The Utility of Mesenteric T1 Mapping on MR Enterography in Crohn’s Disease: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. MR Imaging Protocol
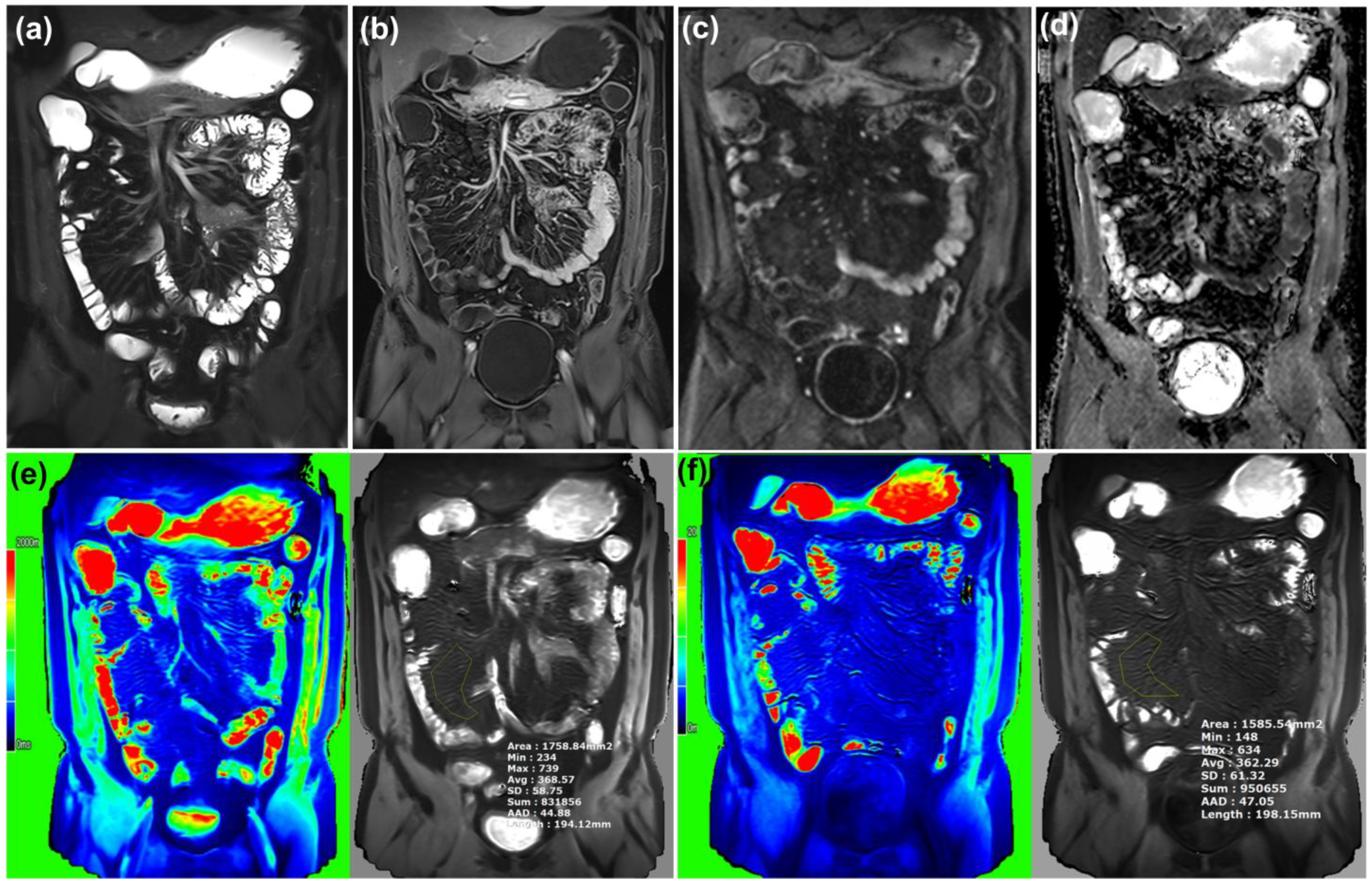
2.2. MR Imaging Analysis
2.3. Clinical and Laboratory Data Collection
2.4. Statistical Analysis
3. Results
3.1. MR Parameters of Bowel Wall and Mesentery
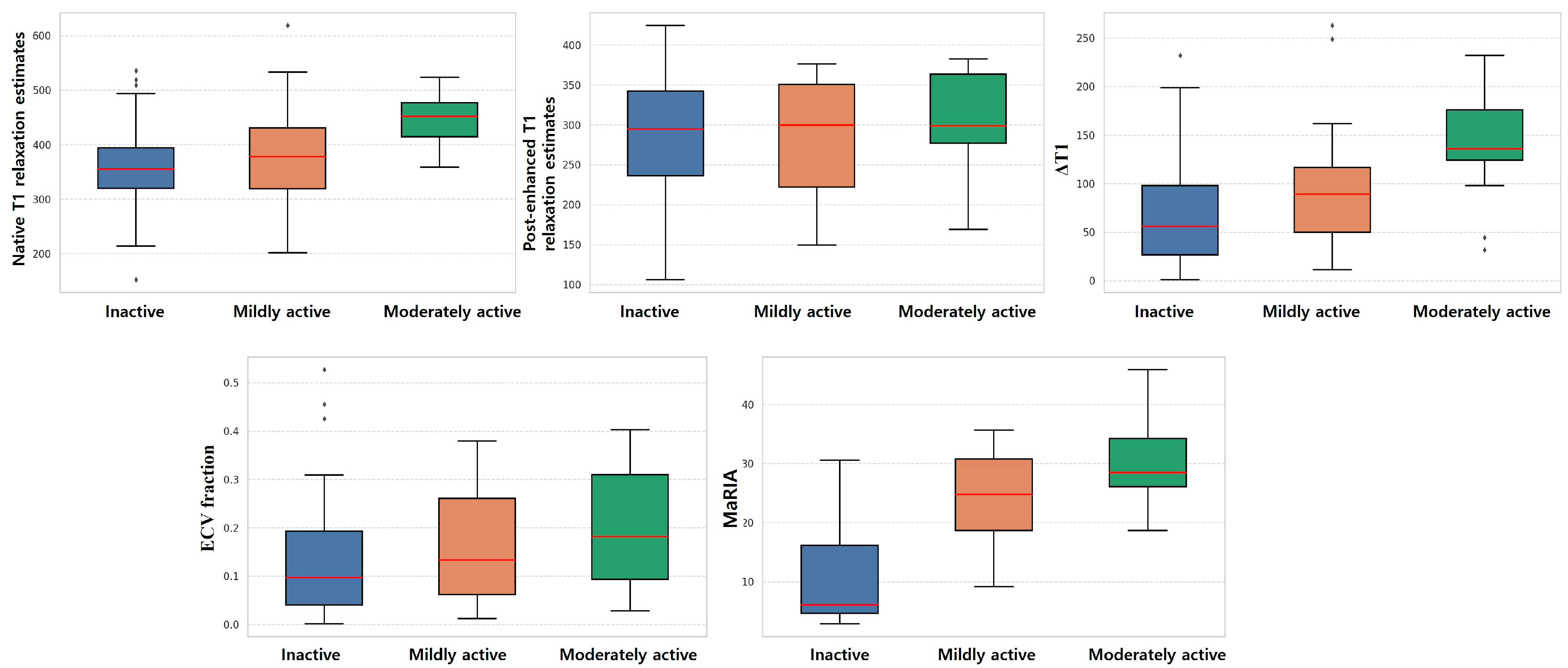
3.2. Subgroup Analysis of MRI Parameter of Bowel Wall and Mesentery
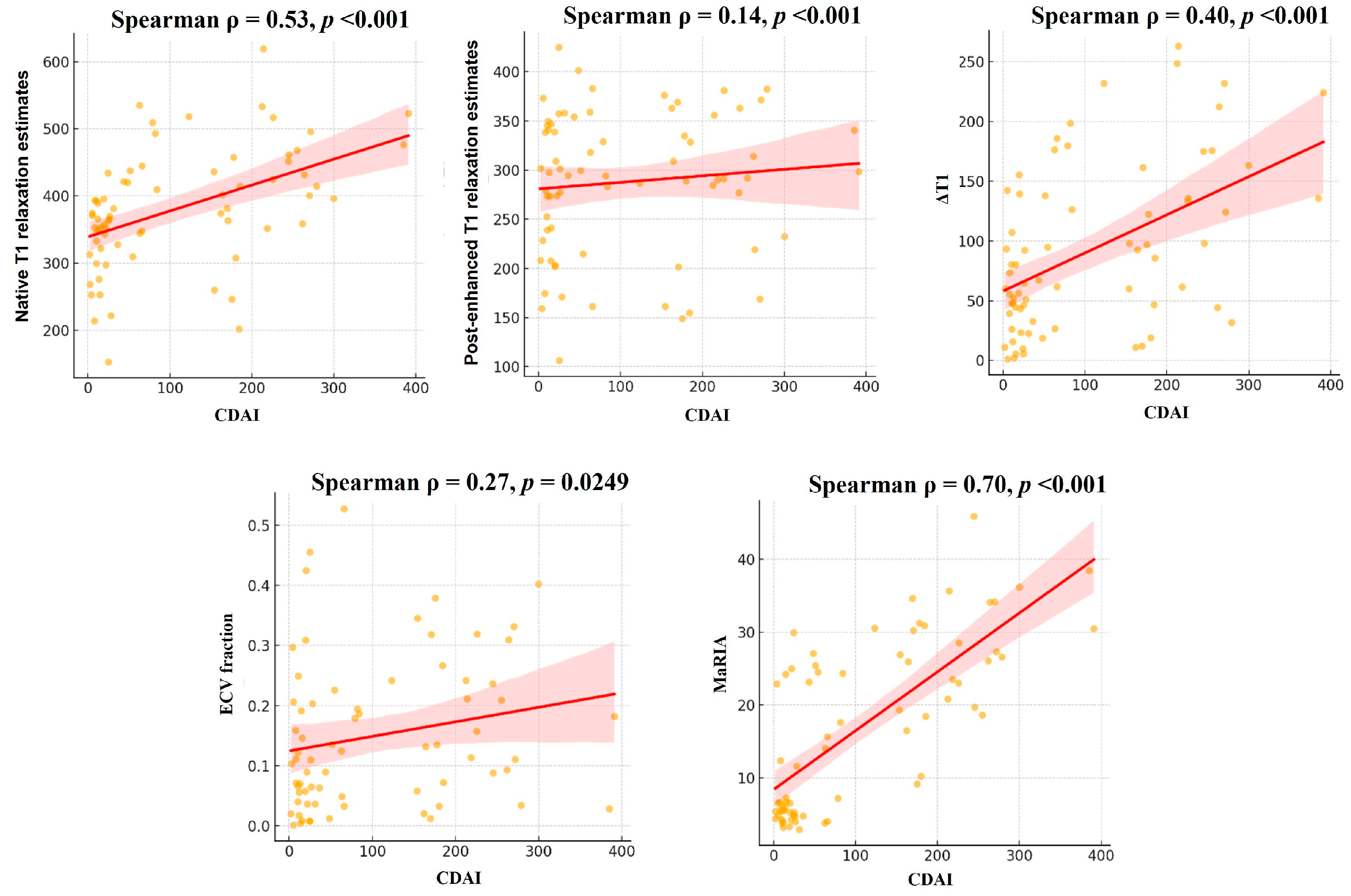
3.3. Correlation Analysis Between Parameters
3.4. Reproducibility of T1 Mapping Measurement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| MRE | Magnetic resonance enterography |
| ECV | extracellular volume |
| CDAI | Crohn’s Disease Activity Index |
References
- Panés, J. Inflammatory bowel disease: Pathogenesis and targets for therapeutic interventions. Acta Physiol. Scand. 2001, 173, 159–165. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Paquet, N.; Glickman, J.; Erturk, S.; Ros, P.; Heverhagen, J.; Patak, M. Crohn’s disease activity: Abdominal computed tomography histopathology correlation. Eur. J. Radiol. Open 2016, 3, 74–78. [Google Scholar] [CrossRef] [PubMed]
- van Schelt, A.-S.; Beek, K.J.; Wassenaar, N.P.M.; Schrauben, E.M.; Runge, J.H.; Gecse, K.B.; van der Bilt, J.D.; Neefjes-Borst, E.A.; Buskens, C.J.; Nederveen, A.J. Viscoelastic properties of small bowel mesentery at MR elastography in Crohn’s disease: A prospective cross-sectional exploratory study. Eur. Radiol. Exp. 2023, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhu, Z.-X.; Li, Z.; Chen, Y.-S.; Zhu, W.-M. Role of mesenteric component in Crohn’s disease: A friend or foe? World J. Gastrointest. Surg. 2021, 13, 1536. [Google Scholar] [CrossRef]
- Seo, N. Comprehensive Review of Magnetic Resonance Enterography-Based Activity Scoring Systems for Crohn’s Disease. Investig. Magn. Reson. Imaging 2025, 29, 1–13. [Google Scholar] [CrossRef]
- Moy, M.P.; Sauk, J.; Gee, M.S. The role of MR enterography in assessing Crohn’s disease activity and treatment response. Gastroenterol. Res. Pract. 2016, 2016, 8168695. [Google Scholar] [CrossRef]
- Biondi, M.; Bicci, E.; Danti, G.; Flammia, F.; Chiti, G.; Palumbo, P.; Bruno, F.; Borgheresi, A.; Grassi, R.; Grassi, F. The role of magnetic resonance enterography in Crohn’s disease: A review of recent literature. Diagnostics 2022, 12, 1236. [Google Scholar] [CrossRef]
