Diagnostic Clinical Predictors of Early Recovery from Stone-Induced Systemic Inflammatory Response Syndrome After Urgent Decompression
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Study Population
2.3. Antibiotic Protocol
2.4. Intervention
2.5. Outcomes
2.6. Statistical Analysis
3. Results
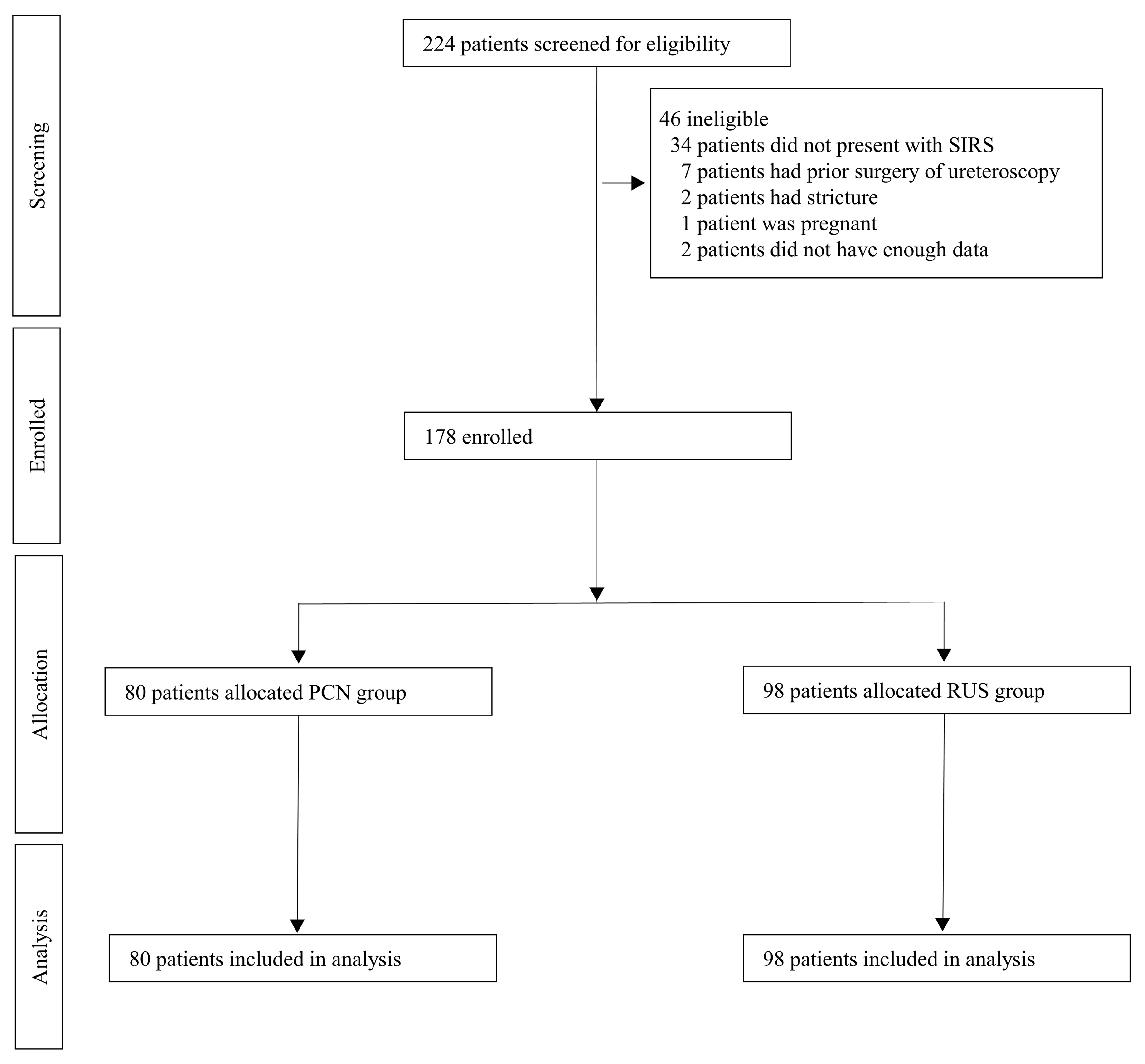
3.1. Patients Characteristics and Drainage Methods
3.2. Factors Associated with Early Recovery
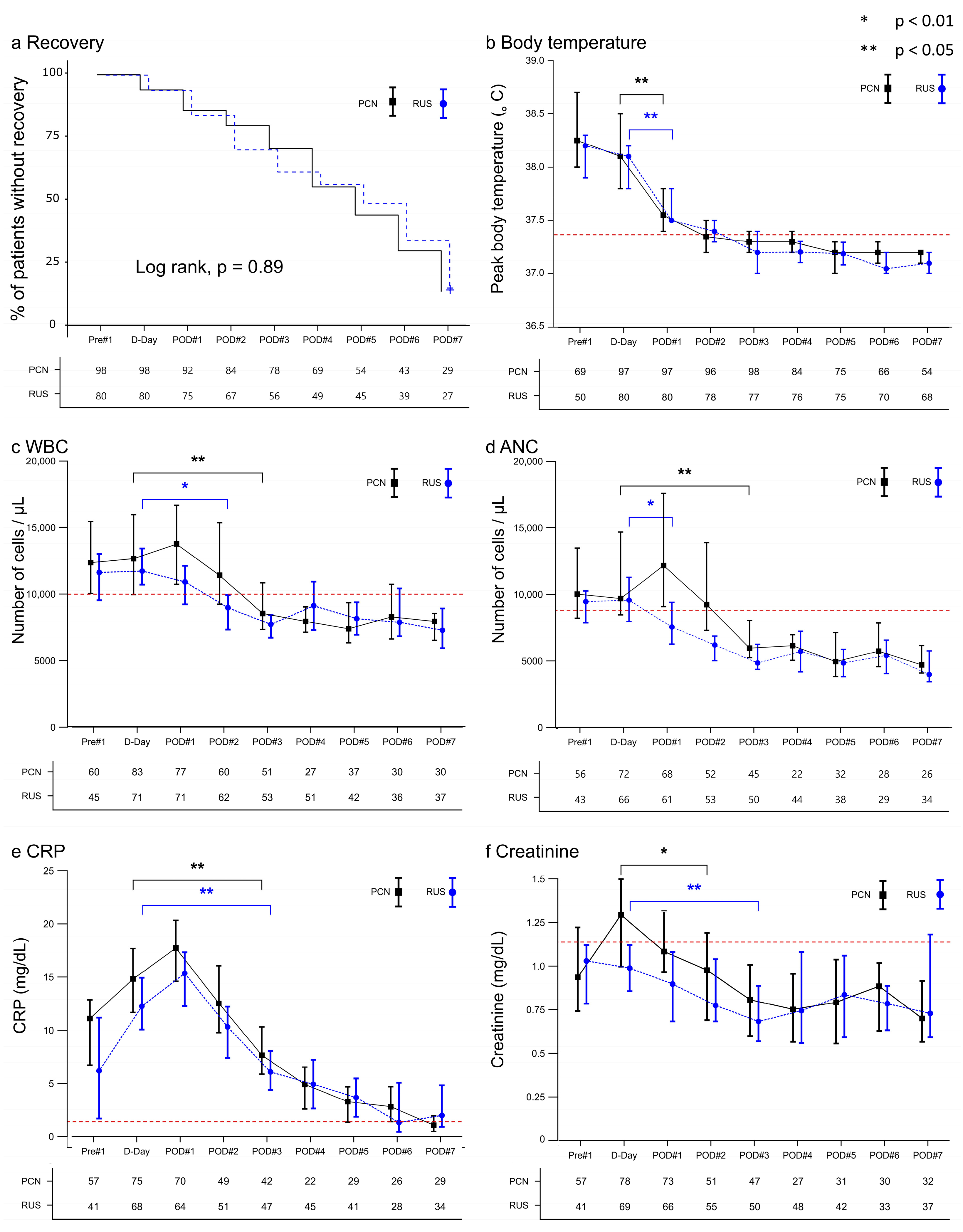
3.3. Inflammatory and Renal Function Trends After Decompression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, Y.; Cook, P.; Roderick, P.; Somani, B.K. Metabolic syndrome and kidney stone disease: A systematic review of literature. J. Endourol. 2016, 30, 246–253. [Google Scholar] [CrossRef]
- Geraghty, R.M.; Proietti, S.; Traxer, O.; Archer, M.; Somani, B.K. Worldwide impact of warmer seasons on the incidence of renal colic and kidney stone disease: Evidence from a systematic review of literature. J. Endourol. 2017, 31, 729–735. [Google Scholar] [CrossRef]
- Sammon, J.D.; Ghani, K.R.; Karakiewicz, P.I.; Bhojani, N.; Ravi, P.; Sun, M.; Sukumar, S.; Trinh, V.Q.; Kowalczyk, K.J.; Kim, S.P.; et al. Temporal trends, practice patterns, and treatment outcomes for infected upper urinary tract stones in the United States. Eur. Urol. 2013, 64, 85–92. [Google Scholar] [CrossRef]
- Borofsky, M.S.; Walter, D.; Li, H.; Shah, O.; Goldfarb, D.S.; Sosa, R.E.; Makarov, D.V. Institutional characteristics associated with receipt of emergency care for obstructive pyelonephritis at community hospitals. J. Urol. 2015, 193, 851–856. [Google Scholar] [CrossRef]
- Borofsky, M.S.; Walter, D.; Shah, O.; Goldfarb, D.S.; Mues, A.C.; Makarov, D.V. Surgical decompression is associated with decreased mortality in patients with sepsis and ureteral calculi. J. Urol. 2013, 189, 946–951. [Google Scholar] [CrossRef]
- Bhanot, R.; Pietropaolo, A.; Tokas, T.; Kallidonis, P.; Skolarikos, A.; Keller, E.X.; De Coninck, V.; Traxer, O.; Gozen, A.; Sarica, K.; et al. Predictors and strategies to avoid mortality following ureteroscopy for stone disease: A systematic review from European association of urologists sections of urolithiasis (EULIS) and Uro-technology (ESUT). Eur. Urol. Focus 2022, 8, 598–607. [Google Scholar] [CrossRef]
