Abstract
Invasive lobular carcinoma (ILC) is the most common special type of breast carcinoma, accounting for 5–15% of all breast carcinoma cases. Its metastatic pattern differs from that of invasive breast carcinoma of no special type, with ILC metastases to the peritoneum, gastrointestinal tract, and female genital tract being more frequent. This literature review focuses on ILC cases with metastasis to the female genital tract (FGT). Searches were conducted in medical databases including PubMed, Scopus, and Web of Science, using specific keywords. Inclusion criteria centered on studies presenting one or more cases of patients with ILC metastasis to the FGT and English language publications. Exclusion criteria included articles that did not present original research findings, studies with insufficient data, and publications in languages other than English. A thorough analysis of 154 results from PubMed, 56 from Scopus, and 173 from Web of Science after the application of inclusion and exclusion criteria resulted in the inclusion of 54 manuscripts describing 61 cases. The demographic, clinicopathological, and therapeutic aspects of ILC metastases to the FGT were reviewed and the differential diagnosis and prognosis for each anatomic location in the FGT were discussed separately. Our analysis of the data showed that the restricted mean survival time was 186 ± 30.7 months and that a negative ER on a secondary tumor was found to be linked to worse patient survival rates. Also of note is the fact that in 37.7% of cases there was involvement of multiple FGT anatomic locations and in 36% of cases there were metastases in organs or anatomic locations other than the FGT. To our knowledge, our study is the only one to describe the features of patients with secondary FGT involvement from ILC.
1. Introduction
Invasive lobular carcinoma (ILC) is the commonest special subtype of invasive breast carcinoma, accounting for 5–15% of breast carcinomas [1], thus being second in frequency only to invasive breast carcinoma of no special type (IBCNST) according to the latest WHO classification of breast tumors [2]. It was first described by Foote and Stewart in 1941 [3]. Its morphological, immunohistochemical, clinical, radiological, and molecular characteristics differ from those of IBCNST. It may remain undetectable or present as a palpable tumor [4]. In imaging studies, it may be detected with difficulty and tumor size may occasionally be underestimated, resulting in positive surgical margins [5]. The metastatic pattern also differs from that of IBCNST, with ILC metastases to the peritoneum, gastrointestinal tract, and female genital tract (FGT) being more frequent [6,7,8]. ILC lacks E-cadherin immunopositivity and displays aberrant β-catenin immunostaining [9]. Its molecular profile is characterized by deleterious mutations in CDH1 paired with allelic loss of the remaining allele [5].
Histologically, ILC is characterized by small cells with discohesive growth patterns forming single-cell files, and a minimal stromal response. Signet ring-like cells may sometimes be found [5]. Apart from the classic variant, several histological variants have been described, including alveolar, tubulolobular, solid, trabecular, signet ring, pleomorphic, and mixed [10,11,12,13,14,15]. Another three rare variants (ILC with extracellular mucin production, ILC with papillary features, and ILC with tubular elements) have been described recently [4].
We herewith review case reports and case series describing ILC metastases to the FGT. We also analyze the demographic, clinicopathological, and therapeutic aspects of ILC metastases to the female genital tract, and we discuss separately the differential diagnosis and prognosis for each anatomic location in the FGT.
2. Materials and Methods
2.1. Search Strategy
A literature review was conducted using PubMed, Scopus, and Web of Science to identify all published cases in the English language of ILC metastasis to the FGT. The research utilized the following terms: “lobular carcinoma” AND “metastasis” AND “female genital tract” OR “ovary” OR “ovarian” OR “vulva” OR “vagina” OR “endometrium” OR “endometrial” OR “uterus” OR “uterine”. We did not set any additional limitations while performing the search.
2.2. Inclusion and Exclusion Criteria
Two authors [MGS, KS] performed the literature review and collected data. Discrepancies were corrected by consensus. In cases where consensus could not be reached, the principal investigator (NK) resolved the disagreement.
The timeline for the selected studies ranged from October 1993 to April 2024.
Both reports with a single case and studies reporting at least two cases of ILC metastases to the FGT were included in the review. At the same time, we excluded narrative or systematic reviews, meta-analyses, opinion pieces, and other articles that did not present original research findings.
Papers available only as abstracts or those with text that was too brief or non-informative were excluded from the present review.
The clinicopathological and treatment parameters analyzed included age (median and range), clinical presentation, primary tumor size, ER, PR, and HER-2 status, both in primary focus and in the metastasis, tumor stage, location of metastatic involvement, metastases in organs or locations other than the FGT, surgical and neoadjuvant or adjuvant treatment, time interval to metastasis, median follow-up, outcome, and tumor grade.
In addition, cases with insufficient or too much aggregated data, as well as manuscripts in languages other than English, were excluded.
After applying inclusion and exclusion criteria, 54 manuscripts describing 61 cases of ILC with metastasis to the female genital tract [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] remained for data extraction.
2.3. Statistical Analysis
As detailed patient characteristics were available in the studied case reports, it was feasible to perform statistical analysis. Specifically, the descriptive characteristics of the quantitative data were expressed as median, Quartile 1 (Q1) to Quartile 3 (Q3), range, and, for completeness reasons mean ± standard deviation (SD). For the qualitative data, the frequency of occurrence and the relevant percentage were reported. It was also possible to evaluate overall survival (OS) via the Kaplan–Meier estimator and perform comparisons of OS with various characteristics via the log-rank method. The statistical analysis was performed using the R language for statistical analysis (version 4.4.0), and the significance level (p-value) was set to 0.05 when applicable tests were two-sided.
3. Results
3.1. Patient Characteristics
In total, 61 patients were reported in the studied case reports and case series. The mean patient age was 57.4 ± 12.2 years (min: 32; max: 86). The detailed descriptive statistics for patient characteristics are presented in Table 1.

Table 1.
Descriptive characteristics of the reported cases in the studied case reports. ANED—alive, no evidence of disease; AWD—alive with the disease; DOD—died of disease; SD—standard deviation.
3.2. Demographic and Clinicopathological Features
Metastasis of ILC to the FGT is uncommon. We were able to retrieve 54 manuscripts describing a total of 61 cases of ILC FGT secondaries. Primary tumor size was mentioned in 22/61 (36%) [17,18,21,22,23,25,28,32,34,36,39,40,41,43,50,53,58,59,63,65,66] cases. The mean tumor size was 36.5 mm (range 9–100 mm). The metastatic site was mentioned in all cases. Some of the patients had more than one FGT metastatic site. The most common metastatic site was the uterine corpus in 30/61 (49.2%) [20,24,27,28,30,35,36,38,39,40,41,43,44,45,46,47,49,50,51,53,54,55,56,59,61,62,64,65,66] patients, followed by the uterine cervix in 25/61 (41%) [21,22,24,27,28,30,31,32,34,35,39,44,46,50,53,54,58,59,62,65,66], and the ovary in 22/61 (36%) [28,29,30,35,36,38,42,44,48,51,53,54,57,63,65,66,67] patients. In 9/61 (14.7%) [16,20,26,27,52,60,68] cases, the metastatic site was an endometrial polyp, in 8/61 (13.1%) [17,23,27,33,37,38,44,45,52,59] a uterine leiomyoma, and in the vulva in 4/61 (6.5%) [18,25,37,42]. Less common metastatic sites were an ovarian granulosa cell tumor [19], the vagina [54], and an ovarian fibroma [69]. Metastases to sites other than the FGT were documented in 21/61 patients (34.4%) [18,20,21,23,36,37,38,40,41,42,43,44,45,46,49,57,59,62,63,64,66,67]. Metastatic spread to the bones was found in 12/21 (57.1%) [18,20,23,38,41,43,49,59,62,63,64,66] patients. Other metastatic sites were the pancreas [21], stomach [21,37,47,67], liver [36,38], pleura [37], peritoneum [37,42,43], lymph nodes [42,43,46], gallbladder [43], omentum [44], orbit [49], large bowel [57], and appendix [57].