- Rimola, J.; Rodríguez, S.; García-Bosch, O.; Ordás, I.; Ayala, E.; Aceituno, M.; Pellisé, M.; Ayuso, C.; Ricart, E.; Donoso, L. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 2009, 58, 1113–1120. [Google Scholar] [CrossRef]
- Borley, N.R.; Mortensen, N.J.; Jewell, D.P.; Warren, B.F. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn’s disease: Evidence for a possible causative link. J. Pathol. 2000, 190, 196–202. [Google Scholar] [CrossRef]
- Mao, R.; Kurada, S.; Gordon, I.O.; Baker, M.E.; Gandhi, N.; McDonald, C.; Coffey, J.C.; Rieder, F. The mesenteric fat and intestinal muscle interface: Creeping fat influencing stricture formation in Crohn’s disease. Inflamm. Bowel Dis. 2019, 25, 421–426. [Google Scholar] [CrossRef]
- Sheehan, A.; Warren, B.; Gear, M.; Shepherd, N. Fat-wrapping in Crohn’s disease: Pathological basis and relevance to surgical practice. Br. J. Surg. 1992, 79, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Koifman, E.; Cohen, I.S.; Lopez-Alonso, R.; Hur, D.B.; Ilivitzki, A.; Waterman, M. Mesenteric fat proliferation on magnetic resonance enterography is a predictor of long-term disease complications in crohn’s disease. BMC Gastroenterol. 2025, 25, 593. [Google Scholar] [CrossRef]
- Guedj, K.; Abitbol, Y.; Cazals-Hatem, D.; Morvan, M.; Maggiori, L.; Panis, Y.; Bouhnik, Y.; Caligiuri, G.; Corcos, O.; Nicoletti, A. Adipocytes orchestrate the formation of tertiary lymphoid organs in the creeping fat of Crohn’s disease affected mesentery. J. Autoimmun. 2019, 103, 102281. [Google Scholar] [CrossRef] [PubMed]
- Evrimler, S.; Swensson, J.K.; Are, V.S.; Tirkes, T.; Vuppalanchi, R.; Akisik, F. Quantitative assessment of disease severity of primary sclerosing cholangitis with T1 mapping and extracellular volume imaging. Abdom. Radiol. 2021, 46, 2433–2443. [Google Scholar] [CrossRef]
- Hoad, C.L.; Palaniyappan, N.; Kaye, P.; Chernova, Y.; James, M.W.; Costigan, C.; Austin, A.; Marciani, L.; Gowland, P.A.; Guha, I.N. A study of T1 relaxation time as a measure of liver fibrosis and the influence of confounding histological factors. NMR Biomed. 2015, 28, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Ntusi, N.A.; Piechnik, S.K.; Francis, J.M.; Ferreira, V.M.; Matthews, P.M.; Robson, M.D.; Wordsworth, P.B.; Neubauer, S.; Karamitsos, T.D. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc. Imaging 2015, 8, 526–536. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 mapping: Basic techniques and clinical applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef]
