Stents and Emerging Alternatives in Upper Gastrointestinal Endoscopy: A Comprehensive Review
Abstract
1. Introduction
2. Type of Stents
2.1. Metallic Stent
- Fully covered (FC-SEMS): Encapsulated in a plastic or silicone membrane to prevent tissue ingrowth, facilitating easier removal or repositioning. However, they are associated with a higher risk of migration.
- Partially covered (PC-SEMS): Designed with uncovered ends to reduce migration risk, though this increases the potential for tissue overgrowth and embedding.
- Uncovered (UC-SEMS): These integrate more firmly with the surrounding tissue, providing long-term support but carrying a significant risk of complications such as tissue ingrowth [7].
2.2. Plastic Stent
2.3. Biodegradable Stents
- Polylactic acid (PLA): Offers a controlled degradation rate, maintaining structural integrity for weeks to months, depending on clinical needs.
- Polyglycolic acid (PGA): Degrades more rapidly but is less mechanically stable than PLA; it is often combined with PLA to optimize performance.
- Polycaprolactone (PCL): Used in specific cases where extended durability is required [10].
2.4. Lumen-Apposing Metal Stent (LAMS)
- Saddle-shaped LAMS: Featuring wide flanges that provide strong tissue apposition, these are commonly used for drainage of pancreatic fluid collections.
- Dumbbell-shaped LAMS: With a more symmetrical design, these are optimized for gastrointestinal bypass procedures.
3. Esophagus
3.1. Esophageal Leak
- Type I: Localized leaks manageable with medical therapy;
- Type II: Requiring radiological or endoscopic intervention;
- Type III: Necessitating surgical intervention [14].
3.1.1. Role of Stent
Indications and Mechanism of Action
Efficacy and Adverse Events
- Type 1: Proximal leaks due to an inadequate seal, often resolved by upsizing the stent or adding an additional one.
- Type 2: Distal retrograde leaks, commonly managed with decompression PEG, additional stenting, or a larger stent.
- Type 3: Leaks through breaches within the stent lining, typically caused by technical difficulties or suction-related trauma during placement, requiring stent replacement.
- Type 4: Leaks between adjacent stents, addressed by using a larger proximal stent.
- Type 5: Migration-related leaks, usually seen in cervical or mid-esophageal stenting without fixation, necessitating a larger stent or anchoring for stability.
3.1.2. Endoscopic Vacuum Therapy (EVT)
SEMS vs. EVT
3.1.3. VAC Stent
SEMS vs. VAC Stent
3.2. Malignant Dysphagia (Esophageal Cancer)
3.2.1. Role of Stent
Indications and Mechanism of Action
Efficacy and Adverse Events
3.3. Benign Strictures
3.3.1. Role of Stent
Indication and Mechanism of Action
Efficacy and Adverse Events
3.3.2. Biodegradable Stents
SEMS vs. BDS
3.3.3. LAMS
SEMS vs. LAMS
3.3.4. Incisional Therapy
Indication and Mechanism of Action
- Radial Incision (RI): 4–8 radial cuts without removal of scar tissue.
- Radial Incision and Cutting (RIC): combines incisions with excision of fibrotic tissue.
- Radial Incision and Selective Cutting (RISC): targets only selected fibrotic segments to minimize the risk of restenosis [95].
Efficacy and Adverse Events
3.4. Esophageal Acute Variceal Bleeding
3.4.1. Role of Stent
Indication and Mechanism of Action
Efficacy and Adverse Events
4. Gastroduodenal Tract—Gastric Outlet Obstruction
4.1. Malignant GOO
- Type I: Stenosis at the duodenal bulb without papillary involvement.
- Type II: Obstruction in the second part of the duodenum involving the papilla—requiring combined palliation of both gastric obstruction and biliary drainage.
- Type III: Obstruction in the third portion of the duodenum, sparing the papilla [115].
4.1.1. Role of Stent
Indications and Mechanism of Action
Efficacy and Adverse Events
4.1.2. LAMS
Indication and Mechanism of Action
- Direct technique: puncture of the jejunal loop with a 19G needle and contrast injection to confirm position [126].
- Device-assisted EUS-GE: balloon or enteroscope passed across the stenosis to aid EUS visualization and targeting [127].
- Wireless Endoscopic Simplified Technique (WEST): described by Bronswijk et al. in 2020 and currently the most widely used technique [128], this approach involves jejunal distension via a nasoenteric tube with saline and dye, followed by “free-hand” single-step LAMS deployment under EUS guidance [128].
Efficacy and Adverse Events
- Type 1 (63.1%): distal flange in the peritoneum, proximal in the stomach, without enterotomy—managed with LAMS removal and OTSC placement.
- Type 2 (30.4%): distal flange in the peritoneum, proximal in the stomach, with confirmed enterotomy—managed with repeat LAMS or LAMS-in-LAMS bridging.
- Type 3 (2.2%): distal flange in the small bowel, proximal in the peritoneum—managed surgically.
- Type 4 (4.3%): distal flange in the colon, proximal in the stomach—managed conservatively or surgically after tract maturation.
Sems vs. LAMS
4.2. Benignant GOO
4.2.1. Role of Stent
4.2.2. LAMS
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Spaander, M.C.W.; Van Der Bogt, R.D.; Baron, T.H.; Albers, D.; Blero, D.; De Ceglie, A.; Conio, M.; Czakó, L.; Everett, S.; Garcia-Pagán, J.-C.; et al. Esophageal Stenting for Benign and Malignant Disease: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2021. Endoscopy 2021, 53, 751–762. [Google Scholar] [CrossRef] [PubMed]
- van Hooft, J.E.; Veld, J.V.; Arnold, D.; Beets-Tan, R.G.H.; Everett, S.; Götz, M.; van Halsema, E.E.; Hill, J.; Manes, G.; Meisner, S.; et al. Self-Expandable Metal Stents for Obstructing Colonic and Extracolonic Cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2020. Endoscopy 2020, 52, 389–407. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, S.W.; van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Huc, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic Endoscopic Ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline|ESGE. Available online: https://www.esge.com/therapeutic-endoscopic-ultrasound-european-society-of-gastrointestinal-endoscopy-guideline (accessed on 18 September 2024).
- Mandarino, F.V.; Barchi, A.; Fanti, L.; Azzolini, F.; Rosati, R.; Danese, S. Endoscopic Vacuum Therapy in the Treatment of Postesophagectomy Leaks: Is Intracavitary the Way? Gastrointest. Endosc. 2022, 96, 873. [Google Scholar] [CrossRef]
