Using Multivariate Adaptive Regression Splines to Estimate Summed Stress Score on Myocardial Perfusion Scintigraphy in Chinese Women with Type 2 Diabetes: A Comparative Study with Multiple Linear Regression
Abstract
1. Introduction
2. Materials and Methods
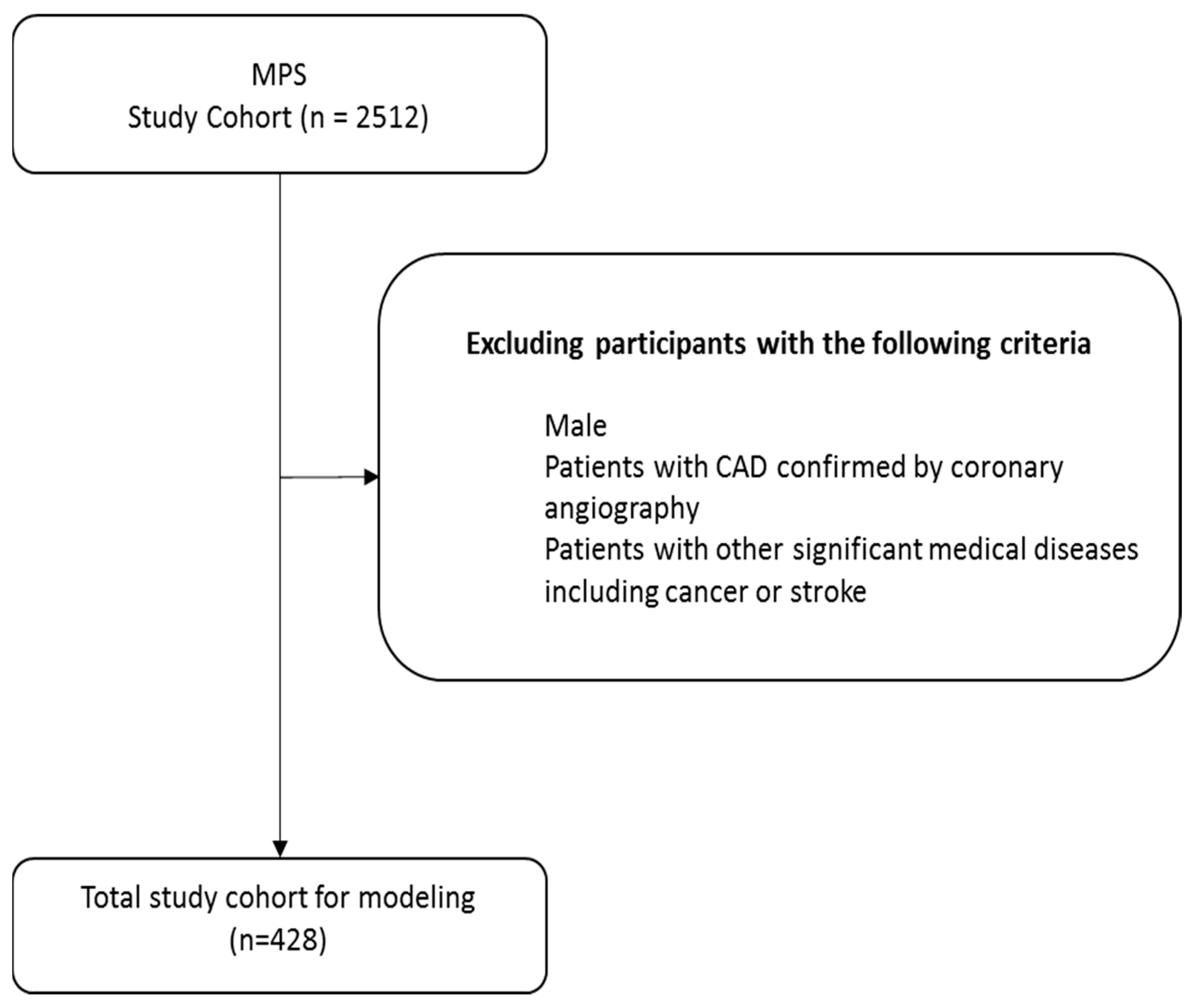
2.1. Participant and Study Design
- Women with T2DM aged 30–95 years.