- Banerjee, R.; Pavlides, M.; Tunnicliffe, E.M.; Piechnik, S.K.; Sarania, N.; Philips, R.; Collier, J.D.; Booth, J.C.; Schneider, J.E.; Wang, L.M. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J. Hepatol. 2014, 60, 69–77. [Google Scholar] [CrossRef]
- Heye, T.; Yang, S.-R.; Bock, M.; Brost, S.; Weigand, K.; Longerich, T.; Kauczor, H.-U.; Hosch, W. MR relaxometry of the liver: Significant elevation of T1 relaxation time in patients with liver cirrhosis. Eur. Radiol. 2012, 22, 1224–1232. [Google Scholar] [CrossRef]
- Katsube, T.; Okada, M.; Kumano, S.; Hori, M.; Imaoka, I.; Ishii, K.; Kudo, M.; Kitagaki, H.; Murakami, T. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Investig. Radiol. 2011, 46, 277–283. [Google Scholar] [CrossRef]
- Besa, C.; Bane, O.; Jajamovich, G.; Marchione, J.; Taouli, B. 3D T1 relaxometry pre and post gadoxetic acid injection for the assessment of liver cirrhosis and liver function. Magn. Reson. Imaging 2015, 33, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.M.; Paek, M.; Han, J.K.; Choi, B.I. Quantitative assessment of hepatic function: Modified look-locker inversion recovery (MOLLI) sequence for T1 mapping on Gd-EOB-DTPA-enhanced liver MR imaging. Eur. Radiol. 2016, 26, 1775–1782. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, J.M.; Kim, E.; Okuaki, T.; Han, J.K. Quantitative Liver Function Analysis: Volumetric T1 Mapping with Fast Multisection B(1) Inhomogeneity Correction in Hepatocyte-specific Contrast-enhanced Liver MR Imaging. Radiology 2017, 282, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.; Tavakoli, M.B. Multiparametric study for glioma grading with FLAIR, ADC map, eADC map, T1 map, and SWI images. Magn. Reson. Imaging 2023, 96, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, N.; Tkach, J.A.; Denson, L.A.; Dillman, J.R. Bowel wall MRI T1 relaxation estimates for assessment of intestinal inflammation in pediatric Crohn’s disease. Abdom. Radiol. 2022, 47, 2730–2738. [Google Scholar] [CrossRef]
