NUAK2 Pathogenic Variants Are Definitively Associated with Neural Tube Defects in Humans: New Genotype-Phenotype Correlation and Review of the Literature
Abstract
1. Introduction
2. Case Description
2.1. Family Assessment
2.2. Exome Sequencing
2.3. Docking Analysis
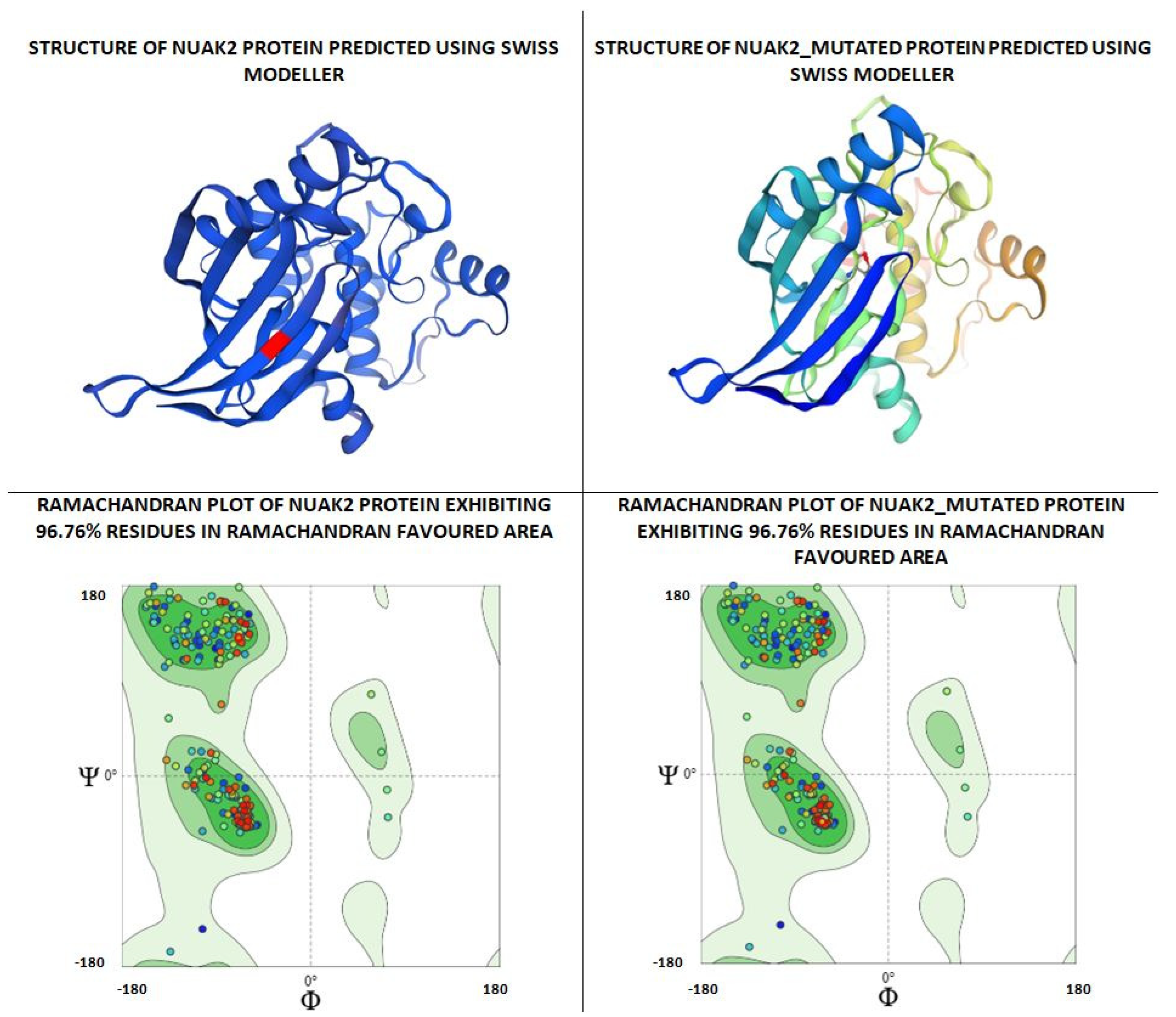
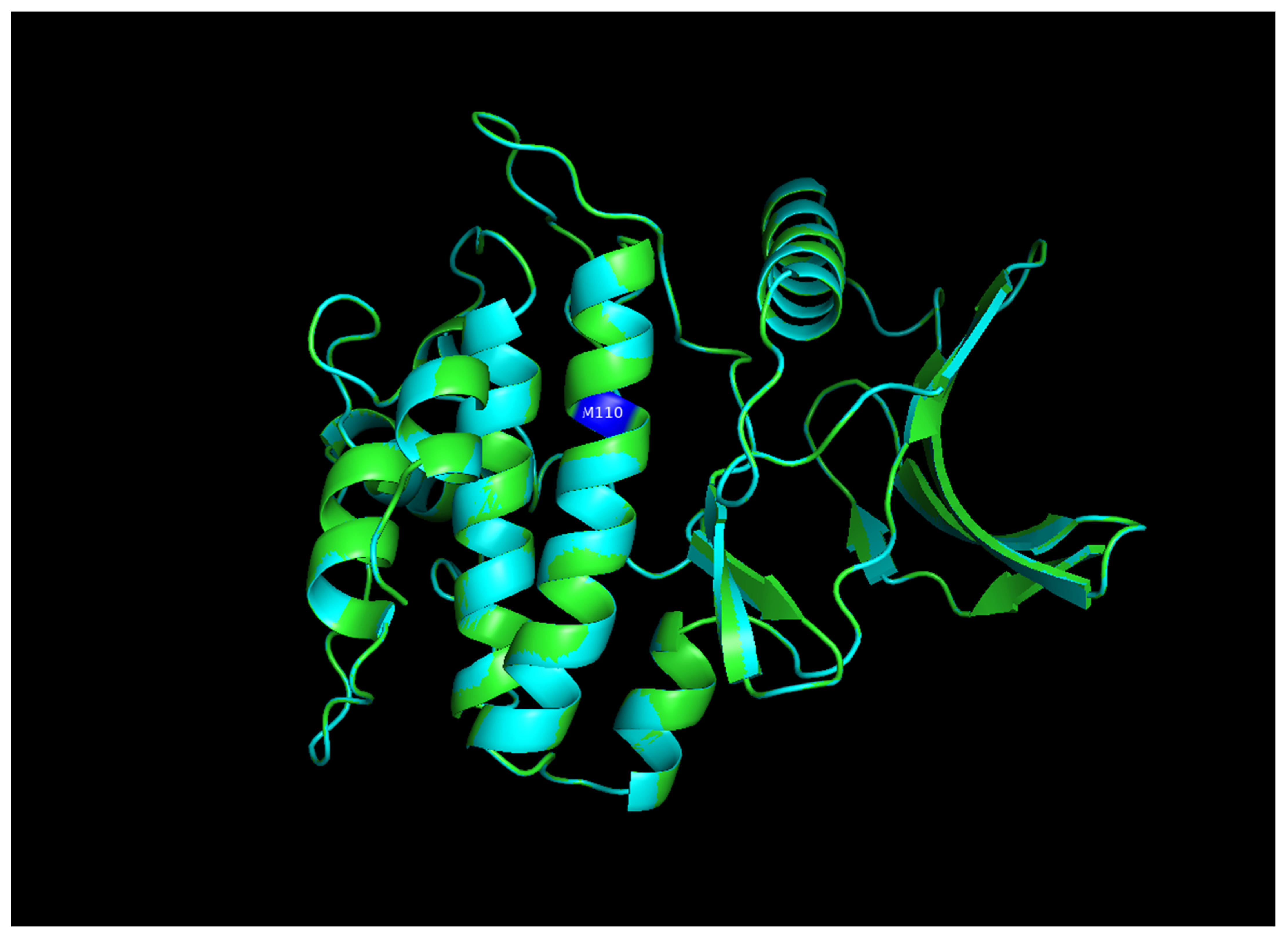
2.4. Results of Genetic Testing and Docking Analysis
3. Discussion
| Reference | Individual | Gene | Variant | Aminoacidic Change | Zigosity | Transmission | Geographic Origin | Consanguineity | Neural Tube Defect |
|---|---|---|---|---|---|---|---|---|---|
| Kibar, 2007 [30] | 19yo female | VANGL1 | c.821G>A | p.Arg274Gln | Heterozygous | A.D, susceptibility to | Italian | No | Myelomeningocele |
| Kibar, 2007 [30] | mother | VANGL1 | c.821G>A | p.Arg274Gln | Heterozygous | A.D., susceptibility to | Italian | No | Vertebral Schisis |
| Kibar, 2007 [30] | maternal aunt | VANGL1 | c.821G>A | p.Arg274Gln | Heterozygous | A.D., susceptibility to | Italian | No | Vertebral Schisis |
| Kibar, 2007 [30] | 21yo female | VANGL1 | c.983T>C | p.Met328Thr | Heterozygous | A.D., susceptibility to | N.A. | No | Myelomeningocele, Chiari II malformation, tethered spinal cord |
| Kibar, 2007 [30] | 10yo female | VANGL1 | c.715G>A | p.Val239Ile | Heterozygous | A.D., susceptibility to | Italian | No | Sacral agenesis with lipomyeloschisis, tethered spinal cord |
| Kibar, 2007 [30] | mother | VANGL1 | c.715G>A | p.Val239Ile | Heterozygous | A.D., susceptibility to | Italian | No | None |
| Kibar, 2007 [30] | brother | VANGL1 | c.715G>A | p.Val239Ile | Heterozygous | A.D., susceptibility to | Italian | No | Dorsal dermal sinus |
| Iliescu, 2014 [31] | male | VANGL1 | c.542G>A | p.Arg181Gln | Heterozygous | A.D., susceptibility to | Italian | No | Myelomeningocele |
| Iliescu, 2014 [31] | mother | VANGL1 | c.542G>A | p.Arg181Gln | Heterozygous | A.D., susceptibility to | Italian | No | None |
| Lei, 2010 [32] | Fetus 1 | VANGL2 | c.251C>T | p.Ser84Phe | Heterozygous | A.D., susceptibility to | Han Chinese | No | Holoprosencephaly |
| Lei, 2010 [32] | Fetus 2 | VANGL2 | c.1057C>T | p.Arg353Cys | Heterozygous | A.D., susceptibility to | Han Chinese | No | Anencephaly, spina bifida |
| Lei, 2010 [32] | Fetus 3 | VANGL2 | c.1310T>C | p.Phe437Ser | Heterozygous | A.D., susceptibility to | Han Chinese | No | Anencephaly |
| Kibar, 2011 [33] | Patient | VANGL2 | c.403C>T | p.Arg135Trp | Heterozygous | A.D., susceptibility to | Italian | N.A. | Lumbo-sacral myelomenigocele, Chiari II malformation, and hydromyelia |
| Kibar, 2011 [33] | Mother | VANGL2 | c.403C>T | p.Arg135Trp | Heterozygous | A.D., susceptibility to | Italian | N.A. | N.A. |
| Kibar, 2011 [33] | Patient | VANGL2 | c.530G>A | p.Arg177His | Heterozygous | A.D., susceptibility to | Italian | N.A. | Diastematomyelia |
| Kibar, 2011 [33] | Patient | VANGL2 | c.809G>A | p.Arg270His | Heterozygous | A.D., susceptibility to | Italian | N.A. | Hydrosyringomyelia, fibrolipoma of the filum terminalis |