- Chugh, S.; Pietropaolo, A.; Montanari, E.; Sarica, K.; Somani, B.K. Predictors of urinary infections and urosepsis after ureteroscopy for stone disease: A systematic review from EAU section of urolithiasis (EULIS). Curr. Urol. Rep. 2020, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Larkin, S.; Johnson, J.; Venkatesh, T.; Vetter, J.; Venkatesh, R. Systemic inflammatory response syndrome in patients with acute obstructive upper tract urinary stone: A risk factor for urgent renal drainage and revisit to the emergency department. BMC Urol. 2020, 20, 77. [Google Scholar] [CrossRef]
- Haas, C.R.; Li, G.; Hyams, E.S.; Shah, O. Delayed decompression of obstructing stones with urinary tract infection is associated with increased odds of death. J. Urol. 2020, 204, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, R.H.; Barton, G.J.; Kothari, A.N.; Zapf, M.A.C.; Flanigan, R.C.; Kuo, P.C.; Gupta, G.N. Early intervention during acute stone admissions: Revealing “the weekend effect” in urological practice. J. Urol. 2016, 196, 124–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramsey, S.; Robertson, A.; Ablett, M.J.; Meddings, R.N.; Hollins, G.W.; Little, B. Evidence-based drainage of infected hydronephrosis secondary to ureteric calculi. J. Endourol. 2010, 24, 185–189. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Seoane, L.M.; Abadia, A.A.S.; Geraghty, R.; Kallidonis, P.; Tailly, T.; Modi, S.; Tzelves, L.; Sarica, K.; Gozen, A.; et al. Emergency upper urinary tract decompression: Double-J stent or nephrostomy? A European YAU/ESUT/EULIS/BSIR survey among urologists and radiologists. World J. Urol. 2022, 40, 1629–1636. [Google Scholar] [CrossRef]
- Pearle, M.S.; Pierce, H.L.; Miller, G.L.; Summa, J.A.; Mutz, J.M.; Petty, B.A.; Roehrborn, C.G.; Kryger, J.V.; Nakada, S.Y. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J. Urol. 1998, 160, 1260–1264. [Google Scholar] [CrossRef]
- Papa, L.; Stiell, I.G.; Wells, G.A.; Ball, I.; Battram, E.; Mahoney, J.E. Predicting intervention in renal colic patients after emergency department evaluation. Can. J. Emerg. Med. 2005, 7, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.W.; McLeod, S.L.; Edmonds, M.L.; Sedran, R.J.; Theakston, K.D. Risk factors associated with urologic intervention in