- Lu, B.; Lin, J.; Du, J.; He, S.; Cao, Q.; Huang, L.; Mao, R.; Sun, C.; Li, Z.; Feng, S. Native T1 mapping and magnetization transfer imaging in grading bowel fibrosis in Crohn’s disease: A comparative animal study. Biosensors 2021, 11, 302. [Google Scholar] [CrossRef]
- Dieringer, M.A.; Deimling, M.; Santoro, D.; Wuerfel, J.; Madai, V.I.; Sobesky, J.; von Knobelsdorff-Brenkenhoff, F.; Schulz-Menger, J.; Niendorf, T. Rapid parametric mapping of the longitudinal relaxation time T1 using two-dimensional variable flip angle magnetic resonance imaging at 1.5 Tesla, 3 Tesla, and 7 Tesla. PLoS ONE 2014, 9, e91318. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Homsi, R.; Sprinkart, A.M.; Doerner, J.; Dabir, D.; Kuetting, D.L.; Block, W.; Andrié, R.; Stehning, C.; Fimmers, R. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 154–161. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Doerner, J.; Thomas, D.K.; Dabir, D.; Gieseke, J.; Sprinkart, A.M.; Fimmers, R.; Stehning, C.; Homsi, R.; Schwab, J.O. Acute myocarditis: Multiparametric cardiac MR imaging. Radiology 2014, 273, 383–392. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Ntusi, N.; Holloway, C.; Choudhury, R.P.; Kardos, A.; Robson, M.D. T1 mapping for the diagnosis of acute myocarditis using CMR: Comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc. Imaging 2013, 6, 1048–1058. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F., Jr. Development of a Crohn’s disease activity index: National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Mao, R.; Li, X. Inflammatory bowel disease cross-sectional imaging: What’s new? United Eur. Gastroenterol. J. 2022, 10, 1179–1193. [Google Scholar] [CrossRef]
- Hoffman, D.H.; Ayoola, A.; Nickel, D.; Han, F.; Chandarana, H.; Shanbhogue, K.P. T1 mapping, T2 mapping and MR elastography of the liver for detection and staging of liver fibrosis. Abdom. Radiol. 2020, 45, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Zhang, C.; Yang, S.; Wang, Y.; Chen, W.; Li, X.; Wang, D. Native T1 mapping compared to ultrasound elastography for staging and monitoring liver fibrosis: An animal study of repeatability, reproducibility, and accuracy. Eur. Radiol. 