- Bemelman, W.A.; Baron, T.H. Endoscopic Management of Transmural Defects, Including Leaks, Perforations, and Fistulae. Gastroenterology 2018, 154, 1938–1946.e1. [Google Scholar] [CrossRef]
- Jackson, C.E.; Johnson, L.S.J.; Williams, D.A.; Laasch, H.-U.; Edwards, D.W.; Harvey, A.G. A Viewpoint on Material and Design Considerations for Oesophageal Stents with Extended Lifetime. J. Mater. Sci. 2022, 57, 3–26. [Google Scholar] [CrossRef]
- Kozarek, R.; Baron, T.H. Self-Expanding Stents: Present and Future. In Gastrointestinal and Pancreatico-Biliary Diseases: Advanced Diagnostic and Therapeutic Endoscopy; Testoni, P.A., Inoue, H., Wallace, M.B., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 829–834. ISBN 978-3-030-56993-8. [Google Scholar]
- Seven, G.; Irani, S.; Ross, A.S.; Gan, S.I.; Gluck, M.; Low, D.; Kozarek, R.A. Partially versus Fully Covered Self-Expanding Metal Stents for Benign and Malignant Esophageal Conditions: A Single Center Experience. Surg. Endosc. 2013, 27, 2185–2192. [Google Scholar] [CrossRef]
- Ahmed, O.; Lee, J.H.; Thompson, C.C.; Faulx, A. AGA Clinical Practice Update on the Optimal Management of the Malignant Alimentary Tract Obstruction: Expert Review. Clin. Gastroenterol. Hepatol. 2021, 19, 1780–1788. [Google Scholar] [CrossRef]
- Lorenzo-Zúñiga, V.; Moreno-de-Vega, V.; Marín, I.; Boix, J. Biodegradable Stents in Gastrointestinal Endoscopy. World J. Gastroenterol. 2014, 20, 2212–2217. [Google Scholar] [CrossRef]
- Köneş, O.; Oran, E. Self-Expanding Biodegradable Stents for Postoperative Upper Gastrointestinal Issues. J. Soc. Laparosc. Robot. Surg. 2018, 22, e2018.00011. [Google Scholar] [CrossRef]
- Mussetto, A.; Fugazza, A.; Fuccio, L.; Triossi, O.; Repici, A.; Anderloni, A. Current Uses and Outcomes of Lumen-Apposing Metal Stents. Ann. Gastroenterol. 2018, 31, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Binmoeller, K.F.; Lau, J.Y.W. Single-Step EUS-Guided Puncture and Delivery of a Lumen-Apposing Stent for Gallbladder Drainage Using a Novel Cautery-Tipped Stent Delivery System. Gastrointest. Endosc. 2014, 80, 1171. [Google Scholar] [CrossRef]
- Low, D.E.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.E.; D’Journo, X.B.; Griffin, S.M.; Hölscher, A.H.; Hofstetter, W.L.; Jobe, B.A.; et al. International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann. Surg. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Kuppusamy, M.K.; Low, D.E.; International Esodata Study Group (IESG). Evaluation of International Contemporary Operative Outcomes and Management Trends Associated with Esophagectomy: A 4-Year Study of >6000 Patients Using ECCG Definitions and the Online Esodata Database. Ann. Surg. 2022, 275, 515–525. [Google Scholar] [CrossRef]
- Rosianu, C.G.; Hoara, P.; Achim, F.; Birla, R.; Bolocan, A.; Mohssen, A.; Copca, N.; Constantinoiu, S. The Use of Esophageal Stents in the Management of Postoperative Fistulas-Current Status, Clinical Outcomes and Perspectives-Review. Life 2023, 13, 966. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Esposito, D.; Spelta, G.N.E.; Cavestro, G.M.; Rosati, R.; Parise, P.; Gemma, M.F.; Fanti, L. Double Layer Stent for the Treatment of Leaks and Fistula after Upper Gastrointestinal Oncologic Surgery: A Retrospective Study. Updates Surg. 2022, 74, 1055–1062. [Google Scholar] [CrossRef]
- Dasari, B.V.M.; Neely, D.; Kennedy, A.; Spence, G.; Rice, P.; Mackle, E.; Epanomeritakis, E. The Role of Esophageal Stents in the Management of Esophageal Anastomotic Leaks and Benign Esophageal Perforations. Ann. Surg. 2014, 259, 852–860. [Google Scholar] [CrossRef]
- Anderloni, A.; Genco, C.; Massidda, M.; Di Leo, M.; Fumagalli, U.R.; Rosati, R.; Correale, L.; Maselli, R.; Ferrara, E.C.; Jovani, M.; et al. Self-Expanding Metal Stents for the Treatment of Post-Surgical Esophageal Leaks: A Tertiary Referral Center Experience. Dig. Surg. 2019, 36, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Plum, P.S.; Herbold, T.; Berlth, F.; Christ, H.; Alakus, H.; Bludau, M.; Chang, D.-H.; Bruns, C.J.; Hölscher, A.H.; Chon, S.-H. Outcome of Self-Expanding Metal Stents in the Treatment of Anastomotic Leaks After Ivor Lewis Esophagectomy. World J. Surg. 2019, 43, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Sanz Segura, P.; Gotor Delso, J.; García Cámara, P.; Sierra Moros, E.; Val Pérez, J.; Soria Santeodoro, M.T.; Uribarrena Amezaga, R. Use of Double-Layered Covered Esophageal Stents in Post-Surgical Esophageal Leaks and Esophageal Perforation: Our Experience. Gastroenterol. Hepatol. Engl. Ed. 2022, 45, 198–203. [Google Scholar] [CrossRef]
- Freeman, R.K.; Ascioti, A.J.; Giannini, T.; Mahidhara, R.J. Analysis of Unsuccessful Esophageal Stent Placements for Esophageal Perforation, Fistula, or Anastomotic Leak. Ann. Thorac. Surg. 2012, 94, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Iglesias Jorquera, E.; Egea Valenzuela, J.; Serrano Jiménez, A.; Carrilero Zaragoza, G.; Ortega Sabater, A.; Sánchez Velasco, E.; Ruiz de Angulo, D.; Munitiz, V.; Parrilla, P.; Alberca de Las Parras, F. Endoscopic Treatment of Postoperative Esophagogastric Leaks with Fully Covered Self-Expanding Metal Stents. Rev. Esp. Enferm. Dig. 2021, 113, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Daly, S.C.; Smith, B.; Hinojosa, M.; Nguyen, N.T. The Role of Endoscopic Stent in Management of Postesophagectomy Leaks. Am. Surg. 2020, 86, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.S.; Thompson, C.C. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 147–161. [Google Scholar] [CrossRef]
- Fujii, L.L.; Bonin, E.A.; Baron, T.H.; Gostout, C.J.; Wong Kee Song, L.M. Utility of an Endoscopic Suturing System for Prevention of Covered Luminal Stent Migration in the Upper GI Tract. Gastrointest. Endosc. 2013, 78, 787–793. [Google Scholar] [CrossRef]
- Wang, T.J.; Aihara, H.; Thompson, A.C.; Schulman, A.R.; Thompson, C.C.; Ryou, M. Choosing the Right Through-the-Scope Clip: A Rigorous Comparison of Rotatability, Whip, Open/Close Precision, and Closure Strength (with Videos). Gastrointest. Endosc. 2019, 89, 77–86.e1. [Google Scholar] [CrossRef]
- Manta, R.; Del Nero, L.; Todd, B.; Parodi, A.; De Ceglie, A.; Zito, F.; Pasquale, L.; Zullo, A.; Conio, M. Newly Designed OTS Clip for Preventing Fully-Covered Self-Expandable Metal Stent Migration in the Gastrointestinal Tract. Endosc. Int. Open 2023, 11, E284–E287. [Google Scholar] [CrossRef]