emergency department patients with suspected renal colic. J. Emerg. Med. 2015, 49, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Kozyrakis, D.; Kratiras, Z.; Soukias, G.; Chatzistamou, S.E.; Zarkadas, A.; Perikleous, S.; Kateris, D.; Katsaros, I.; Skriapas, K.; Karagiannis, D. Clinical outcome and prognostic factors of sepsis, septic shock and prolonged hospitalization, of patients presented with acute obstructive pyelonephritis. J. Endourol. 2020, 34, 516–522. [Google Scholar] [CrossRef]
- Hamasuna, R.; Takahashi, S.; Nagae, H.; Kubo, T.; Yamamoto, S.; Arakawa, S.; Matsumoto, T. Obstructive pyelonephritis as a result of urolithiasis in Japan: Diagnosis, treatment and prognosis. Int. J. Urol. 2015, 22, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Offner, P.J.; Moore, E.E.; Biffl, W.L. Male gender is a risk factor for major infections after surgery. Arch. Surg. 1999, 134, 935–938; discussion 938–940. [Google Scholar] [CrossRef]
- Kisat, M.; Villegas, C.V.; Onguti, S.; Zafar, S.N.; Latif, A.; Efron, D.T.; Haut, E.R.; Schneider, E.B.; Lipsett, P.A.; Zafar, H.; et al. Predictors of sepsis in moderately severely injured patients: An analysis of the National Trauma Data Bank. Surg. Infect. (Larchmt) 2013, 14, 62–68. [Google Scholar] [CrossRef]
- Xu, J.; Tong, L.; Yao, J.; Guo, Z.; Lui, K.Y.; Hu, X.; Cao, L.; Zhu, Y.; Huang, F.; Guan, X.; et al. Association of sex with clinical outcome in critically ill sepsis patients: A retrospective analysis of the large clinical database MIMIC-III. Shock 2019, 52, 146–151. [Google Scholar] [CrossRef]
- Samuelsson, C.; Sjöberg, F.; Karlström, G.; Nolin, T.; Walther, S.M. Gender differences in outcome and use of resources do exist in Swedish intensive care, but to no advantage for women of premenopausal age. Crit. Care 2015, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.E.; Kang, D.; Cho, J.; Na, S.J.; Chung, C.R.; Lim, S.Y.; Lee, Y.J.; Park, S.; Oh, D.K.; Lee, S.Y.; et al. Influence of gender on age-associated in-hospital mortality in patients with sepsis and septic shock: A prospective nationwide multicenter cohort study. Crit. Care 2023, 27, 229. [Google Scholar] [CrossRef] [PubMed]