- Hemoglobin A1c (HbA1c) 6.5–10%.
- BMI 22–30 kg/m2.
- Patients with confirmed CAD, myocardial infarction, valvular heart disease, or non-ischemic cardiomyopathy.
- Other significant diseases (e.g., cancer, stroke).
2.2. MPS
2.3. Laboratory Evaluation
2.4. Machine Learning Method
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stamler, J.; Vaccaro, O.; Neaton, J.D.; Wentworth, D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993, 16, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Schramm, T.K.; Gislason, G.H.; Køber, L.; Rasmussen, S.; Rasmussen, J.N.; Abildstrøm, S.Z.; Hansen, M.L.; Folke, F.; Buch, P.; Madsen, M.; et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: A population study of 3.3 million people. Circulation 2008, 117, 1945–1954. [Google Scholar] [CrossRef]
- Alabas, O.A.; Hall, M.; Dondo, T.B.; Rutherford, M.J.; Timmis, A.D.; Batin, P.D.; Deanfield, J.E.; Hemingway, H.; Gale, C.P. Long-term excess mortality associated with diabetes following acute myocardial infarction: A population-based cohort study. J. Epidemiol. Community Health 2017, 71, 25–32. [Google Scholar] [CrossRef]
- Milan Study on Atherosclerosis and Diabetes (MiSAD) Group. Prevalence of unrecognized silent myocardial ischemia and its association with atherosclerotic risk factors in noninsulin-dependent diabetes mellitus. Am. J. Cardiol. 1997, 79, 134–139. [Google Scholar] [CrossRef] [PubMed]
- May, O.; Arildsen, H.; Damsgaard, E.M.; Mickley, H. Prevalence and prediction of silent ischaemia in diabetes mellitus: A population-based study. Cardiovasc. Res. 1997, 34, 241–247. [Google Scholar] [CrossRef]
- Passa, P.; Drouin, P.; Issa-Sayegh, M.; Blasco, A.; Masquet, C.; Monassier, J.P.; Paillole, C. Coronary disease and diabetes. Diabete Metab. 1995, 21, 446–451. [Google Scholar]
- American Diabetes Association. Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10-11 February 1998, Miami, Florida. Diabetes Care 1998, 21, 1551–1559. [Google Scholar] [CrossRef]
- Leber, A.W.; Knez, A.; von Ziegler, F.; Becker, A.; Nikolaou, K.; Paul, S.; Wintersperger, B.; Reiser, M.; Becker, C.R.; Steinbeck, G.; et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: A comparative study with quantitative coronary angiography and intravascular ultrasound. J. Am. Coll. Cardiol. 2005, 46, 147–154. [Google Scholar] [CrossRef]
- Hoffmann, M.H.; Shi, H.; Manzke, R.; Schmid, F.T.; De Vries, L.; Grass, M.; Brambs, H.J.; Aschoff, A.J. Noninvasive coronary angiography with 16-detector row CT: Effect of heart rate. Radiology 2005, 234, 86–97. [Google Scholar] [CrossRef]
- Mark, D.B.; Hlatky, M.A.; Harrell, F.E.; Lee, K.L., Jr.; Califf, R.M.; Pryor, D.B. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann. Intern. Med. 1987, 106, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.B.; Shaw, L.; Harrell, F.E.; Hlatky, M.A., Jr.; Lee, K.L.; Bengtson, J.R.; McCants, C.B.; Califf, R.M.; Pryor, D.B. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N. Engl. J. Med. 1991, 325, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Shaw, L.J.; Murthy, D.R.; Travin, M.I.; Miller, D.D.; Hachamovitch, R.; Borges-Neto, S.; Berman, D.S.; Waters, D.D.; Heller, G.V. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation 2002, 105, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Scholte, A.J.H.A.; Schuijf, J.D.; Kharagjitsingh, A.V.; Dibbets-Schneider, P.; Stokkel, M.P.; van der Wall, E.E.; Bax, J.J. Prevalence and predictors of an abnormal stress myocardial perfusion study in asymptomatic patients with type 2 diabetes mellitus. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 567–575. [Google Scholar] [CrossRef]