| Kibar, 2011 [33] | Patient | VANGL2 | c.724C>G | p.Leu242Val | Heterozygous | A.D., susceptibility to | Italian | N.A. | Lumbar myeolocystocele |
| Kibar, 2011 [33] | Mother | VANGL2 | c.724C>G | p.Leu242Val | Heterozygous | A.D., susceptibility to | Italian | N.A. | N.A. |
| Kibar, 2011 [33] | Patient | VANGL2 | c.724C>G | p.Leu242Val | Heterozygous | A.D., susceptibility to | Caucasian white American | N.A. | Myelomeningocele |
| Kibar, 2011 [33] | Patient | VANGL2 | c.740C>T | p.Thr247Met | Heterozygous | A.D., susceptibility to | Caucasian white American | N.A. | Lipoma of the filum terminalis, tethered cord |
| Kibar, 2011 [33] | Patient | VANGL2 | c.532G>A | p.Val178Ile | Heterozygous | A.D., susceptibility to | N.A. | N.A. | Lipoma and tethered cord |
| Kibar, 2011 [33] | Father | VANGL2 | c.532G>A | p.Val178Ile | Heterozygous | A.D., susceptibility to | N.A. | N.A. | N.A. |
| Seo, 2011 [34] | Neonate | FUZ | c.115C>T | p.Pro39Ser | Heterozygous | A.D., susceptibility to | Italian | No | Myelomeningocele, Chiari II malformation |
| Seo, 2011 [34] | Female child | FUZ | c.1060G>T | p.Asp354Tyr | Heterozygous | A.D., susceptibility to | Italian | No | Myelomeningocele, Chiari II malformation |
| Seo, 2011 [34] | Father | FUZ | c.1060G>T | p.Asp354Tyr | Heterozygous | A.D., susceptibility to | Italian | No | None |
| Seo, 2011 [34] | Male | FUZ | c.1211G>A | p.Arg404Gln | Heterozygous | A.D., susceptibility to | Caucasian | No | Hemimyelomeningocele, diastematomyelia, Chiari II malformation |
| Seo, 2011 [34] | Father | FUZ | c.1211G>A | p.Arg404Gln | Heterozygous | A.D., susceptibility to | Caucasian | No | None |
| Mastromoro, 2025 [35] | Fetus 1 | CCL2 | MIM*601156:arr[GRCh37]17q12(32104747_ arr[GRCh37]17q12(32104747_32744698)x3pat | _ | Heterozygous | A.D. | Italian | No | Anencephaly |
| Mastromoro, 2025 [35] | Fetus 2 | CCL2 | MIM*601156:arr[GRCh37]17q12(32104747_ arr[GRCh37]17q12(32104747_32744698)x3pat | _ | Heterozygous | A.D. | Italian | No | Anencephaly |
| Mastromoro, 2025 [35] | Father | CCL2 | MIM*601156:arr[GRCh37]17q12(32104747_ arr[GRCh37]17q12(32104747_32744698)x3pat | _ | Heterozygous | A.D. | Italian | No | Dorsal dermal sinus |
| Postma, 2014 [36] | Stillborn | TBXT | c.796A>G | p.His171Arg | Homozygous | A.R. | N.A. | Yes | Sacral agenesis and abnormal ossification of all vertebral bodies |
| Postma, 2014 [36] | Neonate | TBXT | c.796A>G | p.His171A.R.g | Homozygous | A.R. | N.A. | Yes | Sacral and complete left renal agenesis, persistent cloaca with anal atresia, and vertical clefting of all vertebral bodies |
| Postma, 2014 [36] | Child | TBXT | c.796A>G | p.His171Ar.g | Homozygous | A.R. | N.A. | Yes | Sacral agenesis and abnormal ossification of all vertebral bodies |
| Postma, 2014 [36] | Child | TBXT | c.796A>G | p.His171Arg | Homozygous | A.R. | N.A. | Yes | Sacral agenesis and abnormal ossification of all vertebral bodies |