- Adrie, C.; Azoulay, E.; Francais, A.; Clec’h, C.; Darques, L.; Schwebel, C.; Nakache, D.; Jamali, S.; Goldgran-Toledano, D.; Garrouste-Orgeas, M.; et al. Influence of gender on the outcome of severe sepsis: A reappraisal. Chest 2007, 132, 1786–1793. [Google Scholar] [CrossRef]
- Uslan, D.Z.; Crane, S.J.; Steckelberg, J.M.; Cockerill, F.R., 3rd; St Sauver, J.L.; Wilson, W.R.; Baddour, L.M. Age- and sex-associated trends in bloodstream infection: A population-based study in Olmsted County, Minnesota. Arch. Intern. Med. 2007, 167, 834–839. [Google Scholar] [CrossRef]
- Dias, S.P.; Brouwer, M.C.; van de Beek, D. Sex and gender differences in bacterial infections. Infect. Immun. 2022, 90, e0028322. [Google Scholar] [CrossRef] [PubMed]
- Frydrych, L.M.; Fattahi, F.; He, K.; Ward, P.A.; Delano, M.J. Diabetes and sepsis: Risk, recurrence, and ruination. Front. Endocrinol. 2017, 8, 271. [Google Scholar] [CrossRef]
- Lynch, M.F.; Anson, K.M.; Patel, U. Current opinion amongst radiologists and urologists in the UK on percutaneous nephrostomy and ureteric stent insertion for acute renal unobstruction: Results of a postal survey. BJU Int. 2006, 98, 1143–1144. [Google Scholar] [CrossRef]
- Azwadi, I.Z.K.; Norhayati, M.N.; Abdullah, M.S. Percutaneous nephrostomy versus retrograde ureteral stenting for acute upper obstructive uropathy: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 6613. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Gao, M.; Zeng, H.; Cui, Y.; Zhu, Z.; Chen, H. Preoperative midstream urine cultures vs renal pelvic urine culture or stone culture in predicting systemic inflammatory response syndrome and urosepsis after percutaneous nephrolithotomy: A systematic review and meta-analysis. J. Endourol. 2021, 35, 1467–1478. [Google Scholar] [CrossRef]
- Nevo, A.; Mano, R.; Baniel, J.; Lifshitz, D.A. Ureteric stent dwelling time: A risk factor for post-ureteroscopy sepsis. BJU Int. 2017, 120, 117–122. [Google Scholar] [CrossRef]
| Variable | Total (n = 178) | PCN (n = 80) | RUS (n = 98) | p-Value |
|---|---|---|---|---|
| Sex, n (%) | 0.470 | |||
| Female | 125 (70.2) | 54 (67.5) | 71 (72.4) | |
| Male | 53 (29.8) | 26 (32.5) | 27 (27.6) | |
| Age, years, median [IQR] | 68.5 [58.0–77.0] | 73.0 [61.5–80.5] | 66.0 [57.0–74.0] | 0.001 |
| BMI, kg/m2, median [IQR] | 24.5 [21.7–27.7] | 23.5 [20.3–25.9] | 25.9 [22.9–29.0] | <0.001 |
| HTN, n (%) | 116 (65.2) | 56 (70.0) | 60 (61.2) | 0.222 |
