[123I]-Meta-Iodobenzylguanidine Scintigraphy in Sarcoidosis: Exploring Cardiac Autonomic Dysfunction in Patients with Unexplained Cardiac Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
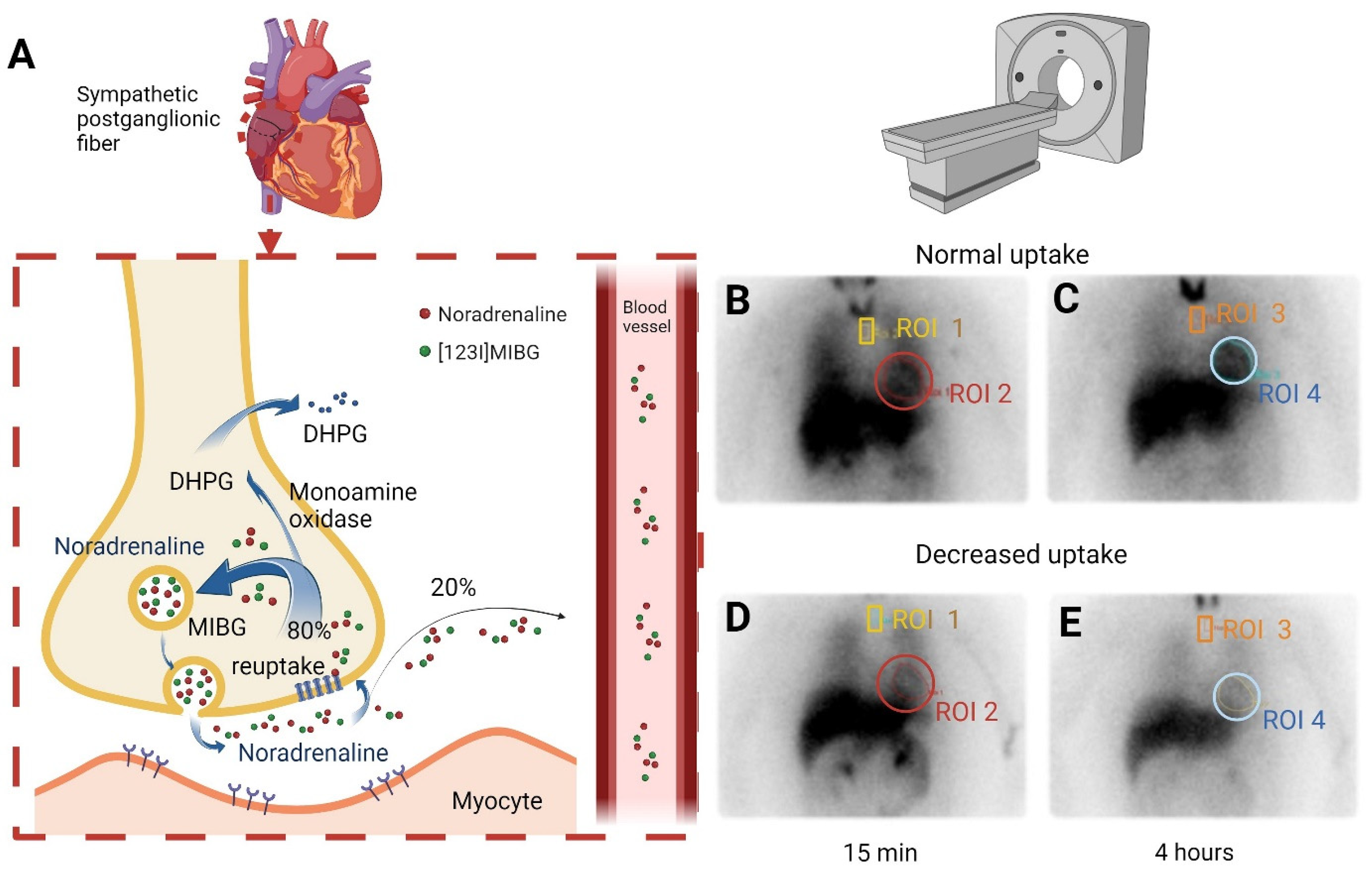
2.2. Measurements
2.2.1. Diagnosis of Cardiac Sarcoidosis
2.2.2. Diagnosis of Cardiac Autonomic Dysfunction
2.2.3. Fluordeoxyglucose Positron Emission Tomography/Computed Tomography ([18F]FDG PET/CT)
2.2.4. Cardiac Magnetic Resonance Imaging
2.3. Clinical Data
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CMR | Cardiac magnetic resonance imaging |
| [18F]FDG PET/CT | [18F]fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography |
| GCP | Good Clinical Practice |
| H/M | Heart-to-mediastinal uptake ratio |
| ILD | Interstitial lung disease |
| LGE | Late gadolinium enhancement |
| [123I]-MIBG | [123I]-meta-iodinebenzylguanidine |
| MDT | Multidisciplinary team |
| ROI | Region of interest |
| SCAD | Sarcoidosis-associated cardiac autonomic dysfunction |
| SFN | Small-fiber neuropathy |
References
- Crouser, E.D.; Maier, L.A.; Baughman, R.P.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Bonham, C.A.; Chen, E.S.; Culver, D.A.; Drake, W.; et al. Diagnosis and detection of sarcoidosis an official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020, 201, E26–E51. [Google Scholar] [CrossRef]
- Sharma, R.; Kouranos, V.; Cooper, L.T.; Metra, M.; Ristic, A.; Heidecker, B.; Baksi, J.; Wicks, E.; Merino, J.L.; Klingel, K.; et al. Management of cardiac sarcoidosis. Eur. Heart J. 2024, 45, 2697–2726. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014, 11, 1305–1323. [Google Scholar] [CrossRef]
- Gawałko, M.; Balsam, P.; Lodziński, P.; Grabowski, M.; Krzowski, B.; Opolski, G.; Kosiuk, J. Cardiac arrhythmias in autoimmune diseases. Circ. J. 2020, 84, 685–694. [Google Scholar] [CrossRef]
- Zalewski, P.; Słomko, J.; Zawadka-Kunikowska, M. Autonomic dysfunction and chronic disease. Br. Med. Bull. 2018, 128, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.M.; Vinik, A.I.; Arezzo, J.C.; Bril, V.; Feldman, E.L.; Freeman, R.; Malik, R.A.; Maser, R.E.; Sosenko, J.M.; Ziegler, D. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care 2005, 28, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, M.; Faber, C.G.; Hoeijmakers, J.G.J.; Lauria, G.; Merkies, I.S.J. Small fibers, large impact: Quality of life in small-fiber neuropathy. Muscle Nerve 2014, 49, 329–336. [Google Scholar] [CrossRef]
- Lacomis, D. Small-fiber neuropathy. Muscle Nerve 2002, 26, 173–188. [Google Scholar] [CrossRef]
- Voortman, M.; Hendriks, C.M.R.; Elfferich, M.D.P.; Bonella, F.; Møller, J.; De Vries, J.; Costabel, U.; Drent, M. The burden of sarcoidosis symptoms from a patient perspective. Lung 2019, 197, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Raasing, L.R.M.; Veltkamp, M.; Datema, M.; Korenromp, I.; Grutters, J.C.; Vogels, O.J.M. Sarcoidosis-Related Small Fiber Neuropathy: Focus on Fatigue, Pain, Restless Legs Syndrome, and Cognitive Function. Sarcoidosis Vasc. Diffus. Lung Dis. 2025, 42, 16214. [Google Scholar] [CrossRef]