| Singh, 2017 [8] | Fetus | TRIM36 | c.1522C>A | p.Pro508Thr | Homozygous | A.R. | Indian | Yes | Anencephaly |
| Bonnard, 2020 [9] | Fetus 1 | NUAK2 | c.412_433delinsG | _ | Homozygous | A.R. | Turkish | Yes | Anencephaly |
| Bonnard, 2020 [9] | Fetus 2 | NUAK2 | c.412_433delinsG | _ | Homozygous | A.R. | Turkish | Yes | Anencephaly |
| Bonnard, 2020 [9] | Fetus 3 | NUAK2 | c.412_433delinsG | _ | Homozygous | A.R. | Turkish | Yes | Anencephaly |
| Bonnard, 2020 [9] | Mother | NUAK2 | c.412_433delinsG | _ | Heterozygous | A.R. | Turkish | Yes | None |
| Bonnard, 2020 [9] | Father | NUAK2 | c.412_433delinsG | _ | Heterozygous | A.R. | Turkish | Yes | None |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Allen, M.I.; Kalousek, D.K.; Chernoff, G.F.; Juriloff, D.; Harris, M.; McGillivray, B.C.; Yong, S.L.; Langlois, S.; MacLeod, P.M.; Chitayat, D. Evidence for multi-site closure of the neural tube in humans. Am. J. Med. Genet. 1993, 47, 723–743. [Google Scholar] [CrossRef]
- Rossi, A.C.; Prefumo, F. Accuracy of ultrasonography at 11–14 weeks of gestation for detection of fetal structural anomalies. Obstet. Gynecol. 2013, 122, 1160–1167. [Google Scholar] [CrossRef]
- Mastromoro, G.; Guadagnolo, D.; Khaleghi Hashemian, N.; Bernardini, L.; Giancotti, A.; Piacentini, G.; De Luca, A.; Pizzuti, A. A Pain in the Neck: Lessons Learnt from Genetic Testing in Fetuses Detected with Nuchal Fluid Collections, Increased Nuchal Translucency versus Cystic Hygroma-Systematic Review of the Literature, Meta-Analysis and Case Series. Diagnostics 2022, 13, 48. [Google Scholar] [CrossRef]
- Thi Pham, X.T.; Nguyen, P.N.; Hoang, X.S. Fraser Syndrome: A Narrative Review Based on a Case from Vietnam and the Past 20 Years of Research. Diagnostics 2025, 15, 1606. [Google Scholar] [CrossRef]
- Buijtendijk, M.F.; Bet, B.B.; Leeflang, M.M.; Shah, H.; Reuvekamp, T.; Goring, T.; Docter, D.; Timmerman, M.G.; Dawood, Y.; Lugthart, M.A.; et al. Diagnostic accuracy of ultrasound screening for fetal structural abnormalities during the first and second trimester of pregnancy in low-risk and unselected populations. Cochrane Database Syst. Rev. 2024, 5, CD014715. [Google Scholar] [CrossRef] [PubMed]
- Dulgheroff, F.F.; Peixoto, A.B.; Petrini, C.G.; Caldas, T.M.R.D.C.; Ramos, D.R.; Magalhães, F.O.; Araujo Júnior, E. Fetal structural anomalies diagnosed during the first, second and third trimesters of pregnancy using ultrasonography: A retrospective cohort study. Sao Paulo Med. J. 2019, 137, 391–400. [Google Scholar] [CrossRef]
- Detrait, E.R.; George, T.M.; Etchevers, H.C.; Gilbert, J.R.; Vekemans, M.; Speer, M.C. Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicol. Teratol. 2005, 27, 515–524. [Google Scholar] [CrossRef]