- Prior, J.O.; Monbaron, D.; Koehli, M.; Calcagni, M.L.; Ruiz, J.; Bischof Delaloye, A. Prevalence of symptomatic and silent stress-induced perfusion defects in diabetic patients with suspected coronary artery disease referred for myocardial perfusion scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 60–69. [Google Scholar] [CrossRef]
- Nakajima, K.; Yamasaki, Y.; Kusuoka, H.; Izumi, T.; Kashiwagi, A.; Kawamori, R.; Shimamoto, K.; Yamada, N.; Nishimura, T. Cardiovascular events in Japanese asymptomatic patients with type 2 diabetes: A 1-year interim report of a J-ACCESS 2 investigation using myocardial perfusion imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 2049–2057. [Google Scholar] [CrossRef]
- Blumenthal, R.S.; Becker, D.M.; Moy, T.F.; Coresh, J.; Wilder, L.B.; Becker, L.C. Exercise thallium tomography predicts future clinically manifest coronary heart disease in a high-risk asymptomatic population. Circulation 1996, 93, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Cambria, R.P.; Brewster, D.C.; Abbott, W.M.; L’Italien, G.J.; Megerman, J.J.; LaMuraglia, G.M.; Moncure, A.C.; Zelt, D.T.; Eagle, K. The impact of selective use of dipyridamole-thallium scans and surgical factors on the current morbidity of aortic surgery. J. Vasc. Surg. 1992, 15, 43–51. [Google Scholar] [CrossRef]
- Damaskos, C.; Garmpis, N.; Kollia, P.; Mitsiopoulos, G.; Barlampa, D.; Drosos, A.; Zelt, D.T.; Diamantis, E. Assessing cardiovascular risk in patients with diabetes: An update. Curr. Cardiol. Rev. 2020, 16, 266–274. [Google Scholar] [CrossRef]
- Hansen, C.L.; Woodhouse, S.; Kramer, M. Effect of patient obesity on the accuracy of thallium-201 myocardial perfusion imaging. Am. J. Cardiol. 2000, 85, 749–752. [Google Scholar] [CrossRef]
- Ağyar, O.; Tırınk, C.; Önder, H.; Şen, U.; Piwczyński, D.; Yavuz, E. Use of multivariate adaptive regression splines algorithm to predict body weight from body measurements of anatolian buffaloes in türkiye. Animals 2022, 12, 2923. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2012, 35, S64–S71. [Google Scholar] [CrossRef]
- Lin, J.-D.; Pei, D.; Chen, F.-Y.; Wu, C.-Z.; Lu, C.-H.; Huang, L.-Y.; Kuo, C.-H.; Kuo, S.-W.; Chen, Y.-L. Comparison between machine learning and multiple linear regression to identify abnormal thallium myocardial perfusion scan in chinese type 2 diabetes. Diagnostics 2022, 12, 1619. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Berman, D.S.; Shaw, L.J.; Kiat, H.; Cohen, I.; Cabico, J.A.; Friedman, J.; George, A. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: Differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998, 97, 535–543. [Google Scholar] [CrossRef]
- Gimelli, A.; Rossi, G.; Landi, P.; Marzullo, P.; Iervasi, G.; L’Abbate, A.; Rovai, D. Stress/Rest Myocardial Perfusion Abnormalities by Gated SPECT: Still the Best Predictor of Cardiac Events in Stable Ischemic Heart Disease. J. Nucl. Med. 2009, 50, 546–553. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Deconinck, E.; Zhang, M.; Petitet, F.; Dubus, E.; Ijjaali, I.; Coomans, D.; Heyden, Y.V. Boosted regression trees, multivariate adaptive regression splines and their two-step combinations with multiple linear regression or partial least squares to predict blood-brain barrier passage: A case study. Anal. Chim. Acta 2008, 609, 13–23. [Google Scholar] [CrossRef]