- Drent, M.; Crouser, E.D.; Grunewald, J. Challenges of sarcoidosis and its management. N. Engl. J. Med. 2021, 385, 1018–1032. [Google Scholar] [CrossRef]
- Drent, M.; Russell, A.M.; Saketkoo, L.A.; Spagnolo, P.; Veltkamp, M.; Wells, A.U.; Goh, N.; Holland, A.; Hochreiter, J.; Kock, S.; et al. Breaking barriers: Holistic assessment of ability to work in patients with sarcoidosis. Lancet Respir. Med. 2024, 12, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.M.; Scheifele, M.; Zacherl, M.J.; Katzdobler, S.; Bernhardt, A.; Brendel, M.; Levin, J.; Höglinger, G.U.; Clauß, S.; Kääb, S.; et al. Diagnostic Efficacy of 123Iodo-Metaiodobenzylguanidine SPECT/CT in Cardiac vs. Neurological Diseases: A Comparative Study of Arrhythmogenic Right Ventricular Cardiomyopathy and α-Synucleinopathies. Diagnostics 2025, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Scholte, A.J.H.A.; Schuijf, J.D.; Delgado, V.; Kok, J.A.; Bus, M.T.J.; Maan, A.C.; Stokkel, M.P.; Kharagitsingh, A.V.; Dibbets-Schneider, P.; Van Der Wall, E.E.; et al. Cardiac autonomic neuropathy in patients with diabetes and no symptoms of coronary artery disease: Comparison of 123I-metaiodobenzylguanidine myocardial scintigraphy and heart rate variability. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1698–1705. [Google Scholar] [CrossRef]
- Bakker, A.L.M.; Mathijssen, H.; Huitema, M.P.; Kapteijns, L.; Grutters, J.C.; Veltkamp, M.; Keijsers, R.G.; Akdim, F.; van Es, H.W.; Peper, J.; et al. Holter monitoring and cardiac biomarkers in screening for cardiac sarcoidosis. Lung 2025, 203, 10. [Google Scholar] [CrossRef] [PubMed]
- Flotats, A.; Carrió, I.; Agostini, D.; Le Guludec, D.; Marcassa, C.; Schäfers, M.; Somsen, G.A.; Unlu, M.; Verberne, H.J. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1802–1812, Erratum in Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 190. [Google Scholar] [CrossRef]
- Somsen, G.A.; Verberne, H.J.; Fleury, E.; Righetti, A. Normal values and within-subject variability of cardiac I-123 MIBG scintigraphy in healthy individuals: Implications for clinical studies. J. Nucl. Cardiol. 2004, 11, 126–133. [Google Scholar] [CrossRef]
- Chirumamilla, A.; Travin, M.I. Cardiac applications of 123I-MIBG imaging. Semin. Nucl. Med. 2011, 41, 374–387. [Google Scholar] [CrossRef]
- Freeman, R. Assessment of cardiovascular autonomic function. Clin. Neurophysiol. 2006, 117, 716–730. [Google Scholar] [CrossRef]
- Mathijssen, H.; Tjoeng, T.W.H.; Keijsers, R.G.M.; Bakker, A.L.M.; Akdim, F.; van Es, H.W.; van Beek, F.T.; Veltkamp, M.V.; Grutters, J.C.; Post, M.C. The usefulness of repeated CMR and FDG PET/CT in the diagnosis of patients with initial possible cardiac sarcoidosis. EJNMMI Res. 2021, 11, 129. [Google Scholar] [CrossRef]