- Singh, N.; Kumble Bhat, V.; Tiwari, A.; Kodaganur, S.G.; Tontanahal, S.J.; Sarda, A.; Malini, K.V.; Kumar, A. A homozygous mutation in TRIM36 causes autosomal recessive anencephaly in an Indian family. Hum. Mol. Genet. 2017, 26, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, C.; Navaratnam, N.; Ghosh, K.; Chan, P.W.; Tan, T.T.; Pomp, O.; Ng, A.Y.J.; Tohari, S.; Changede, R.; Carling, D.; et al. A loss-of-function NUAK2 mutation in humans causes anencephaly due to impaired Hippo-YAP signaling. J. Exp. Med. 2020, 217, e20191561. [Google Scholar] [CrossRef] [PubMed]
- Copp, A.J.; Stanier, P.; Greene, N.D. Neural tube defects: Recent advances, unsolved questions, and controversies. Lancet Neurol. 2013, 12, 799–810. [Google Scholar] [CrossRef]
- Moldenhauer, J.S.; Adzick, N.S. Fetal surgery for myelomeningocele: After the Management of Myelomeningocele Study (MOMS). Semin. Fetal Neonatal Med. 2017, 22, 360–366. [Google Scholar] [CrossRef]
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W.; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef]
- Belfort, M.A.; Whitehead, W.E.; Shamshirsaz, A.A.; Ruano, R.; Cass, D.L.; Olutoye, O.O.; Mann, A.G.; Espinoza, J.; Williams, E.; Lee, T.C.; et al. Fetoscopic open neural tube defect repair: Development and refinement of a two-port, carbon dioxide insufflation technique. Ultrasound Obstet. Gynecol. 2017, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.G.; Friedman, J.M.; Kenna, B.A.; Popkin, J.; Jawanda, M.; Arnold, W. Clinical, genetic, and epidemiological factors in neural tube defects. Am. J. Hum. Genet. 1988, 43, 827–837. [Google Scholar]
- Rampersaud, E.; Melvin, E.C.; Speer, M.C. Nonsyndromic neural tube defects: Genetic basis and genetic investigations. In Neural Tube Defects: From Origin to Treatment; Wyszynski, D.F., Ed.; Oxford University Press: Oxford, UK, 2006; pp. 165–175. [Google Scholar]
- Seller, M.J. Risks in spina bifida. Dev. Med. Child Neurol. 1994, 36, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, B.M.; Hibbard, E.D.; Jeffcoate, T.N. Folic acid and reproduction. Acta Obs. Gynecol. Scand. 1965, 44, 375–400. [Google Scholar] [CrossRef] [PubMed]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Greene, N.D.; Leung, K.Y.; Copp, A.J. Inositol, neural tube closure and the prevention of neural tube defects. Birth Defects Res. 2017, 109, 68–80. [Google Scholar] [CrossRef]
- Greene, N.D.; Leung, K.Y.; Gay, V.; Burren, K.; Mills, K.; Chitty, L.S.; Copp, A.J. Inositol for the prevention of neural tube defects: A pilot randomised controlled trial. Br. J. Nutr. 2016, 115, 974–983. [Google Scholar] [CrossRef]
- Carter, C.O.; Evans, K.A. Spina bifida and anencephalus in Greater London. J. Med. Genet. 1973, 10, 209–234. [Google Scholar] [CrossRef]
- Elwood, J.H.; Nevin, N.C. Factors associated with anencephalus and spina bifida in Belfast. Br. J. Prev. Soc. Med. 1973, 27, 73–80. [Google Scholar] [CrossRef]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Schubach, M.; Maass, T.; Nazaretyan, L.; Röner, S.; Kircher, M. CADD v1.7: Using protein language models, regulatory CNNs and other nucleotide-level scores to improve genome-wide variant predictions. Nucleic Acids Res. 2024, 52, D1143–D1154. [Google Scholar] [CrossRef]