- Rashijane, L.T.; Mokoena, K.; Tyasi, T.L. Using multivariate adaptive regression splines to estimate the body weight of savanna goats. Animals 2023, 13, 1146. [Google Scholar] [CrossRef] [PubMed]
- Toutounji, H.; Durstewitz, D. Detecting multiple change points using adaptive regression splines with application to neural recordings. Front. Neuroinform 2018, 12, 67. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Asadollahi-Baboli, M.; Mani-Varnosfaderani, A. Shuffling multivariate adaptive regression splines and adaptive neuro-fuzzy inference system as tools for QSAR study of SARS inhibitors. J. Pharm. Biomed. Anal. 2009, 50, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Pfob, A.; Lu, S.C.; Sidey-Gibbons, C. Machine learning in medicine: A practical introduction to techniques for data pre-processing, hyperparameter tuning, and model comparison. BMC Med. Res. Methodol 2022, 22, 282. [Google Scholar] [CrossRef]
- Gackowski, M.; Szewczyk-Golec, K.; Pluskota, R.; Koba, M.; Mądra-Gackowska, K.; Woźniak, A. Application of Multivariate Adaptive Regression Splines (MARSplines) for predicting antitumor activity of anthrapyrazole derivatives. Int. J. Mol. Sci. 2022, 23, 5132. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Bhat, G.; Saade, G.R.; Spratt, H. Multivariate adaptive regression splines analysis to predict biomarkers of spontaneous preterm birth. Acta Obs. Gynecol Scand 2014, 93, 382–391. [Google Scholar] [CrossRef]
- Peters-Sanders, L.; Sanders, H.; Goldstein, H.; Ramachandran, K. Using multivariate adaptive regression splines to predict lexical characteristics’ influence on word learning in first through third graders. J. Speech Lang. Hear. Res. 2023, 66, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, I.F.; Adolph, A.L.; Puyau, M.R.; Vohra, F.A.; Butte, N.F. Multivariate adaptive regression splines models for the prediction of energy expenditure in children and adolescents. J. Appl. Physiol. 2010, 108, 128–136. [Google Scholar] [CrossRef]
- Friedman, J.H.; Roosen, C.B. An introduction to multivariate adaptive regression splines. Stat. Methods Med. Res. 1995, 4, 197–217. [Google Scholar] [CrossRef]
- Milborrow, S. R Package, Version, 5.3.3; Derived from Mda: MARS by T. Hastie and R. Tibshirani. Earth: Multivariate Adaptive Regression Splines. 2024. Available online: http://CRAN.R-project.org/package=earth (accessed on 26 February 2024).
- Kuhn, M. R Package, Version, 6.0–94; Caret: Classification and Regression Training. 2023. Available online: https://CRAN.R-project.org/package=caret (accessed on 21 March 2023).
- Gleissner, C.A.; Galkina, E.; Nadler, J.L.; Ley, K. Mechanisms by which diabetes increases cardiovascular disease. Drug Discov. Today Dis. Mech. 2007, 4, 131–140. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Litvinova, L.; Poggio, P.; Sukhorukov, V.N.; Orekhov, A.N. Effect of glucose levels on cardiovascular risk. Cells 2022, 11, 3034. [Google Scholar] [CrossRef]
- Nielson, C.; Lange, T.; Hadjokas, N. Blood glucose and coronary artery disease in nondiabetic patients. Diabetes Care 2006, 29, 998–1001. [Google Scholar] [CrossRef][Green Version]
- Siddiqi, T.J.; Khan, M.S.; Pandey, A.; Greene, S. Duration of Diabetes and Risk of Incident Heart Failure: Using the Past to Predict the Future; American College of Cardiology: Washington, DC, USA, 2021; Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2021/12/01/13/34/Duration-of-Diabetes-and-Risk-of-Incident-Heart-Failure (accessed on 24 July 2025).