2020, 30, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Liang, W.; Wu, M.; Lai, C.; Mei, Y.; Li, Y.; Xu, J.; Luo, L.; Quan, X. Comparison of T1 mapping and T1rho values with conventional diffusion-weighted imaging to assess fibrosis in a rat model of unilateral ureteral obstruction. Acad. Radiol. 2019, 26, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ugander, M.; Oki, A.J.; Hsu, L.-Y.; Kellman, P.; Greiser, A.; Aletras, A.H.; Sibley, C.T.; Chen, M.Y.; Bandettini, W.P.; Arai, A.E. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur. Heart J. 2012, 33, 1268–1278. [Google Scholar] [CrossRef]
- Dall’Armellina, E.; Ferreira, V.M.; Kharbanda, R.K.; Prendergast, B.; Piechnik, S.K.; Robson, M.D.; Jones, M.; Francis, J.M.; Choudhury, R.P.; Neubauer, S. Diagnostic value of pre-contrast T1 mapping in acute and chronic myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 739–742. [Google Scholar] [CrossRef]
- Hinojar, R.; Foote, L.; Arroyo Ucar, E.; Jackson, T.; Jabbour, A.; Yu, C.Y.; McCrohon, J.; Higgins, D.M.; Carr-White, G.; Mayr, M.; et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: A proposed diagnostic algorithm using CMR. JACC Cardiovasc. Imaging 2015, 8, 37–46. [Google Scholar] [CrossRef]
- Bohnen, S.; Radunski, U.K.; Lund, G.K.; Kandolf, R.; Stehning, C.; Schnackenburg, B.; Adam, G.; Blankenberg, S.; Muellerleile, K. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ. Cardiovasc. Imaging 2015, 8, e003073. [Google Scholar] [CrossRef]
- Nezafat, R. Native T1 mapping for myocardial infarction: Time to throw out the gadolinium? JACC Cardiovasc. Imaging 2015, 8, 1031–1033. [Google Scholar] [CrossRef]
- Mather, A.N.; Lockie, T.; Nagel, E.; Marber, M.; Perera, D.; Redwood, S.; Radjenovic, A.; Saha, A.; Greenwood, J.P.; Plein, S. Appearance of microvascular obstruction on high resolution first-pass perfusion, early and late gadolinium enhancement CMR in patients with acute myocardial infarction. J. Cardiovasc. Magn. Reson. 2009, 11, 33. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Treibel, T.A.; Moon, J.C. Myocardial T1 mapping: Where are we now and where are we going? Res. Rep. Clin. Cardiol. 2014, 5, 339–347. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2016, 19, 75. [Google Scholar]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Rimola, J.; Ordás, I.; Rodriguez, S.; García-Bosch, O.; Aceituno, M.; Llach, J.; Ayuso, C.; Ricart, E.; Panés, J. Magnetic resonance imaging for evaluation of Crohn’s disease: Validation of parameters of severity and quantitative index of activity. Inflamm. Bowel Dis. 2011, 17, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Luglio, G.; Rispo, A.; Imperatore, N.; Giglio, M.C.; Amendola, A.; Tropeano, F.P.; Peltrini, R.; Castiglione, F.; De Palma, G.D.; Bucci, L. Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn’s disease: The SuPREMe-CD study-a randomized clinical trial. Ann. Surg. 2020, 272, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Reiter, U.; Reiter, C.; Kräuter, C.; Fuchsjäger, M.; Reiter, G. Cardiac magnetic resonance T1 mapping. Part 2: Diagnostic potential and applications. Eur. J. Radiol. 2018, 109, 235–247. [Google Scholar] [CrossRef]
- Lurz, J.A.; Luecke, C.; Lang, D.; Besler, C.; Rommel, K.-P.; Klingel, K.; Kandolf, R.; Adams, V.; Schöne, K.; Hindricks, G. CMR–derived extracellular volume fraction as a marker for myocardial fibrosis: The importance of coexisting myocardial inflammation. JACC Cardiovasc. Imaging 2018, 11, 38–45. [Google Scholar] [CrossRef]
- Panes, J.; Bouhnik, Y.; Reinisch, W.; Stoker, J.; Taylor, S.A.; Baumgart, D.C.; Danese, S.; Halligan, S.; Marincek, B.; Matos, C.; et al. Imaging techniques for assessment of inflammatory bowel disease: Joint ECCO and ESGAR evidence-based consensus guidelines. J. Crohns Colitis 2013, 7, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Iima, M. Diffusion Magnetic Resonance Imaging: What Water Tells Us about Biological Tissues. PLoS Biol. 2015, 13, e1002203. [Google Scholar] [CrossRef]


| T2 HASTE Coronal | T2 HAST Coronal (Fat Suppression) | T2 Truefisp Coronal (Fat Suppression) | T2 Truefisp Axial (Fat Suppression) | DWI | Coronal Gb-Enhanced 3D T1 Weighted VIBE | |
|---|---|---|---|---|---|---|
| Repetition time (ms) | 1000 | 1000 | 520.85 | 300.8 | 2000 | 3.95 |
| Echo time (ms) | 81 | 79 | 2 | 2 | 49 | 1 |
| Image matrix | 384 × 307 | 320 × 320 | 256 × 154 | 256 × 156 | 100 × 130 | 320 × 260 |
| Flip angle | 137 | 170 | 44 | 51 | 90 | 9 |
| Section thickness (mm) | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 2.5 |
| Section gap (mm) | 0.5 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Variables | Total (n = 71) | |
|---|---|---|
| Age (y), mean ± SD) | 37.1 ± 12.5 | |