Symptoms were mentioned in 60/61 (98.4%) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] cases. Bleeding from the genitalia was the commonest symptom, being reported in 24/60 (40%) [17,20,21,24,26,28,33,35,37,41,43,44,49,52,53,54,55,56,58,60,61,64,65,68] cases, followed by abdominal pain in 6/60 (10%) [19,22,57,59,60,69] patients, a mass in 5/60 (8.3%) [18,23,25,51] cases, abdominal distention in 3/60 (5%) [44,63,69] cases, and abdominal discomfort in 3/60 (%) [39,67] patients. Less common symptoms included abdominal fullness [29], loss of appetite [29], urinary incontinence [38], polyuria [39], abdominal bloating [42,57], abdominal compression [45], postcoital bleeding [46], vaginal fullness and discomfort [51], altered bowel habits [57], and right shoulder pain [66] in 1/60 (1.7%) of patients each. Finally, 14/60 (23.3%) [16,27,30,31,32,34,36,40,47,48,49,50,51,62] patients were asymptomatic. In 42 cases, the FGT metastasis was metachronous, while in 12 cases it was concurrent with the primary tumor. The interval to metastasis ranged from 2 to 360 months (mean 65.6 months). The detailed demographic and clinicopathological features of the cases are shown in Table 2.

Table 2.
Demographic and clinicopathological features of the reported cases.
3.3. Histological Findings
No information regarding the subtype of ILC was available, apart from one case of ILC with extracellular mucin production [69], which is a very rare subtype with around forty cases in the English literature [1,3,4].
3.4. Estrogen Receptors (ER)/Progesterone Receptors (PR)/HER-2 Status
Concerning hormonal and HER-2 status, 36/61 (59%) [18,20,21,23,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,54,55,56,58,59,63,65,66,68] cases reported ER and PR in the primary focus and 34/61 (55.7%) [18,19,20,21,23,26,27,28,29,32,35,37,39,40,41,42,44,45,46,47,48,50,52,56,57,58,59,62,63,64,66,68] in the metastatic setting. HER-2 status was reported in 24/61 (39.3%) cases [29,30,32,34,35,37,38,39,40,41,43,44,45,47,50,54,55,56,58,59,63,65,66,68] in the primary focus and in 12/61 cases (19.7%) [28,29,32,35,46,47,52,58,62,64] in the metastatic location. ER, PR, and HER-2 status of the cases and the histological grades are shown in Table 3.

Table 3.
ER, PR, and HER-2 status and histological grades of the reported cases.
3.5. Treatment
Surgical treatment information was available in 51/61 (83.6%) [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,52,54,55,56,57,58,59,61,62,63,64,65,66,67,68] cases. In 32/51 (62.7%) [17,20,21,22,23,25,26,27,28,29,30,31,35,39,40,42,44,47,49,54,55,56,57,58,61,62,65,66,67,68] cases, surgical treatment consisted of a modified radical mastectomy. Breast-conserving surgery was performed in 9/51 (17.6%) [18,32,34,36,41,45,46,48,53] cases, hysterectomy and bilateral salpingo-oophorectomy in 5/51 [23,36,39,59,63] (9.8%) cases, and breast biopsies in 3/51 (5.9%) [33,43,44] cases. Omentectomy was performed in 2/51 (3.9%) [36,63] cases. Endometrial and cervical biopsies were performed in 2/51 (3.9%) [34,49] cases, cervical biopsy in 1/51 (1.9%) [34] cases, and partial vulvectomy in 1/51 (1.9%) [18] cases. Additionally, two manuscripts reported that surgical treatment was performed without any additional detail concerning the type of intervention [52,64]. In cases with metachronous FGT metastasis, second-line treatment was reported in 43/49 (87.7%) [16,19,20,21,22,23,25,27,28,29,30,31,32,33,35,37,38,40,41,42,44,45,46,47,48,49,52,53,54,55,56,57,58,60,61,62,64,65,66,68] cases. Surgical treatment involved hysterectomy and bilateral salpingo-oophorectomy in 22/43 (51.2%) [16,21,26,28,29,30,32,35,38,41,42,45,48,49,52,53,54,55,58,60,64,65,66,68] patients, bilateral oophorectomy in 2/43 (4.6%) [19,57] cases, pancreatoduodenectomy in 1/43 (2.3%) [21] cases, wide tumor excision in 1/43 (2.3%) [25] cases, omentectomy in 4/43 (9.3%) [29,30,48,64] cases, pelvic lymph node sampling in 1/43 (2.3%) [29] cases, pelvic lymphadenectomy in 2/43 (4.6%) [30,32] cases, excision of endometrial polyps in 2/43 (4.6%) [33,37] cases, appendectomy in 2/43 (4.6%) [42,57] cases, endometrial biopsy in 1/43 (2.3%) [47] cases, peritoneal biopsies in 2/43 (4.6%) [48,64] cases, partial colectomy in 1/43 (2.3%) [52] cases, and anterior resection in 1/43 (2.3%) [57] cases. Biopsy of the metastatic lesion was performed in 5/43 (11.6%) cases [20,46,56,61,62].
Information regarding adjuvant treatment was provided in 50/61 (83.6%) [17,19,20,21,22,23,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,52,53,54,55,56,57,58,59,62,63,64,65,66,67,68] cases. Chemotherapy either in the adjuvant or neoadjuvant setting was offered in 43/50 (86%) [17,19,20,22,23,25,27,28,29,31,33,34,35,36,38,40,41,42,43,44,45,46,47,48,49,52,53,54,55,56,57,58,59,62,63,64,65,66,67,68] cases and radiotherapy in 27/50 (54%) [19,20,22,27,28,29,31,32,34,35,42,44,45,46,47,48,49,52,53,54,55,57,58,62,65,67,68] cases. In two cases, patients refused chemotherapy [30,50], and in another two, radiotherapy [30,66]. The most common regimen used consisted of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF regimen). In contrast, the second most common regimen consisted of adriamycin, cyclophosphamide (AC regimen), and paclitaxel administered in four [20,25,31,42] and three cases [54,58,63], respectively. Hormonal treatment was provided to 48/50 (96%) [17,19,20,21,22,23,25,26,27,28,29,30,31,32,34,35,36,37,38,39,40,41,42,43,44,45,46,47,49,52,53,54,55,56,57,58,59,62,63,64,65,66,67,68] patients. Among 43 cases with metachronous metastasis, 13 received additional chemotherapy [21,25,30,32,40,42,44,46,47,48,54,56,66,67], one received targeted therapy [65], two received additional radiotherapy [23,41], and 13 received additional hormonal treatment [19,21,23,32,35,41,42,45,52,58,64,65,68].