- Papaefthymiou, A.; Gkolfakis, P.; Basiliya, K.; Ramai, D.; Tziatzios, G.; Sehgal, V.; Telese, A.; Norton, B.; Aslam, N.; Johnson, G.; et al. Success Rates of Fixation Techniques on Prevention of Esophageal Stent Migration: A Systematic Review and Meta-Analysis. Endoscopy 2024, 56, 22–30. [Google Scholar] [CrossRef]
- van Boeckel, P.G.A.; Dua, K.S.; Weusten, B.L.A.M.; Schmits, R.J.H.; Surapaneni, N.; Timmer, R.; Vleggaar, F.P.; Siersema, P.D. Fully Covered Self-Expandable Metal Stents (SEMS), Partially Covered SEMS and Self-Expandable Plastic Stents for the Treatment of Benign Esophageal Ruptures and Anastomotic Leaks. BMC Gastroenterol. 2012, 12, 19. [Google Scholar] [CrossRef]
- Speer, E.; Dunst, C.M.; Shada, A.; Reavis, K.M.; Swanström, L.L. Covered Stents in Cervical Anastomoses Following Esophagectomy. Surg. Endosc. 2016, 30, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Bohle, W.; Louris, I.; Schaudt, A.; Koeninger, J.; Zoller, W.G. Predictors for Treatment Failure of Self-Expandable Metal Stents for Anastomotic Leak after Gastro-Esophageal Resection. J. Gastrointest. Liver Dis. 2020, 29, 145–149. [Google Scholar] [CrossRef]
- Eisendrath, P.; Cremer, M.; Himpens, J.; Cadière, G.-B.; Le Moine, O.; Devière, J. Endotherapy Including Temporary Stenting of Fistulas of the Upper Gastrointestinal Tract after Laparoscopic Bariatric Surgery. Endoscopy 2007, 39, 625–630. [Google Scholar] [CrossRef]
- Salminen, P.; Gullichsen, R.; Laine, S. Use of Self-Expandable Metal Stents for the Treatment of Esophageal Perforations and Anastomotic Leaks. Surg. Endosc. 2009, 23, 1526–1530. [Google Scholar] [CrossRef]
- Schoppmann, S.F.; Langer, F.B.; Prager, G.; Zacherl, J. Outcome and Complications of Long-Term Self-Expanding Esophageal Stenting. Dis. Esophagus 2013, 26, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Stephens, E.H.; Correa, A.M.; Kim, M.P.; Gaur, P.; Blackmon, S.H. Classification of Esophageal Stent Leaks: Leak Presentation, Complications, and Management. Ann. Thorac. Surg. 2014, 98, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Fanti, L.; Barchi, A.; Vespa, E.; Danese, S. Endoscopic Vacuum Therapy for Esophageal Perforations: Is It Risk Effective for Every Size of Defect? Endoscopy 2023, 55, 971. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Huh, C.W.; Min, Y.W.; Park, J.C. Endoscopic Vacuum Therapy for the Management of Upper GI Leaks and Perforations: A Multicenter Retrospective Study of Factors Associated with Treatment Failure (with Video). Gastrointest. Endosc. 2022, 95, 281–290. [Google Scholar] [CrossRef]
- Momblan, D.; Gimeno Garcia, A.Z.; Busquets, D.; Juzgado, D.; García Lledó, J.; Ferrero, E.; Tejedor-Tejada, J.; Junquera, F.; Díaz-Tasende, J.; Moris, M.; et al. Endoscopic Vacuum Therapy for Upper Gastrointestinal Leaks and Perforations: Analysis from a Multicenter Spanish Registry. Am. J. Gastroenterol. 2023, 118, 1797–1806. [Google Scholar] [CrossRef]
- Luttikhold, J.; Pattynama, L.M.D.; Seewald, S.; Groth, S.; Morell, B.K.; Gutschow, C.A.; Ida, S.; Nilsson, M.; Eshuis, W.J.; Pouw, R.E. Endoscopic Vacuum Therapy for Esophageal Perforation: A Multicenter Retrospective Cohort Study. Endoscopy 2023, 55, 859–864. [Google Scholar] [CrossRef]
- Richter, F.; Hendricks, A.; Schniewind, B.; Hampe, J.; Heits, N.; von Schönfels, W.; Reichert, B.; Eberle, K.; Ellrichmann, M.; Baumann, P.; et al. Eso-Sponge® for Anastomotic Leakage after Oesophageal Resection or Perforation: Outcomes from a National, Prospective Multicentre Registry. BJS Open 2022, 6, zrac030. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Barchi, A.; Fanti, L.; D’Amico, F.; Azzolini, F.; Esposito, D.; Biamonte, P.; Lauri, G.; Danese, S. Endoscopic Vacuum Therapy for Post-Esophagectomy Anastomotic Dehiscence as Rescue Treatment: A Single Center Case Series. Esophagus 2022, 19, 417–425. [Google Scholar] [CrossRef]
- Jung, D.H.; Yun, H.-R.; Lee, S.J.; Kim, N.W.; Huh, C.W. Endoscopic Vacuum Therapy in Patients with Transmural Defects of the Upper Gastrointestinal Tract: A Systematic Review with Meta-Analysis. J. Clin. Med. 2021, 10, 2346. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Barchi, A.; D’Amico, F.; Fanti, L.; Azzolini, F.; Viale, E.; Esposito, D.; Rosati, R.; Fiorino, G.; Bemelman, W.A.; et al. Endoscopic Vacuum Therapy (EVT) versus Self-Expandable Metal Stent (SEMS) for Anastomotic Leaks after Upper Gastrointestinal Surgery: Systematic Review and Meta-Analysis. Life 2023, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Laukoetter, M.G.; Mennigen, R.; Neumann, P.A.; Dhayat, S.; Horst, G.; Palmes, D.; Senninger, N.; Vowinkel, T. Successful Closure of Defects in the Upper Gastrointestinal Tract by Endoscopic Vacuum Therapy (EVT): A Prospective Cohort Study. Surg. Endosc. 2017, 31, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M.; Schulte, T.; Egberts, J.; Schafmayer, C.; Hampe, J.; Fritscher-Ravens, A.; Broering, D.C.; Schniewind, B. Drainage of Esophageal Leakage Using Endoscopic Vacuum Therapy: A Prospective Pilot Study. Endoscopy 2010, 42, 693–698. [Google Scholar] [CrossRef]
- Pournaras, D.J.; Hardwick, R.H.; Safranek, P.M.; Sujendran, V.; Bennett, J.; Macaulay, G.D.; Hindmarsh, A. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J. Surg. 2018, 42, 2507–2511. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Barchi, A.; Leone, L.; Fanti, L.; Azzolini, F.; Viale, E.; Esposito, D.; Salmeri, N.; Puccetti, F.; Barbieri, L.; et al. Endoscopic Vacuum Therapy versus Self-Expandable Metal Stent for Treatment of Anastomotic Leaks < 30 Mm Following Oncologic Ivor-Lewis Esophagectomy: A Matched Case-Control Study. Surg. Endosc. 2023, 37, 7039–7050. [Google Scholar] [CrossRef]
- Baltin, C.; Kron, F.; Urbanski, A.; Zander, T.; Kron, A.; Berlth, F.; Kleinert, R.; Hallek, M.; Hoelscher, A.H.; Chon, S.-H. The Economic Burden of Endoscopic Treatment for Anastomotic Leaks Following Oncological Ivor Lewis Esophagectomy. PLoS ONE 2019, 14, e0221406. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Barchi, A.; Biamonte, P.; Esposito, D.; Azzolini, F.; Fanti, L.; Danese, S. The Prophylactic Use of Endoscopic Vacuum Therapy for Anastomotic Dehiscence after Rectal Anterior Resection: Is It Feasible for Redo Surgery? Tech. Coloproctol. 2022, 26, 319–320. [Google Scholar] [CrossRef]