- Raasing, L.R.M. Insights and Innovations in Sarcoidosis-Associated Small Fiber Neuropathy. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2025. Available online: https://researchinformation.umcutrecht.nl/en/publications/insights-and-innovations-in-sarcoidosis-associated-small-fiber-ne (accessed on 8 August 2025).
- Hoitsma, E.; Faber, C.G.; Van Kroonenburgh, M.J.P.G.; Gorgels, A.P.M.; Halders, S.G.E.A.; Heidendal, G.A.K.; Kessels, A.G.H.; Reulen, J.P.H.; Drent, M. Association of small fiber neuropathy with cardiac sympathetic dysfunction in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2005, 22, 43–50. [Google Scholar]
- Gimelli, A.; Liga, R.; Agostini, D.; Bengel, F.M.; Ernst, S.; Hyafil, F.; Saraste, A.; Scholte, A.J.H.A.; Verberne, H.J.; Verschure, D.O.; et al. The role of myocardial innervation imaging in different clinical scenarios: An expert document of the European Association of Cardiovascular Imaging and Cardiovascular Committee of the European Association of Nuclear Medicine. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 480–490. [Google Scholar] [CrossRef]
- Tamaki, N.; Manabe, O. Current status and perspectives of nuclear cardiology. Ann. Nucl. Med. 2024, 38, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Misumi, I.; Kimura, Y.; Hokamura, Y.; Honda, Y.; Yasunaga, T.; Nakashima, K.; Takemura, N.; Asoshina, M.; Uranaka, N.; Takenaka, S.; et al. Scintigraphic detection of regional disruption of the adrenergic nervous system in sarcoid heart disease. Jpn. Circ. J. 1996, 60, 774–778. [Google Scholar] [CrossRef][Green Version]
- Nakata, T.; Miyamoto, K.; Doi, A.; Sasao, H.; Wakabayashi, T.; Kobayashi, H.; Tsuchihashi, K.; Shimamoto, K. Cardiac death prediction and impaired cardiac sympathetic innervation assessed by MIBG in patients with failing and nonfailing hearts. J. Nucl. Cardiol. 1998, 5, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Smulders, N.M.; Bast, A.; van Kroonenburgh, M.J.P.G.; Drent, M. Improvement of cardiac sympathetic nerve function in sarcoidosis. Sarcoidosis, Vasc. Diffus. Lung Dis. 2008, 25, 140–142. [Google Scholar]
- Oishi, M.; Mukaino, A.; Kunii, M.; Saito, A.; Arita, Y.; Koike, H.; Higuchi, O.; Maeda, Y.; Abiru, N.; Yamaguchi, N.; et al. Association between neurosarcoidosis with autonomic dysfunction and anti-ganglionic acetylcholine receptor antibodies. J. Neurol. 2021, 268, 4265–4279. [Google Scholar] [CrossRef]
- Parker, B.M.; Rogers, S.L.; Lymperopoulos, A. Clinical pharmacogenomics of carvedilol: The stereo-selective metabolism angle. Pharmacogenomics 2018, 19, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Low, P.A.; Tomalia, V.A.; Park, K.J. Autonomic Function Tests: Some Clinical Applications. J. Clin. Neurol. 2013, 9, 1–8. [Google Scholar] [CrossRef]

| Total Group (n = 40) | [123I]MIBG Normal (n = 21) | [123I]MIBG Abnormal (n = 19) | p-Value | |
|---|---|---|---|---|