3.6. Outcome
Follow-up information was available in 40/61 (65.6%) [17,18,19,21,22,23,25,28,30,31,32,34,35,36,37,38,39,40,41,42,43,45,47,48,50,51,52,53,54,58,60,62,63,64,66,67,68] cases. Briefly, 16/40 (40%) [17,19,32,35,36,37,38,39,45,48,52,53,58,60,64,67,68] patients were alive without evidence of disease, 9/40 (22.5%) [18,23,25,30,34,41,42,47,51] were alive with disease, and 10/40 (25%) [21,28,31,40,43,51,54,66] died of disease in a period of time that ranged from 1 to 308 months. In 2/40 (5%) [22,50] cases, patients were lost to follow-up. Treatment and follow-up data are shown in Table 4.

Table 4.
Treatment and follow-up features of the reported cases.
3.7. Patient Survival
Patient survival time information was available for 31 patients. Of these patients, 10 were deceased due to their disease. The restricted mean survival time was 186 ± 30.7 months. Kaplan–Meier curves for overall survival are shown in Figure 1.
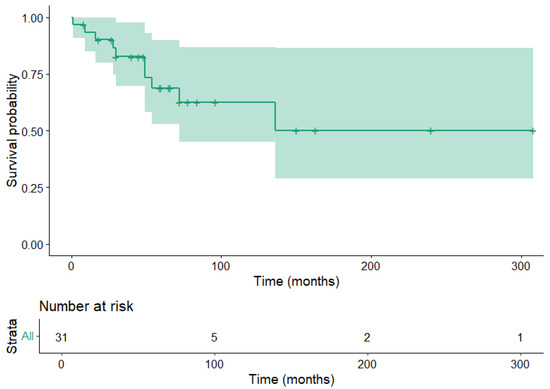
Figure 1.
Kaplan–Meier estimator for patient overall survival. The shaded area corresponds to the 95% confidence interval.
Further analysis was based on the evaluation of the role of all recorded characteristics in patient survival. The results are depicted in Table 5. Notably, a negative ER on a secondary tumor was found to be linked to worse patient survival (HR: 0.13, 95% CI: 0.02–0.8, p = 0.01); see Figure 2.

Table 5.
Role of the study variables in patient overall survival. HR—hazard ratio, CI—confidence interval; N—number of valid cases.
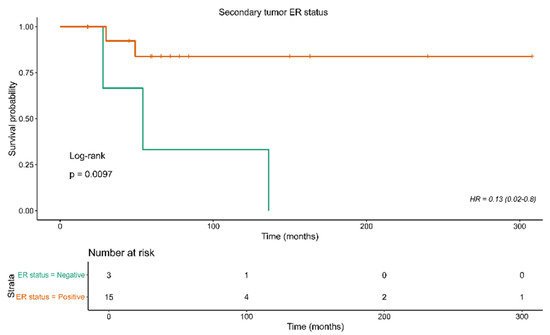
Figure 2.
Kaplan–Meier curves for overall survival in relation to ER status of the secondary tumor.
4. Discussion
Extragenital metastases to the FGT are relatively uncommon. Concerning specific locations of secondaries, one study reported that, among 149 metastatic neoplasms to the FGT from primary extragenital tumors reported in one study, the ovary (75.8%) and vagina (13.4%) were the most frequent locations, followed by the endometrium (4.7%) and cervix (3.4%) [70]. In our review the most common metastatic location was the uterine corpus in 30 (49.2%) cases, followed by the uterine cervix in 25 (41%) patients, and the ovary in 22 (36%) patients. We believe this difference can be explained by the fact that we analyzed mostly case reports. We excluded from our search some cases series reporting ovarian metastases due to the fact that they did not mention the subtype of metastatic breast carcinoma. The majority of FGT metastases from breast cancer occur in advanced cases during hormonal treatment or follow-up [53]. Similarly, in several of our cases metastasis of ILC occurred while patients were under hormonal treatment.
In the literature, the incidence of ILC metastasis to the FGT ranges from 2% to 5% in clinical series [5] and from 36% to 52% in autopsy series [71,72]. This difference is probably due to the fact that autopsy may discover clinically occult micrometastases. In our review, there was involvement of more than one FGT site in 37.7% of cases, and in 36% of cases there were metastases in organs or anatomic locations other than the FGT. The most common locations were skeletal metastases, which occurred in more than half of these cases, followed by the stomach.
The ovaries frequently receive metastases from primary malignant tumors both of genital and extragenital sites [73,74]. This can be explained by the fact that they provide an excellent environment for malignant cell implantation due to their rich vasculature and extensive lymphatic network, as well as due to a favorable pH and oxygen pressure in the ovarian stroma [31]. In a number of studies, the most common primary site varies between the gastrointestinal tract and the breast [75]. Ovarian metastasis from breast carcinoma constitutes 3–38% of all ovarian neoplasms, with a variable incidence depending on diagnostic methods, geographic distributions, and other variables [76]. Studies have shown that breast cancer patients have an incidence of ovarian metastases of 13–47%, either in autopsy or surgical material [77,78]. ILC ovarian metastases usually manifest as bilateral solid and cystic masses, the so-called Krükenberg tumor(s). Micrometastatic disease may remain undetected both on clinical examination and in imaging studies [79].
Metastases in the ovaries may occasionally mimic the clinical and histological characteristics of primary ovarian carcinomas [73]. The distinction between metastatic versus primary ovarian carcinoma is of paramount importance since their management differs [72,75]. To date, there are no clear guidelines concerning the management of carcinomas metastatic to the ovary. However, surgical resection may increase patient survival rates [79].
Some clinicopathologic factors of primary breast carcinoma have been identified to be related to increased risk of ovarian metastasis. ILC has an increased metastatic potential to the ovaries [5]. Also, young age and premenopausal status are factors related to increased risk [80]. Other factors related to the development of ovarian metastases are other co-existent metastatic sites [81], large primary tumor size [82,83], inflammatory breast cancer [84], positive lymph nodes [85,86], higher stage (III-IV) [87], and bilaterality [81].
The incidental finding of an ovarian mass in an asymptomatic patient may be the first sign of ovarian metastasis [77]. Usually, they are bilateral, small, and solid [88,89]. Other symptoms, including gastrointestinal symptoms, ascites, pelvic pain, and vaginal bleeding, can be observed in some patients [77]. However, none of these clinical manifestations is related to either breast metastasis or primary ovarian carcinoma [90]. In our review, only 3/20 (15%) [30,48,51] cases were asymptomatic.
Imaging examinations are also widely used for the diagnosis, staging, and monitoring of curative effects.
The pathologic examination of a specimen includes gross, microscopic, and immunohistochemical testing. These are considered the ‘gold standard’ in the diagnosis of metastatic breast cancer to the ovaries [91].
On gross examination, bilateral involvement, small size, and a solid mass are clues related to metastatic breast carcinoma [92,93,94]. Metastases in the ovaries are usually located in the ovarian medulla and/or cortex [88]. On the other hand, primary tumors are typically located in the ovarian surface epithelium and superficial cortex [95].
Microscopically, ovarian metastases sometimes mimic histological features of primary ovarian carcinomas [96,97], which makes their distinction difficult. The characteristic pattern of ILC of small, discohesive cells forming single-cell files will usually allow diagnosis on hematoxylin and eosin stains. In difficult cases, immunohistochemical analysis typically resolves any diagnostic problem. Immunostaining for TRPS1, GATA-3, GCDFP-15, and mammaglobin favors metastasis of breast origin [98], whereas PAX-8, WT-1 p53, and p16 staining favors primary ovarian carcinoma. It is important to remember that mammaglobin can be expressed in gynaecologic malignancies [99].