- Guadagnolo, D.; Mastromoro, G.; Di Palma, F.; Pizzuti, A.; Marchionni, E. Prenatal Exome Sequencing: Background, Current Practice and Future Perspectives-A Systematic Review. Diagnostics 2021, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Mastromoro, G.; Guadagnolo, D.; Khaleghi Hashemian, N.; Marchionni, E.; Traversa, A.; Pizzuti, A. Molecular Approaches in Fetal Malformations, Dynamic Anomalies and Soft Markers: Diagnostic Rates and Challenges-Systematic Review of the Literature and Meta-Analysis. Diagnostics 2022, 12, 575. [Google Scholar] [CrossRef]
- Harris, M.J.; Juriloff, D.M. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res. Part A Clin. Mol. Teratol. 2010, 88, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.E.; Etheredge, A.J.; Brown, K.S.; Mitchell, L.E.; Whitehead, A.S. Maternal genotype for the monocyte chemoattractant protein 1 A(-2518)G promoter polymorphism is associated with the risk of spina bifida in offspring. Am. J. Med. Genet. A 2006, 140, 1114–1118. [Google Scholar] [CrossRef]
- Kibar, Z.; Torban, E.; McDearmid, J.R.; Reynolds, A.; Berghout, J.; Mathieu, M.; Kirillova, I.; De Marco, P.; Merello, E.; Hayes, J.M.; et al. Mutations in VANGL1 associated with neural-tube defects. N. Engl. J. Med. 2007, 356, 1432–1437. [Google Scholar] [CrossRef]
- Iliescu, A.; Gravel, M.; Horth, C.; Gros, P. Independent mutations at Arg181 and Arg274 of Vangl proteins that are associated with neural tube defects in humans decrease protein stability and impair membrane targeting. Biochemistry 2014, 53, 5356–5364. [Google Scholar] [CrossRef]
- Lei, Y.P.; Zhang, T.; Li, H.; Wu, B.L.; Jin, L.; Wang, H.Y. VANGL2 mutations in human cranial neural-tube defects. N. Engl. J. Med. 2010, 362, 2232–2235. [Google Scholar] [CrossRef]
- Kibar, Z.; Salem, S.; Bosoi, C.M.; Pauwels, E.; De Marco, P.; Merello, E.; Bassuk, A.G.; Capra, V.; Gros, P. Contribution of VANGL2 mutations to isolated neural tube defects. Clin. Genet. 2011, 80, 76–82. [Google Scholar] [CrossRef]
- Seo, J.H.; Zilber, Y.; Babayeva, S.; Liu, J.; Kyriakopoulos, P.; De Marco, P.; Merello, E.; Capra, V.; Gros, P.; Torban, E. Mutations in the planar cell polarity gene, Fuzzy, are associated with neural tube defects in humans. Hum. Mol. Genet. 2011, 20, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Mastromoro, G.; Darelli, D.; Mariani, S.; Colantoni, F.; Bucossi, S.; Canestrelli, M.; Russo, C.D.; Squitti, R.; Micalizzi, A.; Zumpano, A.; et al. Microduplication encompassing CCL2 segregates in a family with recurrent anencephaly and dorsal dermal sinus: A possible link between chemokine and neural tube defects? Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 313, 114617. [Google Scholar] [CrossRef]
- Postma, A.V.; Alders, M.; Sylva, M.; Bilardo, C.M.; Pajkrt, E.; van Rijn, R.R.; Schulte-Merker, S.; Bulk, S.; Stefanovic, S.; Ilgun, A.; et al. Mutations in the T (brachyury) gene cause a novel syndrome consisting of sacral agenesis, abnormal ossification of the vertebral bodies and a persistent notochordal canal. J. Med. Genet. 2014, 51, 90–97. [Google Scholar] [CrossRef]