| sex n (%) | male | 53 (74.6) |
| female | 18 (25.4) | |
| Montreal classification | ||
| Age at diagnosis n (%) | A1 (≤16) | 5 (7.0) |
| A2 (17–40) | 55 (77.5) | |
| A3 (>40) | 11 (15.5) | |
| Behavior, n (%) | B1-non-strictureingm non-penetrating | 27 (38.0) |
| B2-stricturing | 19 (26.8) | |
| B3-penetrating | 16 (22.5) | |
| p | 9 (12.7) | |
| Location, n (%) | L1-terminal ileum | 42 (59.2) |
| L2-colon | 3 (4.2) | |
| L3-ileocolic | 26 (36.6) | |
| Current medication, n (%) | Anti-inflammatory drug | 8 (11.3) * |
| Immunomodulator | 21 (29.6) * | |
| Biologics | 42 (59.2) * | |
| CDAI, median (IQR) | 54 (15, 184) | |
| Fecal calprotectin, median (IQR) | 110.0 (19.5, 651.0) | |
| CRP, median (IQR) | 0.10 (0.03, 0.33) |
| All | Inactive (n = 44) | Active (n = 27) | p-Value | |
|---|---|---|---|---|
| Native T1 relaxation estimates (ms) | 374.2 (332.9, 434.6) | 355.2 (314.9, 394.9) | 414.3 (363.4, 467.9) | <0.001 |
| Post-enhanced T1 relaxation estimates (ms) | 294.6 (232.3, 347.1) | 294.5 (231.2, 344.3) | 298.7 (232.3, 363.0) | 0.546 |
| ΔT1 (ms) | 73.5 (39.4, 138.2) | 55.9 (26.2, 104.1) | 122.5 (59.9, 174.9) | 0.008 |
| ECV fraction (%) | 11.4 (4.9, 22.6) | 9.7 (3.8, 19.4) | 15.7 (7.3, 31.0) | 0.066 |
| MaRIA | 16.4 (5.4, 26.6) | 6.1 (4.5, 17.1) | 26.9 (19.7, 34.1) | <0.001 |
| Inactive (n = 43) | Mildly Active (n = 14) | Moderately Active (n = 13) | p-Value | Inactive vs. Mildly | Inactive vs. Moderate | Mildly vs. Moderate | |
|---|---|---|---|---|---|---|---|
| native T1 relaxation estimates (ms) | 374.2 (332.9, 434.6) | 377.8 (295.8, 441.4) | 451.9 (407.6, 486.1) | 0.001 | 0.414 | <0.001 | 0.038 |
| Post-enhanced T1 relaxation estimates (ms) | 294.6 (232.3, 347.1) | 299.7 (191.6, 357.6) | 298.7 (254.7, 367.4) | 0.677 | 0.877 | 0.413 | 0.458 |
| ΔT1 (ms) | 73.5 (39.4, 138.2) | 89.3 (39.7, 132.3) | 135.9 (111.1, 194.1) | 0.007 | 0.245 | 0.002 | 0.048 |
| ECV fraction (%) | 11.4 (4.9, 22.6) | 13.4 (5.2, 28.0) | 18.2 (9.1, 31.4) | 0.155 | 0.261 | 0.074 | 0.616 |
| MaRIA | 16.4 (5.4, 24.7) | 24.7 (17.9, 31.0) | 28.5 (24.6, 35.2) | <0.001 | <0.001 | <0.001 | 0.105 |
| Univariable | ||||||
|---|---|---|---|---|---|---|
| Variable | B (SE) | p-value | 95% CI | |||
| MaRIA | 6.68 (0.71) | <0.001 | 5.28–8.07 | |||