| DM, n (%) | 67 (37.6) | 34 (42.5) | 33 (33.7) | 0.226 |
| Stroke, n (%) | 17 (9.6) | 9 (11.2) | 8 (8.2) | 0.483 |
| Visit type, n (%) | 0.189 | |||
| Emergency room | 169 (94.9) | 78 (97.5) | 91 (92.9) | |
| Outpatient | 9 (5.1) | 2 (2.5) | 7 (7.1) | |
| Hospital days, median [IQR] | 10.5 [7.0–15.0] | 13.0 [8.5–16.0] | 8.0 [6.0–12.0] | <0.001 |
| Prior to other hospital treatment, n (%) | 55 (30.9) | 24 (30.0) | 31 (31.6) | 0.823 |
| Onset of fever, n (%) | 0.123 | |||
| 1–3 days | 146 (82.0) | 72 (90.0) | 74 (75.5) | |
| 3 days–1 week | 16 (9.0) | 4 (5.0) | 12 (12.2) | |
| ~1 week | 4 (2.3) | 1 (1.2) | 3 (3.0) | |
| Unknown | 12 (6.7) | 3 (3.8) | 9 (9.2) | |
| Location of stone, n (%) | 0.268 | |||
| Low-ureter | 47 (26.4) | 24 (30.0) | 23 (23.5) | |
| Mid-ureter | 32 (18.0) | 16 (20.0) | 16 (16.3) | |
| Upper-ureter | 92 (51.7) | 39 (48.8) | 53 (54.1) | |
| Kidney | 7 (3.9) | 1 (1.2) | 6 (6.1) | |
| Size of stone, n (%) | 0.616 | |||
| approximately 5 mm | 34 (19.1) | 13 (16.2) | 21 (21.4) | |
| 5–9 mm | 87 (48.9) | 41 (51.2) | 46 (46.9) | |
| 10–20 mm | 43 (24.2) | 18 (22.5) | 25 (25.5) | |
| >20 mm | 14 (7.9) | 8 (10.0) | 6 (6.1) | |
| Coexistence of renal stones, n (%) | 0.491 | |||
| Free | 75 (42.1) | 31 (38.8) | 44 (44.9) | |
| Both | 40 (22.5) | 19 (23.8) | 21 (21.4) | |
| Ipsilateral | 41 (23.0) | 22 (27.5) | 19 (19.4) | |
| Contralateral | 22 (12.4) | 8 (10.0) | 14 (14.3) | |
| Surgery performed, n (%) | 131 (73.6) | 61 (76.2) | 69 (70.4) | 0.482 |
| Timing of definite surgery, n (%) | 0.006 | |||
| Re-admission surgery | 47 (36.2) | 14 (23.0) | 33 (47.8) | |
| In-hospital surgery | 83 (63.8) | 47 (77.0) | 36 (52.2) | |
| Drainage location, n (%) | 0.342 | |||
| Right | 80 (44.9) | 36 (45.0) | 44 (44.9) | |
| Left | 91 (51.1) | 39 (48.8) | 52 (53.1) | |
| Both | 7 (3.9) | 5 (6.2) | 2 (2.0) |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% Wald CI) | p-Value | |
| Age | 1.02 (0.99–1.04) | 0.217 | ||
| Female sex | 0.39 (0.19–0.80) | 0.01 | 0.44 (0.20–0.95) | 0.036 |
| + culture from any positive sites | 0.58 (0.29–1.15) | 0.118 | ||
| + urine positive culture | 0.72 (0.36–1.42) | 0.341 | ||
| + E. coli positive culture | 0.35 (0.12–0.83) | 0.026 | ||
| HTN | 1.37 (0.67–2.95) | 0.397 | ||