- Yabuta, N.; Okada, N.; Ito, A.; Hosomi, T.; Nishihara, S.; Sasayama, Y.; Fujimori, A.; Okuzaki, D.; Zhao, H.; Ikawa, M.; et al. Lats2 is an essential mitotic regulator required for the coordination of cell division. J. Biol. Chem. 2007, 282, 19259–19271. [Google Scholar] [CrossRef]
- Suzuki, A.; Kusakai, G.; Kishimoto, A.; Minegichi, Y.; Ogura, T.; Esumi, H. Induction of cell-cell detachment during glucose starvation through F-actin conversion by SNARK, the fourth member of the AMP-activated protein kinase catalytic subunit family. Biochem. Biophys. Res. Commun. 2003, 311, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Legembre, P.; Schickel, R.; Barnhart, B.C.; Peter, M.E. Identification of SNF1/ AMP kinase-related kinase as an NFkappaB-regulated anti-apoptotic kinase involved in CD95-induced motility and invasiveness. J. Biol. Chem. 2004, 279, 46742–46747. [Google Scholar] [CrossRef]
- Suzuki, A.; Kusakai, G.; Kishimoto, A.; Shimojo, Y.; Miyamoto, S.; Ogura, T.; Ochiai, A.; Esumi, H. Regulation of caspase-6 and FLIP by the AMPK family member ARK5. Oncogene 2004, 23, 7067–7075. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liu, J.E.; Liu, W.; Liu, C.Y.; Liu, Z.Y.; Sun, Z.Y. A new role of NUAK1: Directly phosphorylating p53 and regulating cell proliferation. Oncogene 2011, 30, 2933–2942. [Google Scholar] [CrossRef]
- Namiki, T.; Tanemura, A.; Valencia, J.C.; Coelho, S.G.; Passeron, T.; Kawaguchi, M.; Vieira, W.D.; Ishikawa, M.; Nishijima, W.; Izumo, T.; et al. AMP kinase-related kinase NUAK2 affects tumor growth, migration, and clinical outcome of human melanoma. Proc. Natl. Acad. Sci. USA 2011, 108, 6597–6602. [Google Scholar] [CrossRef]
- Miner, J.H.; Cunningham, J.; Sanes, J.R. Roles for laminin in embryogenesis: Exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J. Cell Biol. 1998, 143, 1713–1723. [Google Scholar] [CrossRef]
- Arikawa-Hirasawa, E.; Watanabe, H.; Takami, H.; Hassell, J.R.; Yamada, Y. Perlecan is essential for cartilage and cephalic development. Nat. Genet. 1999, 23, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Nandadasa, S.; Yamamoto, T.S.; Terasaka-Iioka, C.; Wylie, C.; Ueno, N. Nectin-2 and N-cadherin interact through extracellular domains and induce apical accumulation of F-actin in apical constriction of Xenopus neural tube morphogenesis. Development 2010, 137, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Copp, A.J.; Greene, N.D.; Murdoch, J.N. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 2003, 4, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, T.; Shioi, G.; Hirano, M.; Aizawa, S. Neural tube defects by NUAK1 and NUAK2 double mutation. Dev. Dyn. 2012, 241, 1350–1364. [Google Scholar] [CrossRef]
- Radswiki, T.; Walizai, T.; Gaillard, F. Dorsal Dermal Sinus. Available online: https://radiopaedia.org/articles/dorsal-dermal-sinus?lang=us (accessed on 3 July 2025).