- Müller, P.C.; Morell, B.; Vetter, D.; Raptis, D.A.; Kapp, J.R.; Gubler, C.; Gutschow, C.A. Preemptive Endoluminal Vacuum Therapy to Reduce Morbidity After Minimally Invasive Ivor Lewis Esophagectomy: Including a Novel Grading System for Postoperative Endoscopic Assessment of GI-Anastomoses. Ann. Surg. 2021, 274, 751–757. [Google Scholar] [CrossRef]
- Lange, J.; Kähler, G.; Bernhardt, J.; Knievel, J.; Dormann, A.; Hügle, U.; Eisenberger, C.F.; Heiss, M.M. The VACStent Trial: Combined Treatment of Esophageal Leaks by Covered Stent and Endoscopic Vacuum Therapy. Surg. Endosc. 2023, 37, 3657–3668. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Eisenberger, C.F.; Knievel, J.; Linderer, A.; Heiss, M.M. Preemptive Endoluminal Vacuum Therapy with the VACStent-A Pilot Study to Reduce Anastomotic Leakage after Ivor Lewis Hybrid Esophagectomy. Front. Surg. 2023, 10, 1133083. [Google Scholar] [CrossRef]
- Pattynama, L.M.D.; Eshuis, W.J.; Van Berge Henegouwen, M.I.; Bergman, J.J.G.H.M.; Pouw, R.E. Vacuum-Stent: A Combination of Endoscopic Vacuum Therapy and an Intraluminal Stent for Treatment of Esophageal Transmural Defects. Front. Surg. 2023, 10, 1145984. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.-H.; Bartella, I.; Bürger, M.; Rieck, I.; Goeser, T.; Schröder, W.; Bruns, C.J. VACStent: A New Option for Endoscopic Vacuum Therapy in Patients with Esophageal Anastomotic Leaks after Upper Gastrointestinal Surgery. Endoscopy 2020, 52, E166–E167. [Google Scholar] [CrossRef]
- Chon, S.-H.; Töx, U.; Lorenz, F.; Rieck, I.; Wagner, B.J.; Kleinert, R.; Fuchs, H.F.; Goeser, T.; Quaas, A.; Bruns, C.J. A Novel Hybrid Stent with Endoscopic Vacuum Therapy for Treating Leaks of the Upper Gastrointestinal Tract. Visc. Med. 2021, 37, 403–409. [Google Scholar] [CrossRef]
- Chon, S.-H.; Scherdel, J.; Rieck, I.; Lorenz, F.; Dratsch, T.; Kleinert, R.; Gebauer, F.; Fuchs, H.F.; Goeser, T.; Bruns, C.J. A New Hybrid Stent Using Endoscopic Vacuum Therapy in Treating Esophageal Leaks: A Prospective Single-Center Experience of Its Safety and Feasibility with Mid-Term Follow-Up. Dis. Esophagus 2022, 35, doab067. [Google Scholar] [CrossRef]
- Bludau, M.; Fuchs, H.F.; Herbold, T.; Maus, M.K.H.; Alakus, H.; Popp, F.; Leers, J.M.; Bruns, C.J.; Hölscher, A.H.; Schröder, W.; et al. Results of Endoscopic Vacuum-Assisted Closure Device for Treatment of Upper GI Leaks. Surg. Endosc. 2018, 32, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Knievel, J.; Wichmann, D.; Kähler, G.; Wiedbrauck, F.; Hellmich, T.; Kandler, M.; Bernhardt, J.; Scholz, D.; Beyna, T.; et al. Clinical Implantation of 92 VACStents in the Upper Gastrointestinal Tract of 50 Patients—Applicability and Safety Analysis of an Innovative Endoscopic Concept. Front. Surg. 2023, 10, 1182094. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Carpentier, D.; Lemmers, A. Refractory Eso-pleural Fistula Following Roux-en-Y Gastric Bypass: VacStent to the Rescue!! Dig. Endosc. 2024, 36, 221. [Google Scholar] [CrossRef]
- Fischer, A.; Bausch, D.; Richter-Schrag, H.-J. Use of a Specially Designed Partially Covered Self-Expandable Metal Stent (PSEMS) with a 40-Mm Diameter for the Treatment of Upper Gastrointestinal Suture or Staple Line Leaks in 11 Cases. Surg. Endosc. 2013, 27, 642–647. [Google Scholar] [CrossRef]
- Mennigen, R.; Harting, C.; Lindner, K.; Vowinkel, T.; Rijcken, E.; Palmes, D.; Senninger, N.; Laukoetter, M.G. Comparison of Endoscopic Vacuum Therapy Versus Stent for Anastomotic Leak After Esophagectomy. J. Gastrointest. Surg. 2015, 19, 1229–1235. [Google Scholar] [CrossRef]
- Licht, E.; Markowitz, A.J.; Bains, M.S.; Gerdes, H.; Ludwig, E.; Mendelsohn, R.B.; Rizk, N.P.; Shah, P.; Strong, V.E.; Schattner, M.A. Endoscopic Management of Esophageal Anastomotic Leaks After Surgery for Malignant Disease. Ann. Thorac. Surg. 2016, 101, 301–304. [Google Scholar] [CrossRef]
- Schweigert, M.; Dubecz, A.; Stadlhuber, R.J.; Muschweck, H.; Stein, H.J. Risk of Stent-Related Aortic Erosion after Endoscopic Stent Insertion for Intrathoracic Anastomotic Leaks after Esophagectomy. Ann. Thorac. Surg. 2011, 92, 513–518. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Ma, Y.-L.; Qin, Q.; Wang, P.-H.; Luo, Y.; Xu, P.-F.; Cui, Y. Epidemiology of Esophageal Cancer in 2020 and Projections to 2030 and 2040. Thorac. Cancer 2023, 14, 3–11. [Google Scholar] [CrossRef]
- Celestin, L.R. Permanent Intubation in Inoperable Cancer of the Oesophagus and Cardia: A New Tube. Ann. R. Coll. Surg. Engl. 1959, 25, 165–170. [Google Scholar]
- Atkinson, M.; Ferguson, R. Fibreoptic Endoscopic Palliative Intubation of Inoperable Oesophagogastric Neoplasms. Br. Med. J. 1977, 1, 266–267. [Google Scholar] [CrossRef]
- Frimberger, E. Expanding Spiral—A New Type of Prosthesis for the Palliative Treatment of Malignant Esophageal Stenoses. Endoscopy 1983, 15 (Suppl. 1), 213–214. [Google Scholar] [CrossRef]
- Dai, Y.; Li, C.; Xie, Y.; Liu, X.; Zhang, J.; Zhou, J.; Pan, X.; Yang, S. Interventions for Dysphagia in Oesophageal Cancer. Cochrane Database Syst. Rev. 2014, 2014, CD005048. [Google Scholar] [CrossRef]
- Wang, C.; Wei, H.; Li, Y. Comparison of Fully-Covered vs Partially Covered Self-Expanding Metallic Stents for Palliative Treatment of Inoperable Esophageal Malignancy: A Systematic Review and Meta-Analysis. BMC Cancer 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N.; Morris, A.I.; Marcon, N.; Segalin, A.; Peracchia, A.; Bethge, N.; Zuccaro, G.; Bosco, J.J.; Jones, W.F. A Prospective, Randomized, Controlled Trial of Covered Expandable Metal Stents in the Palliation of Malignant Esophageal Obstruction at the Gastroesophageal Junction. Am. J. Gastroenterol. 2001, 96, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Saranovic, D.; Djuric-Stefanovic, A.; Ivanovic, A.; Masulovic, D.; Pesko, P. Fluoroscopically Guided Insertion of Self-Expandable Metal Esophageal Stents for Palliative Treatment of Patients with Malignant Stenosis of Esophagus and Cardia: Comparison of Uncovered and Covered Stent Types. Dis. Esophagus 2005, 18, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Chou, I.-T.; Yu, F.-J.; Shih, H.-Y.; Liu, Y.-W.; Lee, J.-Y.; Chou, S.-H.; Hsu, J.-S.; Chen, W.-C.; Wu, I.-C. Risk factors of stent migration in esophageal cancer patients who underwent fully-covered self-expanding metal stents for malignant dysphagia or tracheoesophageal fistula. J. Formos. Med. Assoc. 2024, 124, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Bick, B.L.; Imperiale, T.F.; Johnson, C.S.; DeWitt, J.M. Endoscopic Suturing of Esophageal Fully Covered Self-Expanding Metal Stents Reduces Rates of Stent Migration. Gastrointest. Endosc. 2017, 86, 1015–1021. [Google Scholar] [CrossRef]