Concerning treatment options, most breast cancer patients have other non-FGT metastases at the time of ovarian metastasis. The treatment should be for systemic disease. The regimen should be tailored to the clinicopathological aspects of the metastatic site, the burden of disease, the eventual visceral crisis, the symptoms, and the performance status of the patient. Drug toxicity profile and patient preferences are of utmost importance [85].
The prognosis of patients with breast carcinoma ovarian metastases is poor since the median progression-free survival ranges from 9 to 30 months, the median overall survival is 16 to 38 months, and the 5-year survival rate is 6 to 26% [79].
Clinicopathologic factors that affect survival are age [96], time interval to ovarian metastasis [82], unilaterality [100], and menstrual status [89].
Uterine metastases from extragenital cancers are much less common than ovarian metastases [52]. Metastases confined to the uterus, without ovarian involvement, are very rare and can occur through hematogenous spread [52]. The myometrium is the most commonly involved location within the corpus uteri that metastatic ILC involves, followed by the endometrium. The first manifestation of metastatic disease may be abnormal uterine bleeding [86]. ILC is, in most cases, ER-positive. Premenopausal patients regularly receive tamoxifen as part of the adjuvant treatment, which increases the risk for endometrioid carcinoma of the endometrium [86]. Differentiating metastatic ILC from a primary endometrial carcinoma can be difficult but is of huge importance since the treatment is different for these carcinomas.
Metastasis from breast carcinoma to the cervix uteri is very rare, with an estimated frequency of 0.8–1.7% [101]. This is possibly due to its small size, its reduced blood flow and distal circulation, and the presence of abundant fibrous tissue [102]. The true incidence of cervical metastasis from ILC remains unknown. Other distant metastases were found at the time of diagnosis of cervical metastasis in 67–89% of cases [103]. In our review, we found 22 cases of ILC metastatic to the cervix. Other distant metastases were found in only 27% of cases. Differential diagnosis between a cervical primary and ILC cervical metastasis may occasionally prove difficult. Dedifferentiation of cervical adenocarcinoma and squamous cell carcinoma with acantholytic changes may result in the simulation of the morphology of ILC [104]. Again, the finding of the characteristic morphology of ILC, i.e., discohesive cells and the formation of single cell files within the cervical stroma, especially when the cervical epithelium is spared, should raise the suspicion of metastatic disease. Appropriate immunohistostaining with TRPS-1, GATA3, mammaglobin, and GCDFP-15 will provide the diagnostic solution in ambiguous cases.
Vaginal metastasis is second in frequency after ovarian involvement in the FGT. Treatment consists of surgical debulking chemotherapy and/or radiotherapy. For patients with vaginal metastasis from breast carcinoma a very important prognostic feature is the finding of metastases in other sites [105]. A lot of times, when vaginal metastases occur, there are already metastases in other organs. Whenever this happens, the prognosis is poor [106]. In our review we found a single case of vaginal metastasis. This case had metastases also to the uterus, bilateral ovaries, and cervix. The patient succumbed to disease 16 months after diagnosis.
Breast cancer metastasis to the vulva is very rare. In these cases, the differential diagnosis is conducted with primary breast carcinoma of the vulva [107]. The most important distinguishing feature is previous history of breast cancer. Additionally, the histological similarity between the primary breast and the metastatic lesion, as well as the absence of an in situ element, will guide the pathologist in diagnosing a metastatic lesion [107].
Our study is unique due to the fact that it analyzes the very rare occurrence of FGT metastases of ILC. However, there are some limitations in our study. Missing information in several papers was frequently encountered. Missing data included hormone receptor status, detailed information on treatment in some cases, and information concerning survival. We propose that in the future, cases of ILC with FGT metastases from many different centers should be gathered, with sufficient demographic, clinical, pathological, treatment, and outcome information to draw more definitive conclusions regarding the prognosis of ILC metastasis to the FGT.
5. Conclusions
In summary, we reviewed cases and case series of ILC metastasis to the FGT, describing clinical, pathological, therapeutic, and follow-up data. We also discussed the current literature focusing on the differential diagnosis, treatment, and prognosis of ILC metastasis to the FGT. Our review showed that the most common site of FGT metastasis from ILC was the uterus followed by the uterine cervix, a finding that is not in line with previous studies. Involvement of more than one FGT site was present in 37.7% of cases, and metastases in locations other than the FGT were present in 36% of cases. These findings show that FGT metastasis from ILC occurs frequently in the setting of disseminated disease.
Author Contributions
Conceptualization, N.I.K. and I.B.; methodology, G.B., R.C. and M.Z. (Maurizio Zizzo).; software, K.S., M.G.S. and A.P. (Abraham Pouliakis); validation, M.G.S., T.N., N.A. and A.P. (Amanda Psyrri); formal analysis, G.B., R.C. and M.Z. (Maurizio Zizzo) investigation, I.S.P. and D.G.; resources, K.S., T.N. and J.S.; data curation, A.P. (Andrea Palicelli), S.S. and M.Z. (Magda Zanelli); writing—original draft preparation, N.I.K., A.P. (Abraham Pouliakis), and J.S.; writing—review and editing, all authors; visualization, I.S.P. and D.G.; supervision, N.A., A.P. (Amanda Psyrri), and I.G.P.; project administration, S.S., I.B. and A.K.; funding acquisition, A.P. (Andrea Palicelli), I.G.P. and M.Z. (Magda Zanelli). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This study was partially supported by the Italian Ministry of Health—Ricerca Corrente Annual Program 2026.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This article is a review and not an original study. All the references are listed.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cserni, G.; Floris, G.; Koufopoulos, N.; Kovács, A.; Nonni, A.; Regitnig, P.; Stahls, A.; Varga, Z. Invasive lobular carcinoma with extracellular mucin production—A novel pattern of lobular carcinomas of the breast. Clinico-pathological description of eight cases. Virchows Arch. 2017, 471, 3–12. [Google Scholar] [CrossRef]