| DM | 2.54 (1.27–5.14) | 0.009 | 2.26 (1.08–4.75) | 0.031 |
| Stroke | 2.35 (0.8–6.55) | 0.106 | ||
| BMI | 0.95 (0.88–1.01) | 0.13 | ||
| Emergency visit | 0.37 (0.02–2.08) | 0.35 | ||
| Microbiology | ||||
| Urine culture positive | 1.67 (0.80–3.74) | 0.189 | ||
| Culture from any positive sites | 1.55 (1.20–2.79) | 0.654 | ||
| E. coli positive culture | 1.06 (0.54–2.10) | 0.864 | ||
| ESBL positive | 1.81 (0.79–3.99) | 0.147 | ||
| RUS (vs. PCN) | 0.6 (0.3–1.19) | 0.142 | ||
| Stone location | ||||
| Low-ureter | Reference | |||
| Mid-ureter | 4.4 (1.41–15.53) | 0.014 | 5.4 (1.58–18.52) | 0.007 |
| Upper-ureter | 3.49 (1.34–10.93) | 0.017 | 3.32 (1.15–9.55) | 0.026 |
| Kidney | 1.4 (0.07–10.92) | 0.775 | 1.48 (0.14–15.99) | 0.747 |
| Stone size | ||||
| 0–5 mm | Reference | |||
| 5–10 mm | 1.13 (0.42–3.41) | 0.812 | ||
| 10–20 mm | 3.05 (1.09–9.55) | 0.042 | ||
| ~20 mm | 1.87 (0.41–8) | 0.401 | ||
| Coexistence of renal stone | ||||
| Free | Reference | |||
| Both | 2.61 (1.1–6.28) | 0.029 | ||
| Ipsilateral | 1.41 (0.55–3.51) | 0.468 | ||
| Contralateral | 1.28 (0.37–3.9) | 0.674 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Jung, Y.; Chae, H.K.; Nam, W.; Yu, H.; Cho, Y.; Kim, S.J. Diagnostic Clinical Predictors of Early Recovery from Stone-Induced Systemic Inflammatory Response Syndrome After Urgent Decompression. Diagnostics 2025, 15, 2282. https://doi.org/10.3390/diagnostics15172282
Yoon S, Jung Y, Chae HK, Nam W, Yu H, Cho Y, Kim SJ. Diagnostic Clinical Predictors of Early Recovery from Stone-Induced Systemic Inflammatory Response Syndrome After Urgent Decompression. Diagnostics. 2025; 15(17):2282. https://doi.org/10.3390/diagnostics15172282
Chicago/Turabian StyleYoon, Sungbin, Yeonuk Jung, Han Kyu Chae, Wook Nam, Hoon Yu, Youngjong Cho, and Sung Jin Kim. 2025. "Diagnostic Clinical Predictors of Early Recovery from Stone-Induced Systemic Inflammatory Response Syndrome After Urgent Decompression" Diagnostics 15, no. 17: 2282. https://doi.org/10.3390/diagnostics15172282
APA StyleYoon, S., Jung, Y., Chae, H. K., Nam, W., Yu, H., Cho, Y., & Kim, S. J. (2025). Diagnostic Clinical Predictors of Early Recovery from Stone-Induced Systemic Inflammatory Response Syndrome After Urgent Decompression. Diagnostics, 15(17), 2282. https://doi.org/10.3390/diagnostics15172282