- Kang, Y. A Review of Self-Expanding Esophageal Stents for the Palliation Therapy of Inoperable Esophageal Malignancies. BioMed Res. Int. 2019, 2019, 9265017. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.S.; Sheridan, M.B. Esophageal Stenting. Semin. Interv. Radiol. 2004, 21, 157–166. [Google Scholar] [CrossRef]
- Adler, D.G.; Siddiqui, A.A. Endoscopic Management of Esophageal Strictures. Gastrointest. Endosc. 2017, 86, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Small, A.J.; Mendelson, A.; Jovani, M.; Correale, L.; Hassan, C.; Ridola, L.; Anderloni, A.; Ferrara, E.C.; Kochman, M.L. Natural History and Management of Refractory Benign Esophageal Strictures. Gastrointest. Endosc. 2016, 84, 222–228. [Google Scholar] [CrossRef]
- Ravich, W.J. Endoscopic Management of Benign Esophageal Strictures. Curr. Gastroenterol. Rep. 2017, 19, 50. [Google Scholar] [CrossRef]
- Siersema, P.D. Stenting for Benign Esophageal Strictures. Endoscopy 2009, 41, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Song, H.-Y.; Choi, E.K.; Kim, K.R.; Shin, J.H.; Lim, J.-O. Temporary Metallic Stent Placement in the Treatment of Refractory Benign Esophageal Strictures: Results and Factors Associated with Outcome in 55 Patients. Eur. Radiol. 2009, 19, 384–390. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Cui, C.; Li, Y.; Lin, X.; Fu, J. Removable, Fully Covered, Self-Expandable Metal Stents for the Treatment of Refractory Benign Esophagogastric Anastomotic Strictures. Dysphagia 2012, 27, 260–264. [Google Scholar] [CrossRef]
- Walter, D.; van den Berg, M.W.; Hirdes, M.M.; Vleggaar, F.P.; Repici, A.; Deprez, P.H.; Viedma, B.L.; Lovat, L.B.; Weusten, B.L.; Bisschops, R.; et al. Dilation or Biodegradable Stent Placement for Recurrent Benign Esophageal Strictures: A Randomized Controlled Trial. Endoscopy 2018, 50, 1146–1155. [Google Scholar] [CrossRef]
- Yano, T.; Yoda, Y.; Nonaka, S.; Abe, S.; Kawata, N.; Yoshio, T.; Sasaki, T.; Tanaka, S.; Sasaki, F.; Maekita, T.; et al. Pivotal Trial of a Biodegradable Stent for Patients with Refractory Benign Esophageal Stricture. Esophagus 2022, 19, 516–524. [Google Scholar] [CrossRef]
- Liu, L.-L.; Qin, J.; Zeng, C.-H.; Du, R.-J.; Pan, T.; Ji, J.-J.; Lu, L.-G.; Chen, L.; Liu, D.-F.; Yang, J.; et al. Biodegradable PTX-PLGA-Coated Magnesium Stent for Benign Esophageal Stricture: An Experimental Study. Acta Biomater. 2022, 146, 495–505. [Google Scholar] [CrossRef]
- Canena, J.M.T.; Liberato, M.J.A.; Rio-Tinto, R.A.N.; Pinto-Marques, P.M.; Romão, C.M.M.; Coutinho, A.V.M.P.; Neves, B.A.H.C.; Santos-Silva, M.F.C.N. A Comparison of the Temporary Placement of 3 Different Self-Expanding Stents for the Treatment of Refractory Benign Esophageal Strictures: A Prospective Multicentre Study. BMC Gastroenterol. 2012, 12, 70. [Google Scholar] [CrossRef]
- Fuccio, L.; Hassan, C.; Frazzoni, L.; Miglio, R.; Repici, A. Clinical Outcomes Following Stent Placement in Refractory Benign Esophageal Stricture: A Systematic Review and Meta-Analysis. Endoscopy 2016, 48, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Imaz-Iglesia, I.; García-Pérez, S.; Nachtnebel, A.; Martín-Águeda, B.; Sánchez-Piedra, C.; Karadayi, B.; Demirbaş, A.R. Biodegradable Stents for the Treatment of Refractory or Recurrent Benign Esophageal Stenosis. Expert Rev. Med. Devices 2016, 13, 583–599. [Google Scholar] [CrossRef]
- Giri, S.; Vaidya, A.; Kale, A.; Jearth, V.; Sundaram, S. Efficacy of Lumen-Apposing Metal Stents for the Management of Benign Gastrointestinal Stricture: A Systematic Review and Meta-Analysis. Ann. Gastroenterol. 2023, 36, 524–532. [Google Scholar] [CrossRef]
- Mohan, B.P.; Chandan, S.; Garg, R.; Mohamed, S.; Shakhatreh, M.; Dugyala, S.; Mashiana, H.S.; Ponnada, S.; Asokkumar, R.; Adler, D.G. Lumen-Apposing Metal Stents, Fully Covered Self-Expanding Metal Stents, and Biodegradable Stents in the Management of Benign of GI Strictures: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2019, 53, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Siersema, P.D. How to Approach a Patient with Refractory or Recurrent Benign Esophageal Stricture. Gastroenterology 2019, 156, 7–10. [Google Scholar] [CrossRef]
- Raskin, J.B.; Manten, H.; Harary, A.; Redlhammer, D.E.; Rogers, A.I. Transendoscopic Electrosurgical Incision of Lower Esophageal (Schatzki) Rings: A New Treatment Modality. Gastrointest. Endosc. 1985, 31, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, S.-H.; Park, J.-Y.; Lee, C.K.; Chung, I.-K.; Kim, H.S.; Park, S.-H.; Kim, S.-J.; Hong, S.J.; Lee, M.S. Primary Incisional Therapy with a Modified Method for Patients with Benign Anastomotic Esophageal Stricture. Gastrointest. Endosc. 2009, 69, 1029–1033. [Google Scholar] [CrossRef]
- Liang, C.; Tan, Y.; Lu, J.; Le, M.; Liu, D. Endoscopic Incision for Treatment of Benign Gastrointestinal Strictures. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 445–452. [Google Scholar] [CrossRef]
- Muto, M.; Ezoe, Y.; Yano, T.; Aoyama, I.; Yoda, Y.; Minashi, K.; Morita, S.; Horimatsu, T.; Miyamoto, S.-I.; Ohtsu, A.; et al. Usefulness of Endoscopic Radial Incision and Cutting Method for Refractory Esophagogastric Anastomotic Stricture (with Video). Gastrointest. Endosc. 2012, 75, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, M.L.; van Hooft, J.E.; Hansen, B.E.; Fockens, P.; Kuipers, E.J. A Randomized Comparison of Electrocautery Incision with Savary Bougienage for Relief of Anastomotic Gastroesophageal Strictures. Gastrointest. Endosc. 2009, 70, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, Z.; Jogiat, U.; Hajjar, A.; Verhoeff, K.; Turner, S.; Wong, C.; Kung, J.Y.; Bédard, E.L.R. Endoscopic Incisional Therapy for Benign Anastomotic Strictures after Esophagectomy or Gastrectomy: A Systematic Review and Meta-Analysis. Surg. Endosc. 2024, 38, 2995–3003. [Google Scholar] [CrossRef]