- WHO. WHO Classification of Tumours. Breast Tumours, 5th ed.; Lokuhetty, D., White, V.A., Watanave, R., Cree, I.A., Eds.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Koufopoulos, N.; Antoniadou, F.; Kokkali, S.; Pigadioti, E.; Khaldi, L. Invasive lobular carcinoma with extracellular mucin production: Description of a case and review of the literature. Cureus 2019, 11, e5550. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Pateras, I.S.; Gouloumis, A.R.; Ieronimaki, A.I.; Zacharatou, A.; Spathis, A.; Leventakou, D.; Economopoulou, P.; Psyrri, A.; Arkadopoulos, N.; et al. Diagnostically Challenging Subtypes of Invasive Lobular Carcinomas: How to Avoid Potential Diagnostic Pitfalls. Diagnostics 2022, 12, 2658. [Google Scholar] [CrossRef]
- Reed, A.E.M.; Kutasovic, J.R.; Lakhani, S.R.; Simpson, P.T. Invasive lobular carcinoma of the breast: Morphology, biomarkers and’omics. Breast Cancer Res. 2015, 17, 12. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Kokkali, S.; Antoniadou, F.; Dimas, D.T.; Missitzis, I.L. Matrix-producing Breast Carcinoma: A Rare Subtype of Metaplastic Breast Carcinoma. Cureus 2019, 11, e5188. [Google Scholar] [CrossRef]
- Mathew, A.; Rajagopal, P.S.; Villgran, V.; Sandhu, G.S.; Jankowitz, R.C.; Jacob, M.; Rosenzweig, M.; Oesterreich, S.; Brufsky, A. Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd. 2017, 77, 660–666. [Google Scholar] [CrossRef]
- Kioleoglou, Z.; Georgaki, E.; Koufopoulos, N.; Kostek, O.; Volakakis, N.; Dimitriadou, A.; Kokkali, S. Gastrointestinal metastases from lobular breast carcinoma: A literature review. Cureus 2024, 16, e65852. [Google Scholar] [CrossRef]
- Cserni, G.; Bori, R.; Ambrózay, É.; Serfőző, O. Histological Patterns and Mammographic Presentation of Invasive Lobular Carcinoma Show No Obvious Associations. Cancers 2024, 16, 1640. [Google Scholar] [CrossRef]
- Cserni, G. Invasive lobular carcinoma of the breast: We diagnose it, but do we know what it is? Pathologica 2024, 116, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Christgen, M.; Cserni, G.; Floris, G.; Marchio, C.; Djerroudi, L.; Kreipe, H.; Derksen, P.W.B.; Vincent-Salomon, A. Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors. Cancers 2021, 13, 3695. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica 2020, 112, 25–41. [Google Scholar] [CrossRef]
- Koufopoulos, N.I.; Boutas, I.; Pouliakis, A.; Samaras, M.G.; Kotanidis, C.; Kontogeorgi, A.; Dimas, D.T.; Ieronimaki, A.I.; Leventakou, D.; Spathis, A.; et al. The “forgotten” subtypes of breast carcinoma: A systematic review of selected histological variants not included or not recognized as distinct entities in the current World Health Organization classification of breast tumors. Int. J. Mol. Sci. 2024, 25, 8382. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Ieronimaki, A.-I.; Zacharatou, A.; Gouloumis, A.R.; Leventakou, D.; Boutas, I.; Dimas, D.T.; Kontogeorgi, A.; Sitara, K.; Khaldi, L.; et al. A Case of Prostatic Signet-Ring Cell-like Carcinoma with Pagetoid Spread and Intraductal Carcinoma and Long-Term Survival: PD-L1 and Mismatch Repair System Proteins (MMR) Immunohistochemical Evaluation with Systematic Literature Review. J. Pers. Med. 2023, 13, 1016. [Google Scholar] [CrossRef]
- Christgen, M.; Steinemann, D.; Kühnle, E.; Länger, F.; Gluz, O.; Harbeck, N.; Kreipe, H. Lobular breast cancer: Clinical, molecular and morphological characteristics. Pathol. Res. Pract. 2016, 212, 583–597. [Google Scholar] [CrossRef]
- Aranda, F.I.; Laforga, J.B.; Martinez, M.A. Metastasis from breast lobular carcinoma to an endometrial polyp Report of a case with immunohistochemical study. Acta Obstet. Gynecol. Scand. 1993, 72, 585–587. [Google Scholar] [CrossRef]
- Sugiyama, T.; Toyoda, N.; Nose, J.; Kihira, N.; Ando, Y.; Ishihara, A. Breast cancer metastatic to uterine leiomyoma: A case report. J. Obstet. Gynaecol. 1995, 21, 349–355. [Google Scholar] [CrossRef]
- Menzin, A.W.; De Risi, D.; Smilari, T.F.; Kalish, P.E.; Vinciguerra, V. Lobular breast carcinoma metastatic to the vulva: A case report and literature review. Gynecol. Oncol. 1998, 69, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Arnould, L.; Franco, N.; Soubeyrand, M.S.; Mege, F.; Belichard, C.; Lizard-Nacol, S.; Collin, F. Breast carcinoma metastasis within granulosa cell tumor of the ovary: Morphologic, immunohistologic, and molecular analyses of the two different tumor cell populations. Hum. Pathol. 2002, 33, 445–448. [Google Scholar] [CrossRef]
- Alvarez, C.; Ortiz-Rey, J.; Estévez, F.; De La Fuente, A. Metastatic lobular breast carcinoma to an endometrial polyp diagnosed by hysteroscopic biopsy. Obstet. Gynecol. 2003, 102, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Ogino, A.; Nomizu, T.; Gonnda, K.; Okouchi, C.; Sakuma, T.; Yamada, M.; Katagata, N.; Watanabe, F.; Yamaguchi, Y.; Yoshida, T. A case of breast cancer metastasizing to cervix after resection of pancreatic metastasis. Breast Cancer 2003, 10, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Rau, A.R.; Saldanha, P.; Raghuveer, C. Metastatic lobular mammary carcinoma diagnosed in cervicovaginal smears: A case report. Diagn. Cytopathol. 2003, 29, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Blecharz, P.; Szpor, J.; Karolewski, K.; Ryś, J. Breast cancer metastases to uterine leiomyomas-a clinical and patomorphological analysis of two cases. Nowotw. J. Oncol. 2004, 54, 488. [Google Scholar]
- Famoriyo, A.; Sawant, S.; Banfield, P.J. Abnormal uterine bleeding as a presentation of metastatic breast disease in a patient with advanced breast cancer on tamoxifen therapy. Arch. Gynecol. Obstet. 2004, 270, 192–193. [Google Scholar] [CrossRef]
- Sheen-Chen, S.M.; Eng, H.L.; Huang, C.C. Breast cancer metastatic to the vulva. Gynecol. Oncol. 2004, 94, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Al-Brahim, N.; Elavathil, L.J. Metastatic breast lobular carcinoma to tamoxifen-associated endometrial polyp: Case report and literature review. Ann. Diagn. Pathol. 2005, 9, 166–168. [Google Scholar] [CrossRef]