- Zamora, A.; Marrugat, J. Prognosis of diabetic patients with coronary heart disease. Rev. Españ. Cardiol. 2002, 55, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Sullivan, L.; D’Agostino, R.B., Sr.; Wilson, P.W. The significant effect of diabetes duration on coronary heart disease mortality: The Framingham Heart Study. Diabetes Care 2004, 27, 704–708. [Google Scholar] [CrossRef]
- Volpe, M.; Gallo, G. Obesity and cardiovascular disease: An executive document on pathophysiological and clinical links promoted by the Italian Society of Cardiovascular Prevention (SIPREC). Front. Cardiovasc. Med. 2023, 10, 1136340. [Google Scholar] [CrossRef]
- Eeg-Olofsson, K.; Cederholm, J.; Nilsson, P.M.; Zethelius, B.; Nunez, L.; Gudbjörnsdóttir, S.; Eliasson, B. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: An observational study in 13,087 patients. Diabetologia 2009, 52, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, M.P.; Raja, R.; Jamil, A.; Csendes, D.; Gutlapalli, S.D.; Prakash, K.; Swarnakari, K.M.; Bai, M.; Desai, D.M.; Desai, A.; et al. Obesity and coronary artery disease: An updated systematic review 2022. Cureus 2022, 14, e29480. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; Hlatky, M.A.; Hodgson, J.M.; Kushner, F.G.; et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular. J. Am. Coll. Cardiol. 2010, 56, e50–e103. [Google Scholar]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012, 60, e44–e164. [Google Scholar]