- Hagiwara, A.; Togawa, T.; Yamasaki, J.; Shirasu, M.; Sakakura, C.; Yamagishi, H. Endoscopic Incision and Balloon Dilatation for Cicatricial Anastomotic Strictures. Hepatogastroenterology 1999, 46, 997–999. [Google Scholar]
- Augustin, S.; González, A.; Genescà, J. Acute Esophageal Variceal Bleeding: Current Strategies and New Perspectives. World J. Hepatol. 2010, 2, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Camus Duboc, M.; Garcia-Pagan, J.C.; Fuccio, L.; Karstensen, J.G.; Hucl, T.; Jovanovic, I.; Awadie, H.; Hernandez-Gea, V.; Tantau, M.; et al. Endoscopic Diagnosis and Management of Esophagogastric Variceal Hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 1094–1120. [Google Scholar] [CrossRef]
- Mohan, B.P.; Chandan, S.; Khan, S.R.; Kotagiri, R.; Kassab, L.L.; Olaiya, B.; Ponnada, S.; Ofosu, A.; Adler, D.G. Self-Expanding Metal Stents versus TIPS in Treatment of Refractory Bleeding Esophageal Varices: A Systematic Review and Meta-Analysis. Endosc. Int. Open 2020, 8, E291–E300. [Google Scholar] [CrossRef]
- Shao, X.-D.; Qi, X.-S.; Guo, X.-Z. Esophageal Stent for Refractory Variceal Bleeding: A Systemic Review and Meta-Analysis. BioMed. Res. Int. 2016, 2016, 4054513. [Google Scholar] [CrossRef]
- McCarty, T.R.; Njei, B. Self-Expanding Metal Stents for Acute Refractory Esophageal Variceal Bleeding: A Systematic Review and Meta-Analysis. Dig. Endosc. 2016, 28, 539–547. [Google Scholar] [CrossRef]
- Songtanin, B.; Kahathuduwa, C.; Nugent, K. Esophageal Stent in Acute Refractory Variceal Bleeding: A Systematic Review and a Meta-Analysis. J. Clin. Med. 2024, 13, 357. [Google Scholar] [CrossRef]
- Escorsell, À.; Pavel, O.; Cárdenas, A.; Morillas, R.; Llop, E.; Villanueva, C.; Garcia-Pagán, J.C.; Bosch, J.; Variceal Bleeding Study Group. Esophageal Balloon Tamponade versus Esophageal Stent in Controlling Acute Refractory Variceal Bleeding: A Multicenter Randomized, Controlled Trial. Hepatology 2016, 63, 1957–1967. [Google Scholar] [CrossRef]
- Rodrigues, S.G.; Cárdenas, A.; Escorsell, À.; Bosch, J. Balloon Tamponade and Esophageal Stenting for Esophageal Variceal Bleeding in Cirrhosis: A Systematic Review and Meta-Analysis. Semin. Liver Dis. 2019, 39, 178–194. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Testoni, S.G.G.; Barchi, A.; Azzolini, F.; Sinagra, E.; Pepe, G.; Chiti, A.; Danese, S. Imaging in Gastroparesis: Exploring Innovative Diagnostic Approaches, Symptoms, and Treatment. Life 2023, 13, 1743. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Elvevi, A.; Sciola, V.; Mandarino, F.V.; Danese, S.; Invernizzi, P.; Massironi, S. Paradoxical Association between Dyspepsia and Autoimmune Chronic Atrophic Gastritis: Insights into Mechanisms, Pathophysiology, and Treatment Options. World J. Gastroenterol. 2023, 29, 3733–3747. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Testoni, S.G.G.; Barchi, A.; Pepe, G.; Esposito, D.; Fanti, L.; Viale, E.; Biamonte, P.; Azzolini, F.; Danese, S. Gastric Emptying Study before Gastric Peroral Endoscopic Myotomy (G-POEM): Can Intragastric Meal Distribution Be a Predictor of Success? Gut 2023, 72, 1019–1020. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Vespa, E.; Barchi, A.; Fasulo, E.; Sinagra, E.; Azzolini, F.; Danese, S. Precision Endoscopy in Peroral Myotomies for Motility Disorders of the Upper Gastrointestinal Tract: Current Insights and Prospective Avenues-A Comprehensive Review. Life 2023, 13, 2143. [Google Scholar] [CrossRef]
- Johnson, C.D. Gastric Outlet Obstruction Malignant until Proved Otherwise. Am. J. Gastroenterol. 1995, 90, 1740. [Google Scholar]
- Chowdhury, A.; Dhali, G.K.; Banerjee, P.K. Etiology of Gastric Outlet Obstruction. Am. J. Gastroenterol. 1996, 91, 1679. [Google Scholar]
- Samad, A.; Khanzada, T.W.; Shoukat, I. Gastric Outlet Obstruction: Change in Etiology. Pak. J. Surg. 2007, 23, 29–32. [Google Scholar]
- Tendler, D.A. Malignant Gastric Outlet Obstruction: Bridging Another Divide. Am. J. Gastroenterol. 2002, 97, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Mutignani, M.; Tringali, A.; Shah, S.G.; Perri, V.; Familiari, P.; Iacopini, F.; Spada, C.; Costamagna, G. Combined Endoscopic Stent Insertion in Malignant Biliary and Duodenal Obstruction. Endoscopy 2007, 39, 440–447. [Google Scholar] [CrossRef]
- Jeurnink, S.M.; Steyerberg, E.W.; van Hooft, J.E.; van Eijck, C.H.J.; Schwartz, M.P.; Vleggaar, F.P.; Kuipers, E.J.; Siersema, P.D.; Dutch SUSTENT Study Group. Surgical Gastrojejunostomy or Endoscopic Stent Placement for the Palliation of Malignant Gastric Outlet Obstruction (SUSTENT Study): A Multicenter Randomized Trial. Gastrointest. Endosc. 2010, 71, 490–499. [Google Scholar] [CrossRef]
- Jeurnink, S.M.; Polinder, S.; Steyerberg, E.W.; Kuipers, E.J.; Siersema, P.D. Cost Comparison of Gastrojejunostomy versus Duodenal Stent Placement for Malignant Gastric Outlet Obstruction. J. Gastroenterol. 2010, 45, 537–543. [Google Scholar] [CrossRef]
- Lokich, J.J.; Kane, R.A.; Harrison, D.A.; McDermott, W.V. Biliary Tract Obstruction Secondary to Cancer: Management Guidelines and Selected Literature Review. J. Clin. Oncol. 1987, 5, 969–981. [Google Scholar] [CrossRef]
- Staub, J.; Siddiqui, A.; Taylor, L.J.; Loren, D.; Kowalski, T.; Adler, D.G. ERCP Performed through Previously Placed Duodenal Stents: A Multicenter Retrospective Study of Outcomes and Adverse Events. Gastrointest. Endosc. 2018, 87, 1499–1504. [Google Scholar] [CrossRef]
- Mosler, P.; Mergener, K.D.; Brandabur, J.J.; Schembre, D.B.; Kozarek, R.A. Palliation of Gastric Outlet Obstruction and Proximal Small Bowel Obstruction with Self-Expandable Metal Stents: A Single Center Series. J. Clin. Gastroenterol. 2005, 39, 124–128. [Google Scholar] [PubMed]
- Masci, E.; Viale, E.; Mangiavillano, B.; Contin, G.; Lomazzi, A.; Buffoli, F.; Gatti, M.; Repaci, G.; Teruzzi, V.; Fasoli, R.; et al. Enteral Self-Expandable Metal Stent for Malignant Luminal Obstruction of the Upper and Lower Gastrointestinal Tract: A Prospective Multicentric Study. J. Clin. Gastroenterol. 2008, 42, 389–394. [Google Scholar] [CrossRef]
- Mintziras, I.; Miligkos, M.; Wächter, S.; Manoharan, J.; Bartsch, D.K. Palliative Surgical Bypass Is Superior to Palliative Endoscopic Stenting in Patients with Malignant Gastric Outlet Obstruction: Systematic Review and Meta-Analysis. Surg. Endosc. 2019, 33, 3153–3164. [Google Scholar] [CrossRef]
