio | 95% CI | p | Adjusted Hazard Ratio | 95% CI | p | |
| Composite of CV death, LVAD implantation, and HTX | ||||||
| 1: <4 n = 151 | 9.03 | 3.56–22.9 | <0.001 | 3.75 | 1.37–10.25 | 0.010 |
| 2: 4–10 n = 142 | 2.51 | 0.87–7.24 | 0.08 | 1.60 | 0.54–4.73 | 0.397 |
| 3: >10 n = 150 | 1.00 | - | - | 1.00 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiori, E.; Corradetti, S.; Gallo, G.; Palazzuoli, A.; Pagliaro, A.; Molle, R.; Tiberi, P.G.; Salvioni, E.; Piotti, A.; Gugliandolo, P.; et al. Clinical and Prognostic Impact of Hemodynamic Gain Index and Heart Hemodynamic Reserve in Heart Failure with Reduced and Mildly Reduced Ejection Fraction: A Multicenter Study. Diagnostics 2025, 15, 2366. https://doi.org/10.3390/diagnostics15182366
Fiori E, Corradetti S, Gallo G, Palazzuoli A, Pagliaro A, Molle R, Tiberi PG, Salvioni E, Piotti A, Gugliandolo P, et al. Clinical and Prognostic Impact of Hemodynamic Gain Index and Heart Hemodynamic Reserve in Heart Failure with Reduced and Mildly Reduced Ejection Fraction: A Multicenter Study. Diagnostics. 2025; 15(18):2366. https://doi.org/10.3390/diagnostics15182366
Chicago/Turabian StyleFiori, Emiliano, Sara Corradetti, Giovanna Gallo, Alberto Palazzuoli, Antonio Pagliaro, Roberta Molle, Pier Giorgio Tiberi, Elisabetta Salvioni, Arianna Piotti, Paola Gugliandolo, and et al. 2025. "Clinical and Prognostic Impact of Hemodynamic Gain Index and Heart Hemodynamic Reserve in Heart Failure with Reduced and Mildly Reduced Ejection Fraction: A Multicenter Study" Diagnostics 15, no. 18: 2366. https://doi.org/10.3390/diagnostics15182366
APA StyleFiori, E., Corradetti, S., Gallo, G., Palazzuoli, A., Pagliaro, A., Molle, R., Tiberi, P. G., Salvioni, E., Piotti, A., Gugliandolo, P., Agostoni, P., Magrì, D., & Barbato, E. (2025). Clinical and Prognostic Impact of Hemodynamic Gain Index and Heart Hemodynamic Reserve in Heart Failure with Reduced and Mildly Reduced Ejection Fraction: A Multicenter Study. Diagnostics, 15(18), 2366. https://doi.org/10.3390/diagnostics15182366