- Lee, T.F.; Wang, Y.L.; Wei, T.S.; Chen, C.P. Incidental detection of metastatic lobular breast carcinoma in the female internal genital organs 2 years following modified radical mastectomy. Taiwan. J. Obstet. Gynecol. 2005, 44, 368–371. [Google Scholar] [CrossRef][Green Version]
- Scopa, C.D.; Aletra, C.; Lifschitz-Mercer, B.; Czernobilsky, B. Metastases of breast carcinoma to the uterus. Report of two cases, one harboring a primary endometrioid carcinoma, with review of the literature. Gynecol. Oncol. 2005, 96, 543–547. [Google Scholar] [CrossRef]
- Chen, P.; Hu, W.M.; Wang, P.H.; Suen, J.H. Recurrent breast cancer presents as a single solid ovarian mass and ascites. Taiwan. J. Obstet. Gynecol. 2006, 45, 356–359. [Google Scholar] [CrossRef]
- Erkanli, S.; Kayaselcuk, F.; Kuscu, E.; Bolat, F.; Sakalli, H.; Haberal, A. Lobular carcinoma of the breast metastatic to the uterus in a patient under adjuvant anastrozole therapy. Breast 2006, 15, 558–561. [Google Scholar] [CrossRef]
- Perišić, D.; Jančić, S.; Kalinović, D.; Čekerevac, M. Metastasis of lobular breast carcinoma to the cervix. J. Obstet. Gynaecol. Res. 2007, 33, 578–580. [Google Scholar] [CrossRef]
- Manci, N.; Marchetti, C.; Esposito, F.; Graziano, M.; Tomao, F.; Pastore, M.; Bellati, F.; Panici, P.B. Late breast cancer recurrence to the uterine cervix with a review of the literature. Int. J. Gynecol. Pathol. 2008, 27, 113–117. [Google Scholar] [CrossRef]
- Manipadam, M.; Walter, N.; Selvamani, B. Lobular carcinoma metastasis to endometrial polyp unrelated to tamoxifen: Report of a case and review of the literature. Apmis 2008, 116, 538–540. [Google Scholar] [CrossRef]
- Bogliolo, S.; Morotti, M.; Valenzano Menada, M.; Fulcheri, E.; Musizzano, Y.; Casabona, F. Breast cancer with synchronous massive metastasis in the uterine cervix: A case report and review of the literature. Arch. Gynecol. Obstet. 2010, 281, 769–773. [Google Scholar] [CrossRef]
- Ustaalioglu, B.B.; Bilici, A.; Seker, M.; Salman, T.; Gumus, M.; Barisik, N.O.; Salepci, T.; Yaylaci, M. Metastasis of lobular breast carcinoma to the uterus in a patient under anastrozole therapy. Oncologie 2009, 32, 424–426. [Google Scholar] [CrossRef]
- Engelstaedter, V.; Mylonas, I. Lower genital tract metastases at time of first diagnosis of mammary invasive lobular carcinoma. Arch. Gynecol. Obstet. 2011, 283, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Hooker, A.; Radder, C.; van De Wiel, B.; Geenen, M. Metastasis from breast cancer to an endometrial polyp; treatment options and follow-up. Report of a case and review of the literature. Eur. J. Gynaecol. Oncol. 2011, 32, 228–230. [Google Scholar]
- Işçi, H.; Güdücü, N.; Basgul, A.; Aydınlı, K.; Calay, Z.; Dünder, I. Lobular carcinoma of the breast metastasızıng to leiomyoma in a patient under letrozole treatment. Eur. J. Gynaecol. Oncol. 2011, 32, 560–562. [Google Scholar] [PubMed]
- Horikawa, M.; Mori, Y.; Nagai, S.; Tanaka, S.; Saito, S.; Okamoto, T. Metastatic breast cancer to the uterine cervix mimicking a giant cervical leiomyoma. Nagoya J. Med. Sci. 2012, 74, 347. [Google Scholar] [PubMed]
- Komeda, S.; Furukawa, N.; Kasai, T.; Washida, A.; Kobayashi, H. Uterine metastasis of lobular breast cancer during adjuvant letrozole therapy. J. Obstet. Gynaecol. 2013, 33, 100–101. [Google Scholar] [CrossRef]
- Vicioso, L.; Ortega, M.V.; Cívico, V.; López-Beltrán, A. Synchronous metastasis from lobular carcinoma and primary carcinoma of the endometrium in a patient after tamoxifen therapy. Int. J. Gynecol. Pathol. 2013, 32, 66–70. [Google Scholar] [CrossRef]
- Alligood-Percoco, N.R.; Kessler, M.S.; Willis, G. Breast cancer metastasis to the vulva 20 years remote from initial diagnosis: A case report and literature review. Gynecol. Oncol. Rep. 2015, 13, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Bezpalko, K.; Mohamed, M.A.; Mercer, L.; McCann, M.; Elghawy, K.; Wilson, K. Concomitant endometrial and gallbladder metastasis in advanced multiple metastatic invasive lobular carcinoma of the breast: A rare case report. Int. J. Surg. Case Rep. 2015, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Lokadasan, R.; Ratheesan, K.; Sukumaran, R.; Nair, S.P. Metastatic lobular carcinoma of breast mimics primary cervix carcinoma: Two case reports and a review of the literature. Ecancermedicalscience. 2015, 9, 571. [Google Scholar] [CrossRef][Green Version]
- Toyoshima, M.; Iwahashi, H.; Shima, T.; Hayasaka, A.; Kudo, T.; Makino, H.; Igeta, S.; Matsuura, R.; Ishigaki, N.; Akagi, K.; et al. Solitary uterine metastasis of invasive lobular carcinoma after adjuvant endocrine therapy: A case report. J. Med. Case Rep. 2015, 9, 47. [Google Scholar] [CrossRef]
- Waks, A.G.; Lennon, J.; Yadav, B.S.; Hwang, H.; dSchapirael Carmen, M.; Johnson, N.B.; Reynolds, K.; Schapira, L.; Gilman, P.B.; Overmoyer, B. Metastasis to the Cervix Uteri 15 Years After Treatment of Lobular Carcinoma of the Breast. Semin. Oncol. 2015, 42, e81–e94. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.J.; Lai, C.L.; Huang, I.H.; Yu, J.C.; Lee, H.S.; Dai, M.S. Synchronous endometrial and gastric metastases of invasive lobular breast carcinomas. Taiwan. J. Obstet. Gynecol. 2016, 55, 131–134. [Google Scholar] [CrossRef][Green Version]
- Makris, G.M.; Marinelis, A.; Battista, M.J.; Chrelias, C.; Papantoniou, N. An ovarian mass after breast cancer: Metachronous carcinoma or metastasis? A case report. Int. J. Surg. Case Rep. 2017, 31, 106–108. [Google Scholar] [CrossRef]
- Martinez, M.R.; Marazuela, M.A.; Vallejo, M.R.; Bernabeu, R.Á.; Medina, T.P. Metastasis of lobular breast cancer to endometrial polyps with and without the presence of vaginal bleeding. Int. J. Gynecol. Obstet. 2016, 134, 101–102. [Google Scholar] [CrossRef]
- Akhtar, A.; Ratra, A.; Puckett, Y.; Sheikh, A.B.; Ronaghan, C.A. Synchronous uterine metastases from breast cancer: Case study and literature review. Cureus 2017, 9, e1840. [Google Scholar] [CrossRef]
- Bennett, J.A.; Young, R.H.; Chuang, A.Y.; Lerwill, M.F. Ovarian metastases of breast cancers with signet ring cells: A report of 17 cases including 14 Krukenberg tumors. Int. J. Gynecol. Pathol. 2018, 37, 507–515. [Google Scholar] [CrossRef]
- Razia, S.; Nakayama, K.; Tsukao, M.; Nakamura, K.; Ishikawa, M.; Ishibashi, T.; Ishikawa, N.; Sanuki, K.; Yamashita, H.; Ono, R.; et al. Metastasis of breast cancer to an endometrial polyp, the cervix and a leiomyoma: A case report and review of the literature. Oncol. Lett. 2017, 14, 4585–4592. [Google Scholar] [CrossRef]