- Tringali, A.; Costa, D.; Anderloni, A.; Carrara, S.; Repici, A.; Adler, D.G. Covered versus Uncovered Metal Stents for Malignant Gastric Outlet Obstruction: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2020, 92, 1153–1163.e9. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Naitoh, I.; Hayashi, K.; Ban, T.; Natsume, M.; Okumura, F.; Nakazawa, T.; Takada, H.; Hirano, A.; Jinno, N.; et al. Predictors of Stent Dysfunction after Self-Expandable Metal Stent Placement for Malignant Gastric Outlet Obstruction: Tumor Ingrowth in Uncovered Stents and Migration of Covered Stents. Surg. Endosc. 2017, 31, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, A.Y.; Kahaleh, M.; Tyberg, A. Clinical Review of EUS-Guided Gastroenterostomy (EUS-GE). J. Clin. Gastroenterol. 2020, 54, 1–7. [Google Scholar] [CrossRef]
- Itoi, T.; Baron, T.H.; Khashab, M.A.; Tsuchiya, T.; Irani, S.; Dhir, V.; Bun Teoh, A.Y. Technical Review of Endoscopic Ultrasonography-Guided Gastroenterostomy in 2017. Dig. Endosc. 2017, 29, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Itokawa, F.; Uraoka, T.; Gotoda, T.; Horii, J.; Goto, O.; Moriyasu, F.; Moon, J.H.; Kitagawa, Y.; Yahagi, N. Novel EUS-Guided Gastrojejunostomy Technique Using a New Double-Balloon Enteric Tube and Lumen-Apposing Metal Stent (with Videos). Gastrointest. Endosc. 2013, 78, 934–939. [Google Scholar] [CrossRef]
- Bronswijk, M.; van Malenstein, H.; Laleman, W.; Van der Merwe, S.; Vanella, G.; Petrone, M.C.; Arcidiacono, P.G. EUS-Guided Gastroenterostomy: Less Is More! The Wireless EUS-Guided Gastroenterostomy Simplified Technique. VideoGIE 2020, 5, 442. [Google Scholar] [CrossRef]
- Vanella, G.; Tamburrino, D.; Capurso, G.; Bronswijk, M.; Reni, M.; Dell’Anna, G.; Crippa, S.; Van Der Merwe, S.; Falconi, M.; Arcidiacono, P.G. Feasibility of Therapeutic Endoscopic Ultrasound in the Bridge-to-Surgery Scenario: The Example of Pancreatic Adenocarcinoma. World J. Gastroenterol. 2022, 28, 976–984. [Google Scholar] [CrossRef]
- Vanella, G.; Dell’Anna, G.; Capurso, G.; Maisonneuve, P.; Bronswijk, M.; Crippa, S.; Tamburrino, D.; Macchini, M.; Orsi, G.; Casadei-Gardini, A.; et al. EUS-Guided Gastroenterostomy for Management of Malignant Gastric Outlet Obstruction: A Prospective Cohort Study with Matched Comparison with Enteral Stenting. Gastrointest. Endosc. 2023, 98, 337–347.e5. [Google Scholar] [CrossRef]
- Trieu, J.A.; Kahlenberg, S.; Gilman, A.J.; Hathorn, K.; Baron, T.H. Long-Term Outcomes of EUS-Guided Gastroenterostomy: A Large, Single-Center Experience. Clin. Transl. Gastroenterol. 2025, 16, e00648. [Google Scholar] [CrossRef]
- Giri, S.; Harindranath, S.; Mohan, B.P.; Jearth, V.; Varghese, J.; Kozyk, M.; Kale, A.; Sundaram, S. Adverse Events with Endoscopic Ultrasound-guided Gastroenterostomy for Gastric Outlet Obstruction—A Systematic Review and Meta-analysis. United Eur. Gastroenterol. J. 2024, 12, 879–890. [Google Scholar] [CrossRef]
- Ghandour, B.; Bejjani, M.; Irani, S.S.; Sharaiha, R.Z.; Kowalski, T.E.; Pleskow, D.K.; Do-Cong Pham, K.; Anderloni, A.A.; Martinez-Moreno, B.; Khara, H.S.; et al. Classification, Outcomes, and Management of Misdeployed Stents during EUS-Guided Gastroenterostomy. Gastrointest. Endosc. 2022, 95, 80–89. [Google Scholar] [CrossRef]
- Chen, Y.-I.; Itoi, T.; Baron, T.H.; Nieto, J.; Haito-Chavez, Y.; Grimm, I.S.; Ismail, A.; Ngamruengphong, S.; Bukhari, M.; Hajiyeva, G.; et al. EUS-Guided Gastroenterostomy Is Comparable to Enteral Stenting with Fewer Re-Interventions in Malignant Gastric Outlet Obstruction. Surg. Endosc. 2017, 31, 2946–2952. [Google Scholar] [CrossRef]
- Ge, P.S.; Young, J.Y.; Dong, W.; Thompson, C.C. EUS-Guided Gastroenterostomy versus Enteral Stent Placement for Palliation of Malignant Gastric Outlet Obstruction. Surg. Endosc. 2019, 33, 3404–3411. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Benchaya, J.A.; Martel, M.; Barkun, A.; Wyse, J.M.; Ferri, L.; Chen, Y.-I. EUS-Guided Gastroenterostomy vs. Surgical Gastrojejunostomy and Enteral Stenting for Malignant Gastric Outlet Obstruction: A Meta-Analysis. Endosc. Int. Open 2023, 11, E660–E672. [Google Scholar] [CrossRef]
- Tran, K.V.; Vo, N.-P.; Nguyen, H.S.; Vo, N.T.; Thai, T.B.T.; Pham, V.A.; Loh, E.-W.; Tam, K.-W. Palliative Procedures for Malignant Gastric Outlet Obstruction: A Network Meta-Analysis. Endoscopy 2024, 56, 780–789. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Lakhtakia, S.; Tarantino, I.; Perez-Miranda, M.; Kunda, R.; Maluf-Filho, F.; Dhir, V.; Basha, J.; Chan, S.M.; Ligresti, D.; et al. Endoscopic Ultrasonography-Guided Gastroenterostomy versus Uncovered Duodenal Metal Stenting for Unresectable Malignant Gastric Outlet Obstruction (DRA-GOO): A Multicentre Randomised Controlled Trial. Lancet Gastroenterol. Hepatol. 2025, 10, e8–e16. [Google Scholar] [CrossRef] [PubMed]
- Ramai, D.; Nelson, R.; Chaiyakunapruk, N.; Ofosu, A.; Fang, J.C. Endoscopic Ultrasound Gastroenterostomy vs Duodenal Stenting for Malignant Gastric Outlet Obstruction: Cost-Effectiveness Study. Endosc. Int. Open 2025, 13, a25097671. [Google Scholar] [CrossRef] [PubMed]
- Cherian, P.T.; Cherian, S.; Singh, P. Long-Term Follow-up of Patients with Gastric Outlet Obstruction Related to Peptic Ulcer Disease Treated with Endoscopic Balloon Dilatation and Drug Therapy. Gastrointest. Endosc. 2007, 66, 491–497. [Google Scholar] [CrossRef]
- Kochhar, R.; Kochhar, S. Endoscopic Balloon Dilation for Benign Gastric Outlet Obstruction in Adults. World J. Gastrointest. Endosc. 2010, 2, 29–35. [Google Scholar] [CrossRef]
- Dormann, A.J.; Deppe, H.; Wigginghaus, B. Self-Expanding Metallic Stents for Continuous Dilatation of Benign Stenoses in Gastrointestinal Tract—First Results of Long-Term Follow-up in Interim Stent Application in Pyloric and Colonic Obstructions. Z. Gastroenterol. 2001, 39, 957–960. [Google Scholar] [CrossRef]
- Fujitani, K.; Ando, M.; Sakamaki, K.; Terashima, M.; Kawabata, R.; Ito, Y.; Yoshikawa, T.; Kondo, M.; Kodera, Y.; Yoshida, K. Multicentre Observational Study of Quality of Life after Surgical Palliation of Malignant Gastric Outlet Obstruction for Gastric Cancer. BJS Open 2017, 1, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Perez-Miranda, M.; Tyberg, A.; Poletto, D.; Toscano, E.; Gaidhane, M.; Desai, A.P.; Kumta, N.A.; Fayad, L.; Nieto, J.; Barthet, M.; et al. EUS-Guided Gastrojejunostomy Versus Laparoscopic Gastrojejunostomy: An International Collaborative Study. J. Clin. Gastroenterol. 2017, 51, 896–899. [Google Scholar] [CrossRef]