- Naidich, T.P.; Zimmerman, R.A.; McLone, D.G.; Raybaud, C.A.; Altman, N.R.; Braffman, B.H. Congenital anomalies of the spine and spinal cord. In Magnetic Resonance Imaging of the Brain and Spine, 2nd ed.; Atlas, S.W., Ed.; Lippincott-Raven: New York, NY, USA, 1996; pp. 1265–1337. [Google Scholar]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
 ) highlights the individual with the sacral dimple. Individuals I:4, II:4, III:1, III:4, III:5 show the phenotype. (B) Sacral dimple of the proband.
) highlights the individual with the sacral dimple. Individuals I:4, II:4, III:1, III:4, III:5 show the phenotype. (B) Sacral dimple of the proband.
 ) highlights the individual with the sacral dimple. Individuals I:4, II:4, III:1, III:4, III:5 show the phenotype. (B) Sacral dimple of the proband.
) highlights the individual with the sacral dimple. Individuals I:4, II:4, III:1, III:4, III:5 show the phenotype. (B) Sacral dimple of the proband.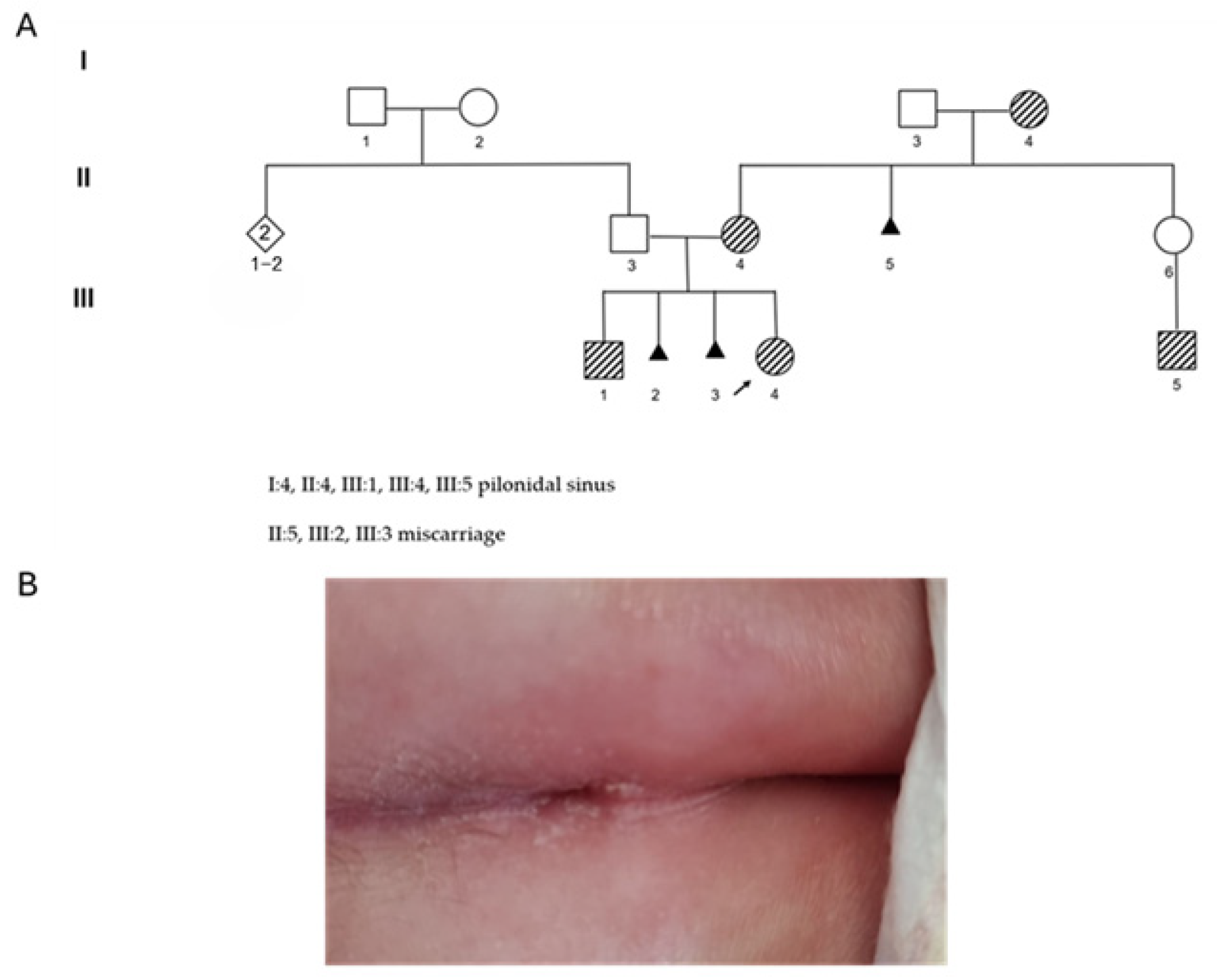
| N. | Site of Docking | Docking Residues Position | Docking Score (kcal/mol) | 3D Structure | 2D Structure * | Interacting Residues | Interaction Type |
|---|---|---|---|---|---|---|---|
| 1. | 6–14 (LGKGTYGKV) Ligand ATP GRID coordinates: 0.542(X) −3.736(y) 17.467(Z) | NUAK2 | −10.595 | 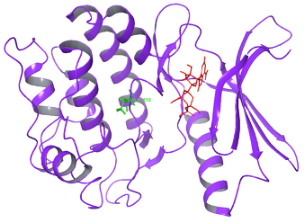 | 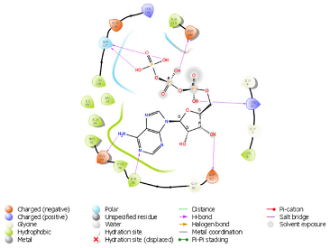 | Lys:8, Asp:83, Ala:79, Glu:77, Asn:127, Asp:140 | H-bond to protein backbone |
| Mutated NUAK2 | −8.881 | 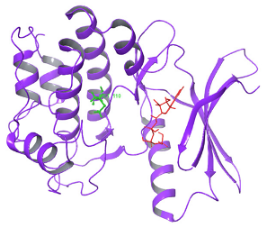 | 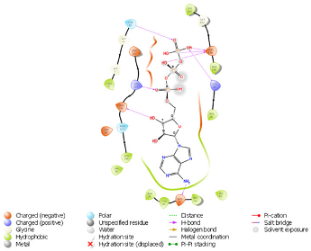 | Thr:10, Lys:28, Glu:77, Lys:124, Glu:126, Asp:140 | H-bond to protein backbone | ||
| 2. | 28 (K) Ligand ATP GRID coordinates: −4.107(X) −0.247(y) 14.504(z) | NUAK2 | −8.088 | 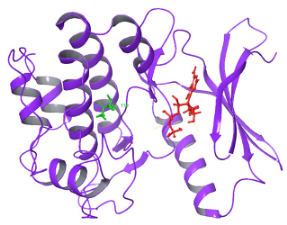 | 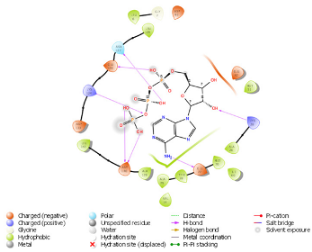 | Lys:28, Glu:77, Lys:124, Glu:126, Asn:127 | H-bond to protein backbone |