- Seo, S.O.; Shin, J.Y.; Ji, Y.I. Metastatic uterine cancer looking as cervical fibroid in recurrent breast cancer woman: A case report. Obstet. Gynecol. Sci. 2017, 60, 481–484. [Google Scholar] [CrossRef][Green Version]
- Aytekin, A.; Bilgetekin, I.; Ciltas, A.; Ogut, B.; Coskun, U.; Benekli, M. Lobular breast cancer metastasis to uterus during adjuvant tamoxifen treatment: A case report and review of the literature. J. Cancer Res. Ther. 2018, 14, 1135–1137. [Google Scholar] [CrossRef]
- Briki, R.; Cherif, O.; Bannour, B.; Hidar, S.; Boughizane, S.; Khairi, H. Uncommon metastases of invasive lobular breast cancer to the endometrium: A report of two cases and review of the literature. Pan Afr. Med. J. 2018, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franco-Márquez, R.; Torres-Gaytán, A.G.; Narro-Martinez, M.A.; Carrasco-Chapa, A.; Núñez, B.G.; Boland-Rodriguez, E. Metastasis of Breast Lobular Carcinoma to Endometrium Presenting as Recurrent Abnormal Uterine Bleeding: A Case Report and Review of Literature. Case Rep. Pathol. 2019, 2019, 5357194. [Google Scholar] [CrossRef] [PubMed]
- Kachi, A.; Nicolas, G.; Semaan, D.B.; Hashem, M.; Abou Sleiman, C. Unusual pattern of invasive lobular carcinoma metastasis: A case report. Am. J. Case Rep. 2019, 20, 1659. [Google Scholar] [CrossRef] [PubMed]
- Fontinele, D.R.S.; Vieira, S.C.; da Silva Júnior, R.G.; Rodrigues, T.S. Lobular carcinoma of the breast with metastasis to the uterine cervix. J. Cancer Res. Ther. 2019, 15, 1411–1414. [Google Scholar] [CrossRef]
- Abdallah, H.; Elwy, A.; Alsayed, A.; Rabea, A.; Magdy, N. Metastatic breast lobular carcinoma to unusual sites: A report of three cases and review of literature. J. Med. Cases 2020, 11, 292. [Google Scholar] [CrossRef]
- Arif, S.H.; Mohammed, A.A.; Mohammed, F.R. Metastatic invasive lobular carcinoma of the breast to the endometrium presenting with abnormal uterine bleeding; Case report. Ann. Med. Surg. 2020, 51, 41–43. [Google Scholar] [CrossRef]
- Gomez, M.; Whitting, K.; Naous, R. Lobular breast carcinoma metastatic to the endometrium in a patient under tamoxifen therapy: A case report. SAGE Open Med. Case Rep. 2020, 8, 2050313X20907208. [Google Scholar] [CrossRef]
- Yuan, L.; Oshilaja, O.; Sierk, A.; Zhang, G.; Booth, C.N.; Brainard, J.; Dyhdalo, K.S. Metastatic breast cancer diagnosed on cervical cytology. Cytopathology 2021, 32, 127–131. [Google Scholar] [CrossRef]
- Akizawa, Y.; Kanno, T.; Horibe, Y.; Shimizu, Y.; Noguchi, E.; Yamamoto, T.; Okamoto, T.; Nagashima, Y.; Tabata, T. Ovarian metastasis from breast cancer mimicking a primary ovarian neoplasm: A case report. Mol. Clin. Oncol. 2021, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Awazu, Y.; Fukuda, T.; Imai, K.; Yamauch, M.; Kasai, M.; Ichimura, T.; Yasui, T.; Sumi, T. Uterine metastasis of lobular breast carcinoma under tamoxifen therapy: A case report. Mol. Clin. Oncol. 2021, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Wang, T.Y.; Lam, H.B.; Chang, C.L. Massive metastasis of breast cancer to female genital organs. Taiwan. J. Obstet. Gynecol. 2021, 60, 563–566. [Google Scholar] [CrossRef]
- Kong, D.; Dong, X.; Qin, P.; Sun, D.; Zhang, Z.; Zhang, Y.; Hao, F.; Wang, M. Asymptomatic uterine metastasis of breast cancer: Case report and literature review. Medicine 2022, 101, e31061. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, X.; Zhang, Z.; Chen, X.; Wang, J.; Zhao, X.; Zhang, J. Case Report: 68Ga-FAPI PET/CT, a more advantageous detection mean of gastric, peritoneal, and ovarian metastases from breast cancer. Front. Oncol. 2022, 12, 1013066. [Google Scholar] [CrossRef]
- Benlghazi, A.; Messaoudi, H.; Benali, S.; Tazi, I.; Elhassani, M.M.; Kouach, J. Lobular carcinoma metastasis to endometrial polyps: Insights from a case report and literature analysis. Int. J. Surg. Case Rep. 2024, 124, 110463. [Google Scholar] [CrossRef]
- Faur, A.C.; Gurban, C.V.; Dăescu, E.; Tîrziu, R.V.; Lazăr, D.C.; Ghenciu, L.A. Mucin-Producing Lobular Breast Carcinoma Metastasis to an Ovarian Fibroma: Histopathological and Immunohistochemical Analysis of a Rare Case and Literature Review. Diagnostics 2024, 14, 953. [Google Scholar] [CrossRef]
- Mazur, M.T.; Hsueh, S.; Gersell, D.J. Metastases to the female genital tract: Analysis of 325 cases. Cancer 1984, 53, 1978–1984. [Google Scholar] [CrossRef]
- Harris, M.; Howell, A.; Chrissohou, M.; Swindell, R.; Hudson, M.; Sellwood, R. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br. J. Cancer 1984, 50, 23–30. [Google Scholar] [CrossRef]
- Lamovec, J.; Bračkko, M. Metastatic pattern of infiltrating lobular carcinoma of the breast: An autopsy study. J. Surg. Oncol. 1991, 48, 28–33. [Google Scholar] [CrossRef]
- Winston, C.B.; Hadar, O.; Teitcher, J.B.; Caravelli, J.F.; Sklarin, N.T.; Panicek, D.M.; Liberman, L. Metastatic lobular carcinoma of the breast: Patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am. J. Roentgenol. 2000, 175, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Guvenal, T.; Salman, M.; Ozyuncu, O.; Sakinci, M.; Basaran, M. The role of cytoreductive surgery in nongenital cancers metastatic to the ovaries. Gynecol. Oncol. 2005, 98, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhou, Y.; Wu, M.; Yao, Y.; Deng, Y. Ovarian metastasis from breast cancer: A comprehensive review. Clin. Transl. Oncol. 2019, 21, 819–827. [Google Scholar] [CrossRef]
- Bigorie, V.; Morice, P.; Duvillard, P.; Antoine, M.; Cortez, A.; Flejou, J.F.; Uzan, S.; Darai, E.; Barranger, E. Ovarian metastases from breast cancer: Report of 29 cases. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2010, 116, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Bastings, L.; Beerendonk, C.; Westphal, J.; Massuger, L.F.; Kaal, S.E.; van Leeuwen, F.E.; Braat, D.D.; Peek, R. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: A systematic review. Hum. Reprod. Update 2013, 19, 483–506. [Google Scholar] [CrossRef]
- Peters, I.T.; van Zwet, E.W.; Smit, V.T.; Liefers, G.J.; Kuppen, P.J.; Hilders, C.G.; Trimbos, J.B. Prevalence and risk factors of ovarian metastases in breast cancer patients <41 years of age in the Netherlands: A nationwide retrospective cohort study. PLoS ONE. 2017, 12, e0168277. [Google Scholar] [CrossRef]
- He, H.; Gonzalez, A.; Robinson, E.; Yang, W.T. Distant metastatic disease manifestations in infiltrating lobular carcinoma of the breast. AJR Am. J. Roentgenol. 2014, 202, 1140–1148. [Google Scholar] [CrossRef]