| native T1 relaxation estimates | 0.56 (0.13) | <0.001 | 0.30–0.83 | |||
| ΔT1 | 0.76 (0.16) | <0.001 | 0.45–1.07 | |||
| post-enhanced T1 relaxation estimates | 0.14 (0.17) | 0.39 | −0.18–0.46 | |||
| ECV fraction | 174.76 (109.78) | 0.11 | −40.42–389.93 | |||
| Fecal calprotectin | 0.04 (0.02) | 0.02 | 0.01–0.08 | |||
| CRP | 22.38 (27.08) | 0.41 | −30.69–75.44 | |||
| Multivariable | ||||||
| Model 1 (R2 = 0.61) | Model 2 (R2 = 0.60) | |||||
| Variable | B (SE) | p-value | 95% CI | B (SE) | p-value | 95% CI |
| MaRIA | 5.29 (1.02) | <0.001 | 3.29–7.29 | 5.55 (1.04) | <0.001 | 3.53–7.58 |
| native T1 relaxation estimates | 0.36 (0.22) | 0.11 | −0.08–0.79 | |||
| ΔT1 | 0.45 (0.20) | 0.02 | 0.06–0.83 | |||
| post-enhanced T1 relaxation estimates | −0.09 (0.34) | 0.79 | −0.77–0.58 | |||
| ECV fraction | 76.11 (136.42) | 0.58 | 343.48–0.09 | −75.40 (99.13) | 0.45 | −269.69–118.88 |
| Fecal calprotectin | 0.00 (0.02) | 0.98 | −0.03–0.03 | 0.00 (0.02) | 0.97 | −0.03–0.03 |
| CRP | 10.23 (11.70) | 0.38 | −12.71–33.17 | 8.08 (12.33) | 0.51 | −16.08–32.24 |
| Intraobserver (95%CI) | Interobserver (95%CI) | ||
|---|---|---|---|
| Observer 1 | Observer 2 | ||
| native T1 relaxation estimates | 0.92 (0.86–0.95) | 0.85 (0.70–0.93) | 0.81 (0.69–0.88) |
| post-enhanced T1 relaxation estimates | 0.73 (0.58–0.83) | 0.84 (0.69–0.92) | 0.74 (0.59–0.84) |
| ECV fraction | 0.85 (0.75–0.91) | 0.81 (0.63–0.91) | 0.76 (0.62–0.85) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Byun, J.; Kim, Y.R. The Utility of Mesenteric T1 Mapping on MR Enterography in Crohn’s Disease: A Preliminary Study. Diagnostics 2025, 15, 2293. https://doi.org/10.3390/diagnostics15182293
Park S, Byun J, Kim YR. The Utility of Mesenteric T1 Mapping on MR Enterography in Crohn’s Disease: A Preliminary Study. Diagnostics. 2025; 15(18):2293. https://doi.org/10.3390/diagnostics15182293
Chicago/Turabian StylePark, Seongkeun, Jieun Byun, and Youe Ree Kim. 2025. "The Utility of Mesenteric T1 Mapping on MR Enterography in Crohn’s Disease: A Preliminary Study" Diagnostics 15, no. 18: 2293. https://doi.org/10.3390/diagnostics15182293
APA StylePark, S., Byun, J., & Kim, Y. R. (2025). The Utility of Mesenteric T1 Mapping on MR Enterography in Crohn’s Disease: A Preliminary Study. Diagnostics, 15(18), 2293. https://doi.org/10.3390/diagnostics15182293