| Age, years, mean ± SD | 48 ± 11 | 45 ± 9 | 52 ± 12 | NS |
| Male, n (%) | 23 (58) | 12 (57) | 11 (58) | NS |
| BMI kg/m2, mean ± SD | 28 ± 5 | 28 ± 4 | 28 ± 5 | NS |
| Time since diagnosis, years, median ± IQR | 11 ± 7 | 11 ± 6 | 12 ± 12 | NS |
| Cardiac sarcoidosis, n (%) | 9 (23) | 3 (14) | 6 (32) | NS |
| Therapy % (n) | ||||
| No immunosuppressive therapy, n (%) | 24 (60) | 14 (67) | 10 (53) | NS |
| Prednisone, n (%) | 7 (18) | 4 (19) | 3 (16) | NS |
| Methotrexate, n (%) | 8 (20) | 3 (14) | 5 (26) | NS |
| TNF-alpha inhibitors, infliximab or adalimumab, n (%) | 2 (5) | 1 (5) | 1 (5) | NS |
| Methylprednisone, n (%) | 1 (3) | 1 (5) | 0 (0) | NS |
| Adalimumab, n (%) | 1 (3) | 0 (0) | 1 (5) | NS |
| Total Group (n = 46) | [123I]MIBG Normal (n = 26) | [123I]MIBG Abnormal (n = 20) | |
|---|---|---|---|
| Chest pain, n (%) | 14 (30) | 7 (27) | 7 (35) |
| Arrhythmia, n (%) | 8 (17) | 6 (23) | 2 (10) |
| Collapse, n (%) | 4 (9) | 1 (4) | 3 (15) |
| Near collapse, n (%) | 3 (7) | 2 (8) | 1 (5) |
| Syncope, n (%) | 1 (2) | 1 (4) | 0 (0) |
| Orthostasis, n (%) | 1 (2) | 1 (4) | 0 (0) |
| Dose (Twice Daily) | n | Alternative Therapy Strategy | Adverse Effects? | n |
|---|---|---|---|---|
| 3.125 mg | 8 | Nebivolol | Yes | 1 |
| 3.125–6.25–3.125 mg | 1 | Valsartran | Yes | 1 |
| 3.125–6.25–12.5 mg | 1 | Promocard | No | 1 |
| 6.25–12.5–25 mg | 1 | Amlodipine | Unknown | 1 |
| 12.5 mg | 2 | Unknown | 2 | |
| 25 mg | 1 | None | 10 | |
| Unknown | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raasing, L.R.M.; Drent, M.; Keijsers, R.G.M.; van den Hoven, A.F.; Post, M.C.; Grutters, J.C.; Veltkamp, M. [123I]-Meta-Iodobenzylguanidine Scintigraphy in Sarcoidosis: Exploring Cardiac Autonomic Dysfunction in Patients with Unexplained Cardiac Symptoms. Diagnostics 2025, 15, 2306. https://doi.org/10.3390/diagnostics15182306
Raasing LRM, Drent M, Keijsers RGM, van den Hoven AF, Post MC, Grutters JC, Veltkamp M. [123I]-Meta-Iodobenzylguanidine Scintigraphy in Sarcoidosis: Exploring Cardiac Autonomic Dysfunction in Patients with Unexplained Cardiac Symptoms. Diagnostics. 2025; 15(18):2306. https://doi.org/10.3390/diagnostics15182306
Chicago/Turabian StyleRaasing, Lisette R. M., Marjolein Drent, Ruth G. M. Keijsers, Andor F. van den Hoven, Marco C. Post, Jan C. Grutters, and Marcel Veltkamp. 2025. "[123I]-Meta-Iodobenzylguanidine Scintigraphy in Sarcoidosis: Exploring Cardiac Autonomic Dysfunction in Patients with Unexplained Cardiac Symptoms" Diagnostics 15, no. 18: 2306. https://doi.org/10.3390/diagnostics15182306
APA StyleRaasing, L. R. M., Drent, M., Keijsers, R. G. M., van den Hoven, A. F., Post, M. C., Grutters, J. C., & Veltkamp, M. (2025). [123I]-Meta-Iodobenzylguanidine Scintigraphy in Sarcoidosis: Exploring Cardiac Autonomic Dysfunction in Patients with Unexplained Cardiac Symptoms. Diagnostics, 15(18), 2306. https://doi.org/10.3390/diagnostics15182306