- Guerriero, S.; Alcazar, J.; Pascual, M.; Ajossa, S.; Olartecoechea, B.; Hereter, L. Preoperative diagnosis of metastatic ovarian cancer is related to origin of primary tumor. Ultrasound Obstet. Gynecol. 2012, 39, 581–586. [Google Scholar] [CrossRef]
- Boutas, I.; Kontogeorgi, A.; Koufopoulos, N.; Dimas, D.T.; Sitara, K.; Kalantaridou, S.N.; Dimitrakakis, K. Breast cancer and fertility preservation in young female patients: A systematic review of the literature. Clin. Pract. 2023, 13, 1413–1426. [Google Scholar] [CrossRef]
- Webb, M.J.; Decker, D.G.; Mussey, E. Cancer metastatic to the ovary: Factors influencing survival. Obstet. Gynecol. 1975, 45, 391–396. [Google Scholar] [PubMed]
- Fujiwara, K.; Ohishi, Y.; Koike, H.; Sawada, S.; Moriya, T.; Kohno, I. Clinical implications of metastases to the ovary. Gynecol. Oncol. 1995, 59, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Roylance, R.; Rosenthal, A. Breast cancer metastasising to the pelvis and abdomen: What the gynaecologist needs to know. BJOG 2012, 119, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.B.; Gangwani, L.; Nahleh, Z.; Subramani, R.; Arumugam, A.; de la Rosa, J.M.; Lakshmanaswamy, R. Recurrence and metastasis of breast cancer is influenced by ovarian hormone’s effect on breast cancer stem cells. Future Oncol. 2015, 11, 983–995. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Hutchins, G.M.; Moore, G.W. Influence of age on the metastatic behavior of breast carcinoma. Hum. Pathol. 1988, 19, 529–534. [Google Scholar] [CrossRef]
- Pimentel, C.; Becquet, M.; Lavoué, V.; Hénno, S.; Lévêque, J.; Ouldamer, L. Ovarian metastases from breast cancer: A series of 28 cases. Anticancer. Res. 2016, 36, 4195–4200. [Google Scholar]
- Abd El hafez, A.; Monir, A. Diagnostic spectrum of ovarian masses in women with breast cancer; magnetic resonance imaging: Histopathology correlation. Ann. Diagn. Pathol. 2013, 17, 441–447. [Google Scholar] [CrossRef]
- Curtin, J.P.; Barakat, R.R.; Hoskins, W.J. Ovarian disease in women with breast cancer. Obstet. Gynecol. 1994, 84, 449–452. [Google Scholar]
- Abu-Rustum, N.R.; Aghajanian, C.A.; Venkatraman, E.S.; Feroz, F.; Barakat, R.R. Metastatic breast carcinoma to the abdomen and pelvis. Gynecol. Oncol. 1997, 66, 41–44. [Google Scholar] [CrossRef]
- Rabban, J.T.; Barnes, M.; Chen, L.M.; Powell, C.B.; Crawford, B.; Zaloudek, C.J. Ovarian pathology in risk-reducing salpingo-oophorectomies from women with BRCA mutations, emphasizing the differential diagnosis of occult primary and metastatic carcinoma. Am. J. Surg. Pathol. 2009, 33, 1125–1136. [Google Scholar] [CrossRef]
- Perrotin, F.; Marret, H.; Bouquin, R.; Fischer-Perrotin, N.; Lansac, J.; Body, G. Incidence, diagnosis and prognosis of ovarian metastasis in breast cancer. Gynecol. Obstet. Fertil. 2001, 29, 308–315. [Google Scholar] [CrossRef]
- Antila, R.; Jalkanen, J.; Heikinheimo, O. Comparison of secondary and primary ovarian malignancies reveals differences in their pre-and perioperative characteristics. Gynecol. Oncol. 2006, 101, 97–101. [Google Scholar] [CrossRef]
- Bruls, J.; Simons, M.; Overbeek, L.I.; Bulten, J.; Massuger, L.F.; Nagtegaal, I.D. A national population-based study provides insight in the origin of malignancies metastatic to the ovary. Virchows Arch. 2015, 467, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kubeček, O.; Laco, J.; Špaček, J.; Petera, J.; Kopecký, J.; Kubečková, A.; Filip, S. The pathogenesis, diagnosis, and management of metastatic tumors to the ovary: A comprehensive review. Clin. Exp. Metastasis 2017, 34, 295–307. [Google Scholar] [CrossRef]
- Yadav, B.S.; Sharma, S.; Robin, T.P.; Sams, S.; Elias, A.D.; Kaklamani, V.; Kelly Marcom, P.; Schaefer, S.; Morris, G.J. Synchronous primary carcinoma of breast and ovary versus ovarian metastases. Semin. Oncol. 2015, 42, e13–e24. [Google Scholar] [CrossRef]
- Tamás, J.; Vereczkey, I.; Tóth, E. Metastatic tumors in the ovary, difficulties of histologic diagnosis. Magy. Onkol. 2015, 59, 205–213. [Google Scholar] [PubMed]
- Zhang, R.; Liu, J.; Jiang, L.; Lang, Z. Application of TRPS1 in ER-negative or low expression distant metastatic breast carcinoma. Pathol. Oncol. Res. 2025, 31, 1612138. [Google Scholar] [CrossRef] [PubMed]
- Zafrakas, M.; Petschke, B.; Donner, A.; Fritzsche, F.; Kristiansen, G.; Knüchel, R.; Dahl, E. Expression analysis of mammaglobin A (SCGB2A2) and lipophilin B (SCGB1D2) in more than 300 human tumors and matching normal tissues reveals their co-expression in gynecologic malignancies. BMC Cancer 2006, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Pauer, H.U.; Viereck, V.; Burfeind, P.; Emons, G.; Krauss, T. Uterine cervical metastasis of breast cancer: A rare complication that may be overlooked. Oncologie 2003, 26, 58–60. [Google Scholar] [CrossRef]
- Pérez-Montiel, D.; Serrano-Olvera, A.; Salazar, L.C.; Cetina-Pérez, L.; Candelaria, M.; Coronel, J.; Montalvo, L.A.; de León, D.C. Adenocarcinoma metastatic to the uterine cervix: A case series. J. Obstet. Gynaecol. Res. 2012, 38, 541–549. [Google Scholar] [CrossRef]
- Abell, M.R.; Gosling, J.R. Gland cell carcinoma (adenocarcinoma) of the uterine cervix. Am. J. Obstet. Gynecol. 1962, 83, 729–755. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Bryson, G.; Jamison, J.; Coutts, M.; McCluggage, W.G. Cervical squamous carcinomas with prominent acantholysis and areas resembling breast lobular carcinoma: An aggressive form of dedifferentation. Int. J. Gynecol. Pathol. 2018, 37, 74–81. [Google Scholar] [CrossRef]
- Mansor, S.; McCluggage, W.G. Cervical adenocarcinoma resembling breast lobular carcinoma: A hitherto undescribed variant of primary cervical adenocarcinoma. Int. J. Gynecol. Pathol. 2010, 29, 594–599. [Google Scholar] [CrossRef]
- Hermi, A.; Chakroun, M.; Saadi, A.; Saidani, B.; Kacem, L.B.; Chebil, M. Upper urinary tract urothelial carcinoma diagnosis by biopsy of a vaginal metastasis. Urol. Case Rep. 2022, 43, 102114. [Google Scholar] [CrossRef]
- Yan, Y.; Guo, T.; Zhang, M.; Cui, G. Vaginal metastasis from breast cancer: A case report. Open Life Sci. 2023, 18, 20220623. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.K.; Roy, S.; Mridha, A.R.; Sharma, D.N. Vulvar metastasis from carcinoma breast unveiling distant metastasis: Exploring an unusual metastatic pattern. J. Egypt. Natl. Cancer Inst. 2015, 27, 243–246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).