- Canakis, A.; Tugarinov, N.; Bapaye, J.; Gilman, A.J.; Hathorn, K.E.; Twery, B.; Sharaiha, R.Z.; Baron, T.H.; Irani, S.S. EUS-Guided Gastroenterostomy for Benign Gastric Outlet Obstruction: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.-M.; Ouazzani, S.; Vanbiervliet, G.; Gasmi, M.; Barthet, M. Endoscopic Ultrasound-Guided Gastrojejunostomy with Wire Endoscopic Simplified Technique: Move towards Benign Indications (with Video). Dig. Endosc. 2024, 37, 167–175. [Google Scholar] [CrossRef] [PubMed]
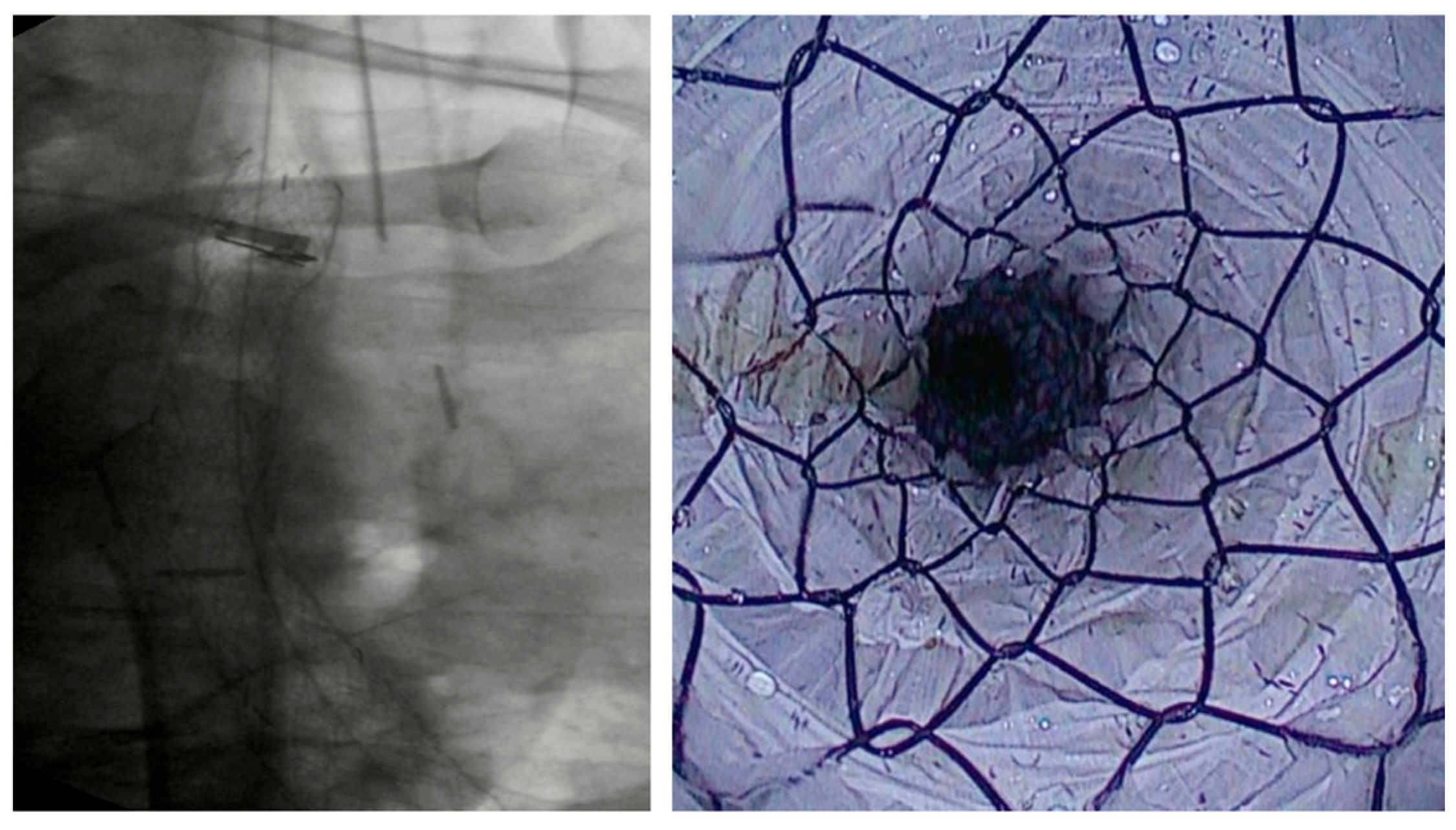

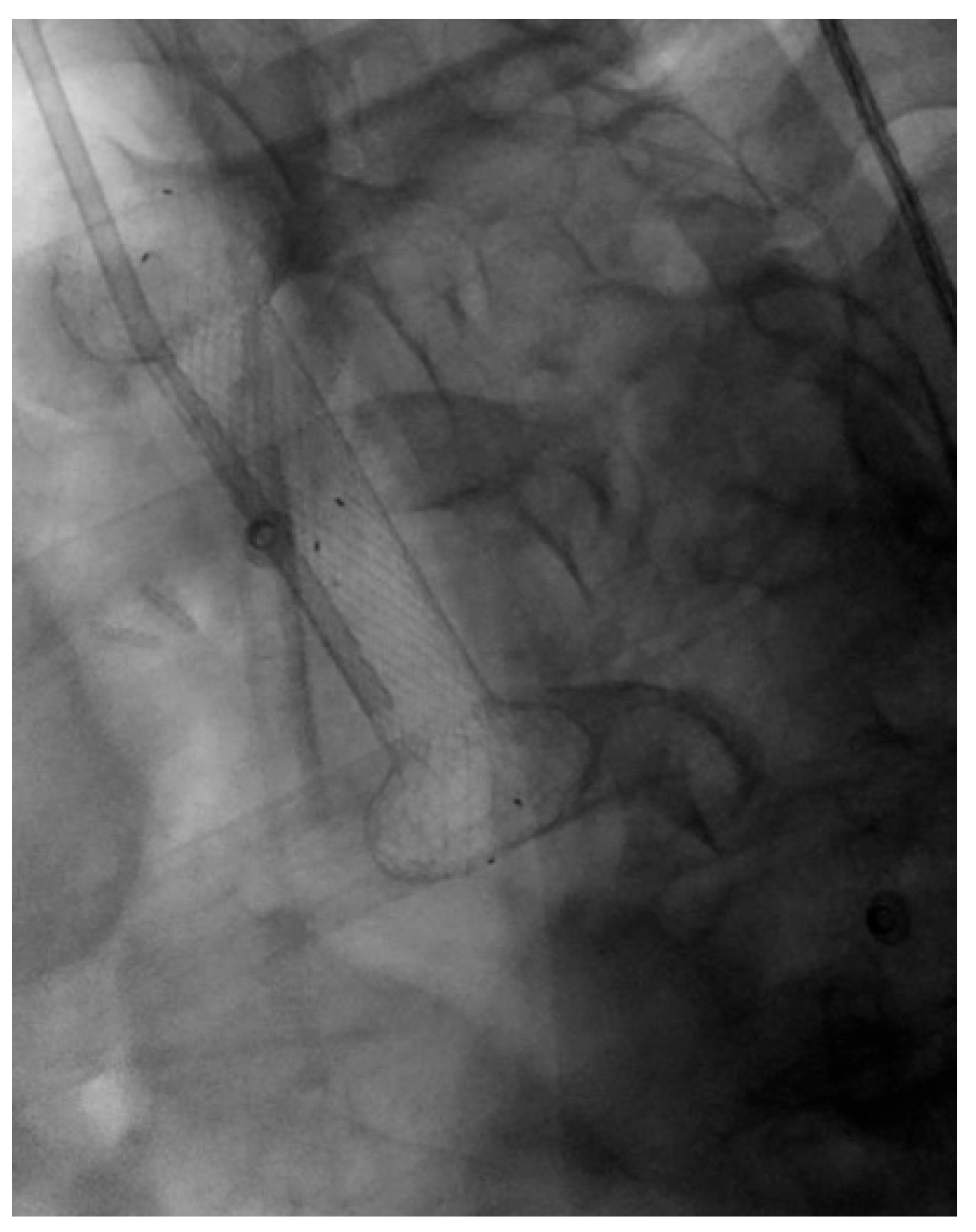
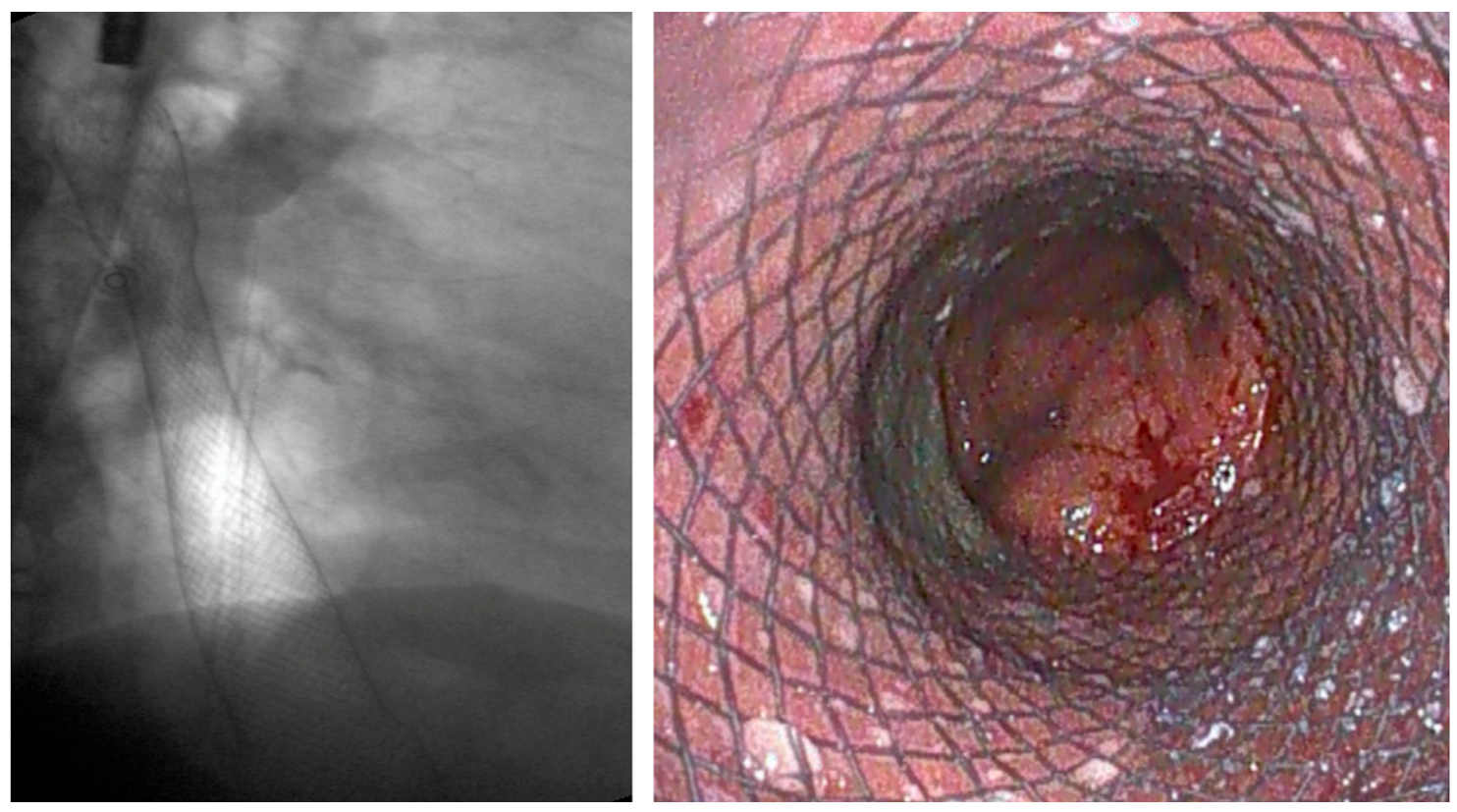
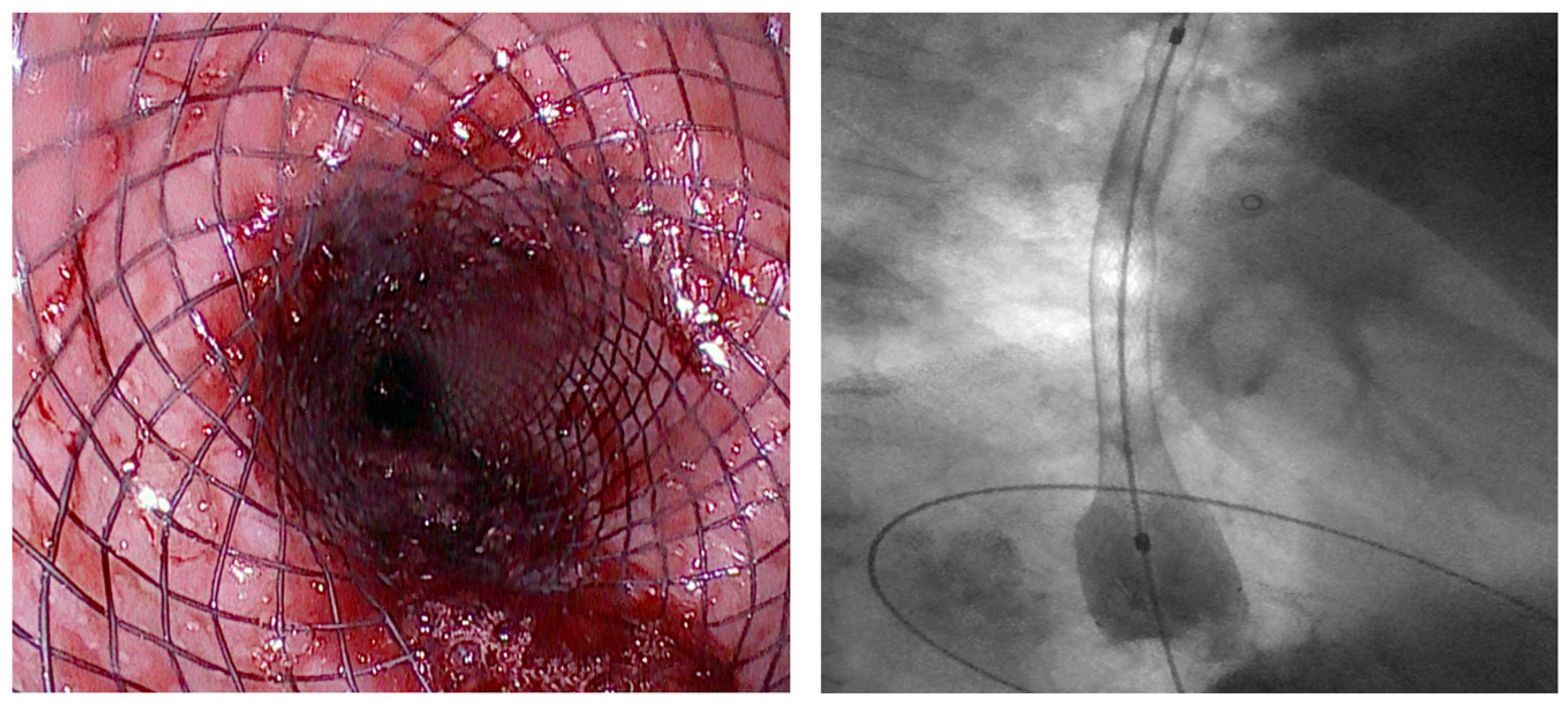
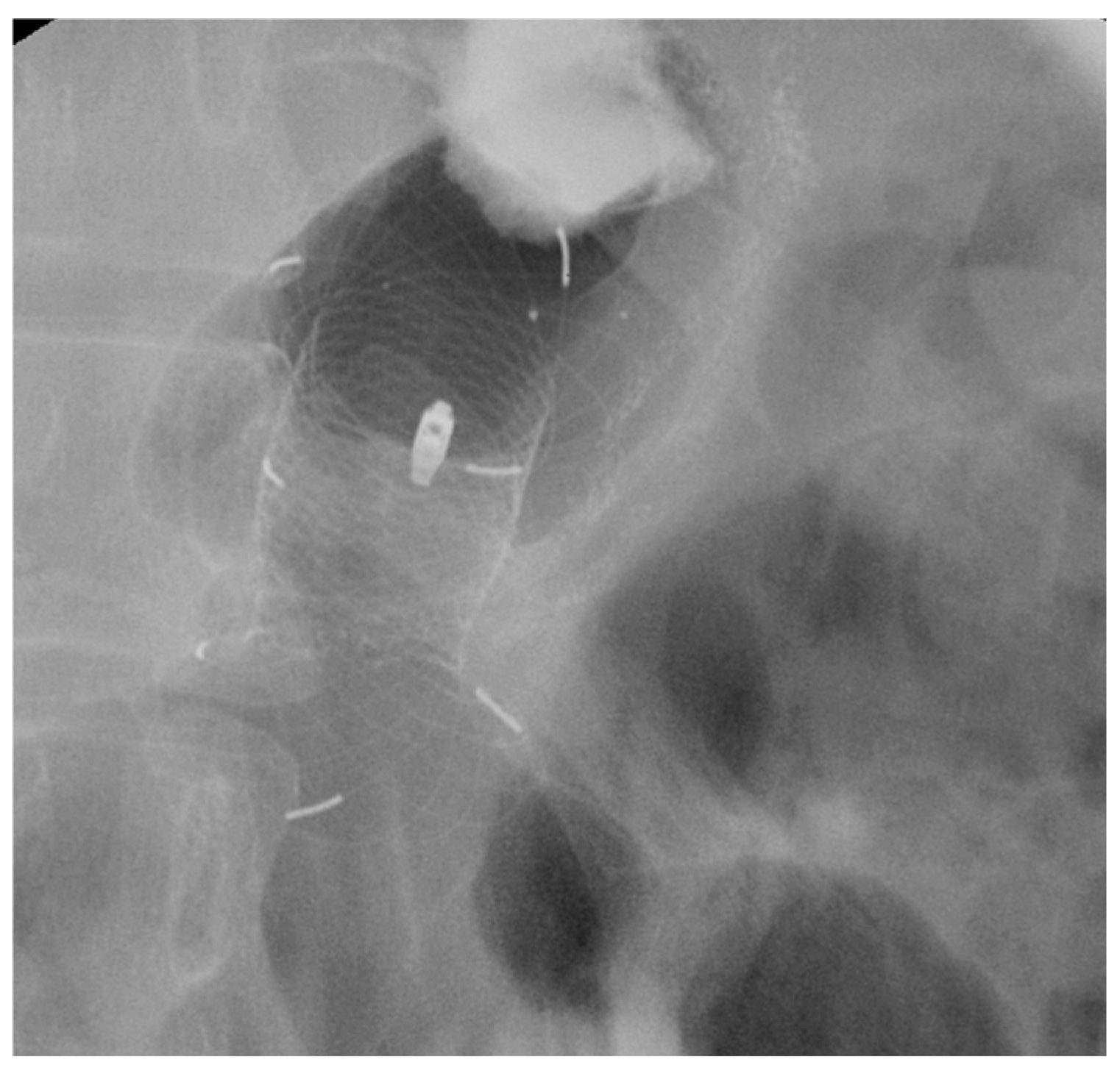
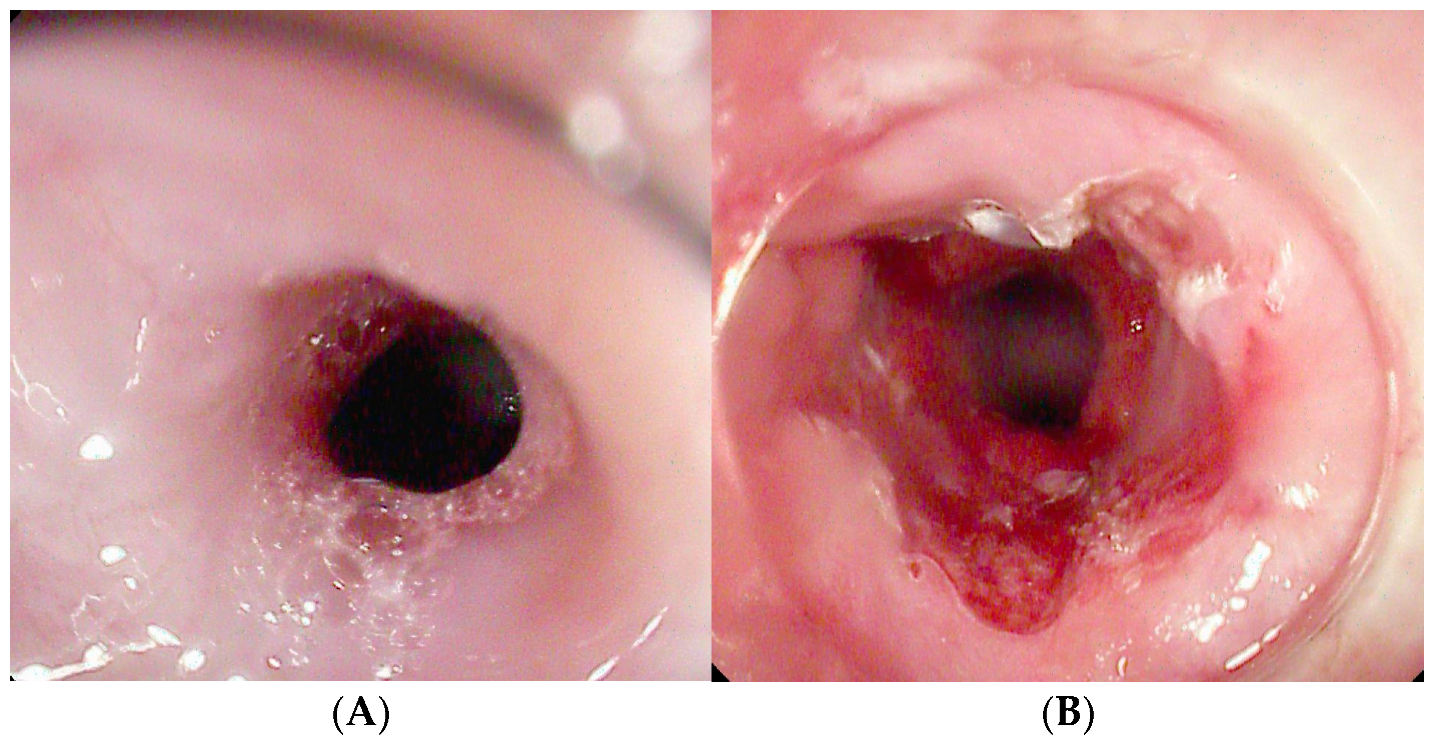
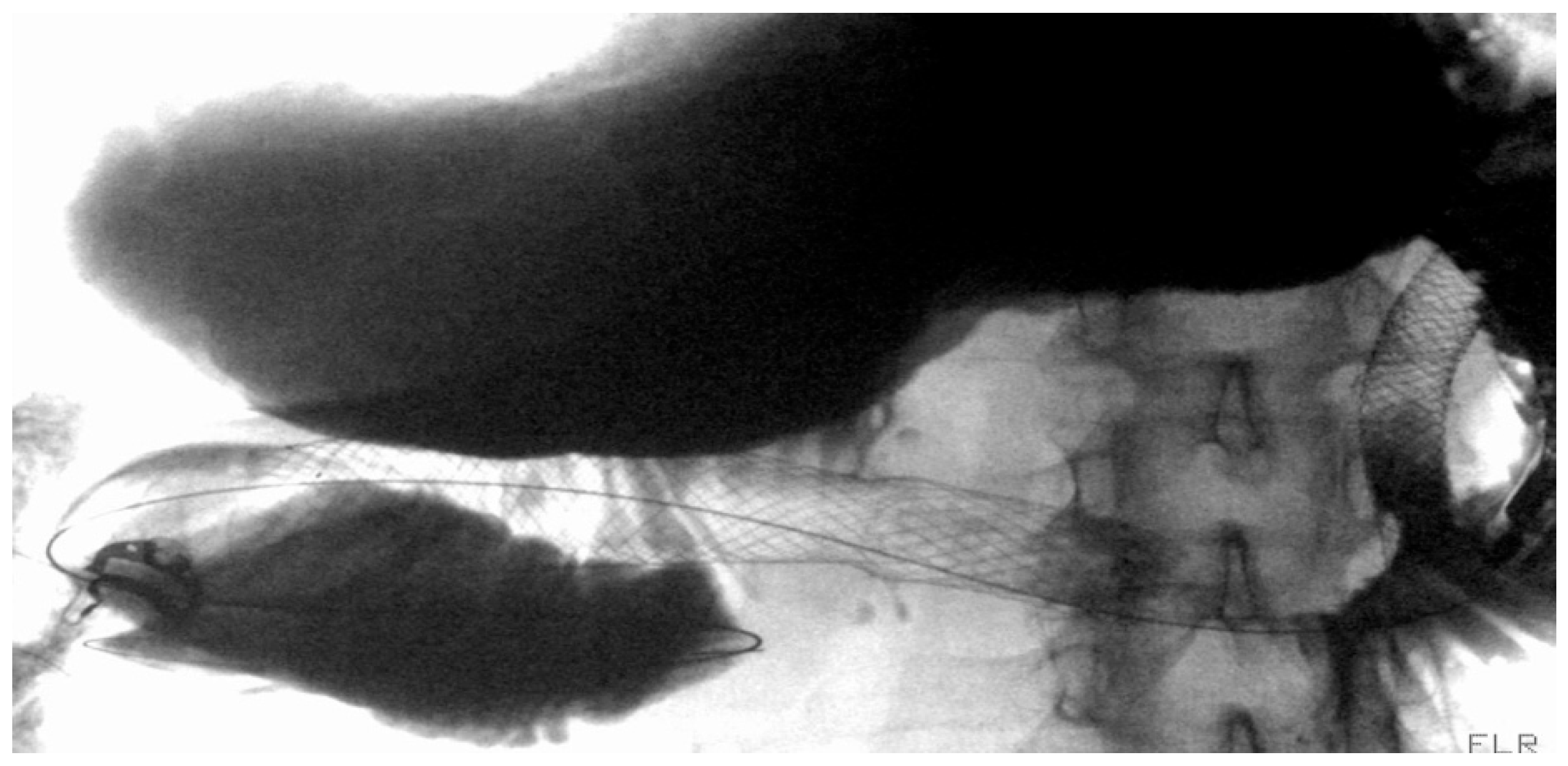
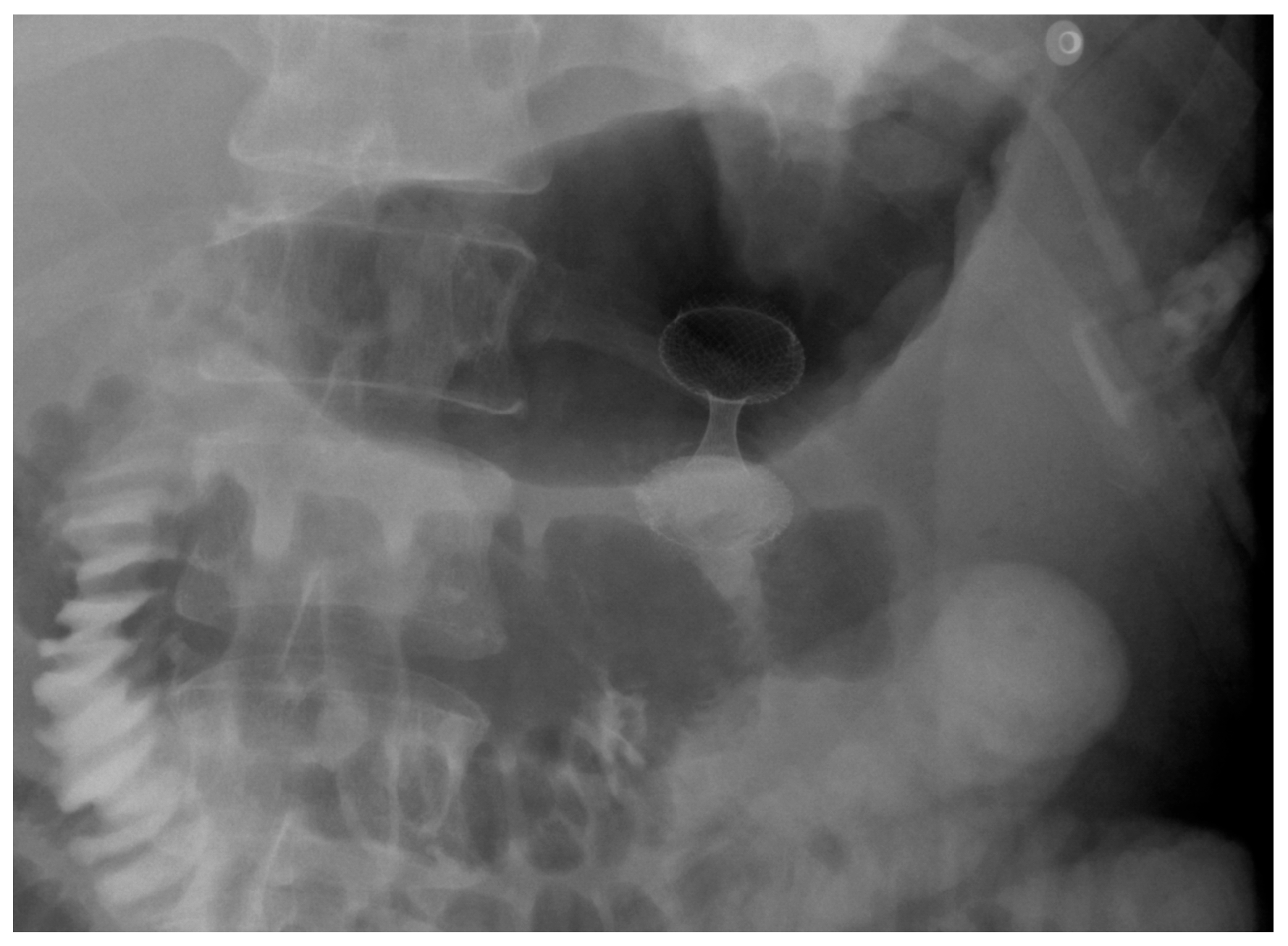
| Authors | Study Design | N° Patients | Indications | Clinical Success % | Adverse Events % |
|---|---|---|---|---|---|
| SEMS | |||||
| Anderloni et al. [19] | Retrospective | 49 | AL post esophageal surgery | 60.5 | 38 |
| Plum et al. [20] | Retrospective | 70 | AL post esophageal surgery | 70 | 28.6 |
| Segura et al. [21] | Retrospective | 20 3 | AL post esophageal surgery Esophageal perforations | 75 | 21.7 |
| Fischer et al. [61] | Retrospective | 11 | AL post esophageal surgery | 100 | 0 |
| Iglesias Jorquera et al. [23] | Retrospective | 25 | AL post esophageal surgery | 84 | 28 |
| Mennigen et al. [62] | Retrospective | 45 | AL post esophageal surgery | 63.3 | 36.7 |
| Mandarino et al. [17] | Retrospective | 37 | AL post esophageal surgery | 62.2 | 22.7 |
| Licht et al. [63] | Retrospective | 49 | AL post esophageal surgery | 88 | 3.2 |
| Schweigert et al. [64] | Retrospective | 25 | AL post esophageal surgery | 76.5 | 23.5 |
| EVT | |||||
| Richter et al. [41] | Prospective | 69 33 | AL post esophageal surgery Esophageal perforations | 91 76 | NA |
| Jung et al. [38] | Retrospective | 119 | AL post esophageal surgery Esophageal perforations | 70.6 | 10.9 |
| Momblan et al. [39] | Prospective | 89 13 | AL post esophageal surgery Esophageal perforations | 82 | 5.9 |
| Luttikhold et al. [40] | Retrospective | 27 | AL post esophageal surgery Esophageal perforations | 89 | 7 |
| VAC STENT | |||||
| Chon et al. [56] | Prospective | 5 5 | AL post esophageal surgery Esophageal perforations | 70 | 0 |
| Chon et al. [57] | Prospective | 18 2 | AL post esophageal surgery Esophageal perforations | 60–71 | 0 |
| Pattynama et al. [54] | Case series | 8 2 | AL post esophageal surgery Esophageal perforations | 100 | 0 |
| Lange et al. [52] | Prospective | 11 4 | AL post esophageal surgery Esophageal perforations | 80 | 7 |
| Blundau et al. [58] | Retrospective | 59 18 | AL post esophageal surgery Esophageal perforations | 78 | NA |
| Authors | Study Design | N° Patients | Indications | Clinical Success % | Adverse Events % |
|---|---|---|---|---|---|
| SEMS | |||||
| Kim et al. [81] | Retrospective | 55 | Benign esophageal strictures | 58—1 month 43—3 months 38—6 months 33—1 year 26—2 years 21—4 years | 31—tissue hyperproliferation 24—severe pain 25—stent migration |
| Liu et al. [82] | Prospective | 24 | Benign anastomotic esophageal strictures | 75—1 year | 72.4—moderate chest pain 3.4—stent migration 17.2—reflux |
| Fuccio et al. [87] | Metanalysis | 227 | Benign esophageal strictures | 40.1 | 21.9 |
| Mohan et al. [90] | Metanalysis | 342 | Benign GI strictures | 48 | 31.5 |
| BDS | |||||
| Tomonori et al. [84] | Non-randomized prospective trial | 30 | Benign esophageal strictures | 46.7 | NA |
| Fuccio et al. [87] | Metanalysis | 77 | Benign esophageal strictures | 32.9 | 21.9 |
| Mohan et al. [90] | Metanalysis | 226 | Benign GI strictures | 34.9 | 11.5 |
| LAMS | |||||
| Giri et al. [89] | Metanalysis | 527 | Benign GI strictures | 93.9 | 13.5 |
| Mohan et al. [90] | Metanalysis | 192 | Benign GI strictures | 78.8 | 29.9 |
| Incisional therapy | |||||
| Lee et al. [93] | Prospective | 24 | Benign anastomotic esophageal strictures | 80.6 | 0 |
| Hordijk et al. [96] | Prospective | 31 | Benign esophageal anastomotic strictures | 78 | NA |
| Authors | Study Design | N° Patients | Indications | Clinical Success % | Adverse Events % |
|---|---|---|---|---|---|
| SEMS | |||||
| Mintziras et al. [122] | Metanalysis | 1306 | Malignant gastric outlet obstruction | 75–100 | 34.2—stent migration 12.5—perforation 14.2—stent disfunction |
| Teoh et al. [138] | RCT | 49 | Malignant gastric outlet obstruction | 92 | 24 |
| LAMS | |||||
| Vanella et al. [130] | Prospective | 70 | Malignant gastric outlet obstruction | 97.1 | 12.9 |
| Trieu et al. [131] | Retrospective | 207 | Gastric outlet obstruction | 97.2 | 4.8—Early AEs 3.4—Late AEs |
| Teoh et al. [138] | RCT | 48 | Malignant gastric outlet obstruction | 100 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardi, F.; Dell’Anna, G.; Biamonte, P.; Barchi, A.; Fanti, L.; Malesci, A.; Fuccio, L.; Sinagra, E.; Calabrese, G.; Facciorusso, A.; et al. Stents and Emerging Alternatives in Upper Gastrointestinal Endoscopy: A Comprehensive Review. Diagnostics 2025, 15, 2344. https://doi.org/10.3390/diagnostics15182344
Bernardi F, Dell’Anna G, Biamonte P, Barchi A, Fanti L, Malesci A, Fuccio L, Sinagra E, Calabrese G, Facciorusso A, et al. Stents and Emerging Alternatives in Upper Gastrointestinal Endoscopy: A Comprehensive Review. Diagnostics. 2025; 15(18):2344. https://doi.org/10.3390/diagnostics15182344
Chicago/Turabian StyleBernardi, Francesca, Giuseppe Dell’Anna, Paolo Biamonte, Alberto Barchi, Lorella Fanti, Alberto Malesci, Lorenzo Fuccio, Emanuele Sinagra, Giulio Calabrese, Antonio Facciorusso, and et al. 2025. "Stents and Emerging Alternatives in Upper Gastrointestinal Endoscopy: A Comprehensive Review" Diagnostics 15, no. 18: 2344. https://doi.org/10.3390/diagnostics15182344
APA StyleBernardi, F., Dell’Anna, G., Biamonte, P., Barchi, A., Fanti, L., Malesci, A., Fuccio, L., Sinagra, E., Calabrese, G., Facciorusso, A., Bruni, A., Donatelli, G., Danese, S., & Mandarino, F. V. (2025). Stents and Emerging Alternatives in Upper Gastrointestinal Endoscopy: A Comprehensive Review. Diagnostics, 15(18), 2344. https://doi.org/10.3390/diagnostics15182344