| Mutated NUAK2 | −9.452 | 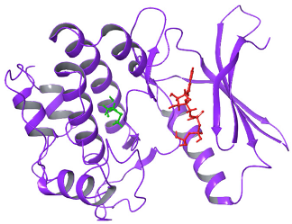 | 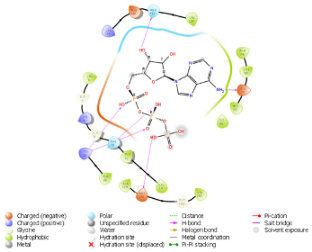 | Thr:10, Lys:28, Glu:77, Asn:127, Asp:140 | H-bond to protein backbone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastromoro, G.; Dello Russo, C.; Mariani, S.; Bucossi, S.; Riccardi, R.; Pal, A.; Squitti, R.; Dangi, M.; Pizzuti, A.; Rongioletti, M.C.A. NUAK2 Pathogenic Variants Are Definitively Associated with Neural Tube Defects in Humans: New Genotype-Phenotype Correlation and Review of the Literature. Diagnostics 2025, 15, 2289. https://doi.org/10.3390/diagnostics15182289
Mastromoro G, Dello Russo C, Mariani S, Bucossi S, Riccardi R, Pal A, Squitti R, Dangi M, Pizzuti A, Rongioletti MCA. NUAK2 Pathogenic Variants Are Definitively Associated with Neural Tube Defects in Humans: New Genotype-Phenotype Correlation and Review of the Literature. Diagnostics. 2025; 15(18):2289. https://doi.org/10.3390/diagnostics15182289
Chicago/Turabian StyleMastromoro, Gioia, Claudio Dello Russo, Stefania Mariani, Serena Bucossi, Riccardo Riccardi, Amit Pal, Rosanna Squitti, Mehak Dangi, Antonio Pizzuti, and Mauro Ciro Antonio Rongioletti. 2025. "NUAK2 Pathogenic Variants Are Definitively Associated with Neural Tube Defects in Humans: New Genotype-Phenotype Correlation and Review of the Literature" Diagnostics 15, no. 18: 2289. https://doi.org/10.3390/diagnostics15182289
APA StyleMastromoro, G., Dello Russo, C., Mariani, S., Bucossi, S., Riccardi, R., Pal, A., Squitti, R., Dangi, M., Pizzuti, A., & Rongioletti, M. C. A. (2025). NUAK2 Pathogenic Variants Are Definitively Associated with Neural Tube Defects in Humans: New Genotype-Phenotype Correlation and Review of the Literature. Diagnostics, 15(18), 2289. https://doi.org/10.3390/diagnostics15182289