- Hachamovitch, R.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Berman, D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003, 107, 2900–2907. [Google Scholar] [CrossRef] [PubMed]


| Metrics | Description | Calculation |
|---|---|---|
| MAPE | Mean Absolute Percentage Error | |
| SMAPE | Symmetric Mean Absolute Percentage Error | |
| RAE | Relative Absolute Error | |
| RRSE | Root Relative Squared Error | |
| RMSE | Root Mean Squared Error |
| Numeric Variables | Unit | Mean ± SD |
|---|---|---|
| Age | year | 69.32 ± 9.64 |
| Body Mass Index | kg/m2 | 26.54 ± 4.43 |
| Duration of Diabetes | year | 14.02 ± 8.31 |
| Systolic Blood Pressure | mmHg | 131.88 ± 15.16 |
| Diastolic Blood Pressure | mmHg | 72.07 ± 10.24 |
| Hemoglobin | g/dL | 12.19 ± 1.38 |
| Hemoglobin A1c | % | 7.66 ± 1.37 |
| Triglycerides | mg/dL | 124.68 ± 74.08 |
| High-Density Lipoprotein Cholesterol | mg/dL | 52.74 ± 14.59 |
| Low-Density Lipoprotein Cholesterol | mg/dL | 94.01 ± 23.75 |
| Alanine Aminotransferase | IU/L | 21.53 ± 11.34 |
| Creatinine | mg/dL | 1.02 ± 0.90 |
| Urine protein | mg/L | 180.36 ± 630.39 |
| Homeostasis Model Assessment of Insulin Resistance | — | 6.26 ± 7.12 |
| Homeostasis Model Assessment of β-cell function | — | 100.29 ± 322.20 |
| Dependent Variable | Unit | Mean ± SD |
| Sum of Stress Score (SSS) | — | 4.56 ± 6.74 |
| Ordinal Variables | n (%) | p-Value |
| Smoking Status | ||
| Smoking | 10 (6.7%) | 0.677 |
| Non-Smoking | 140 (93.3%) |
| Variable | Age | BMI | DD | SBP | DBP | Hb | HbA1c | TG |
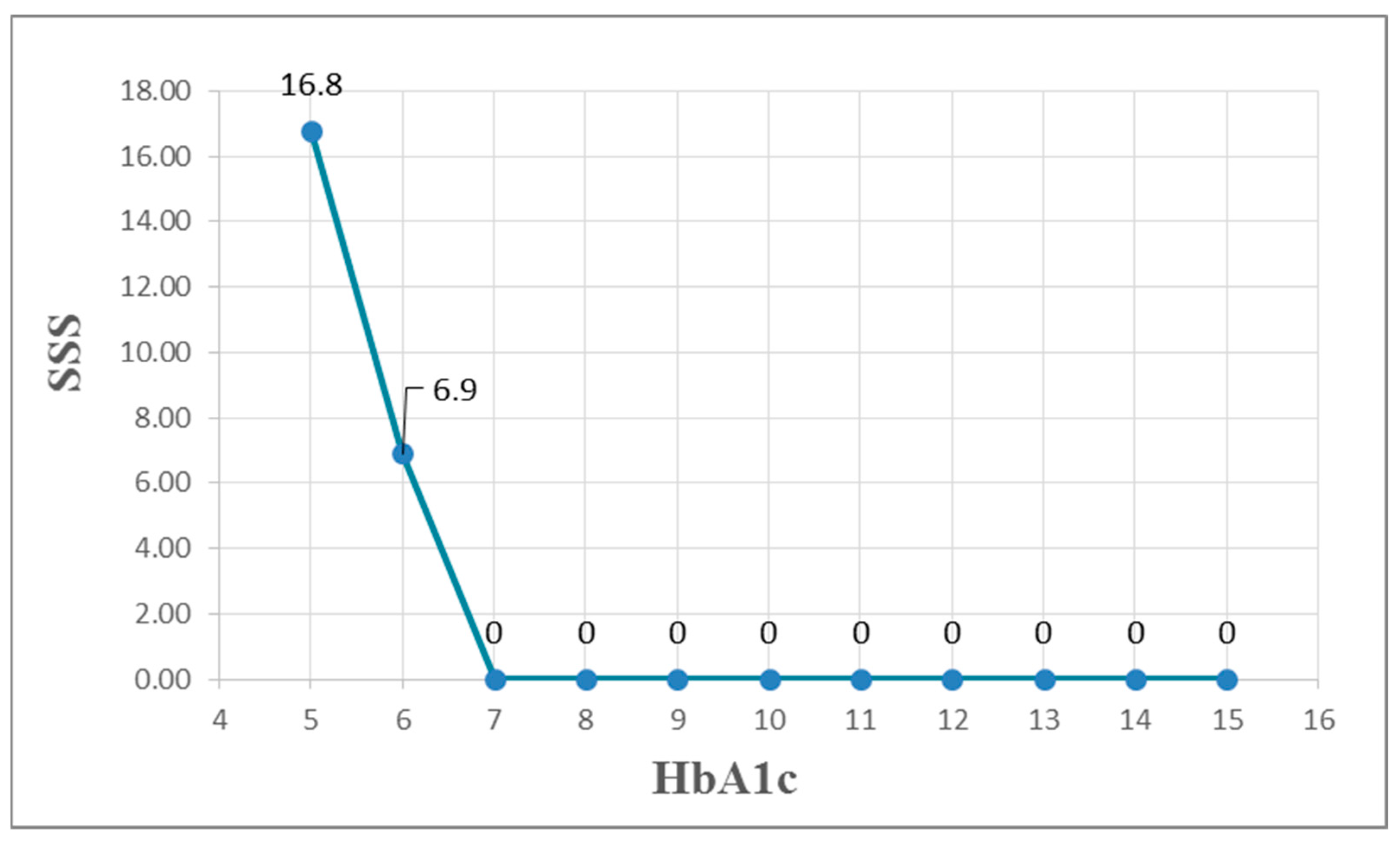
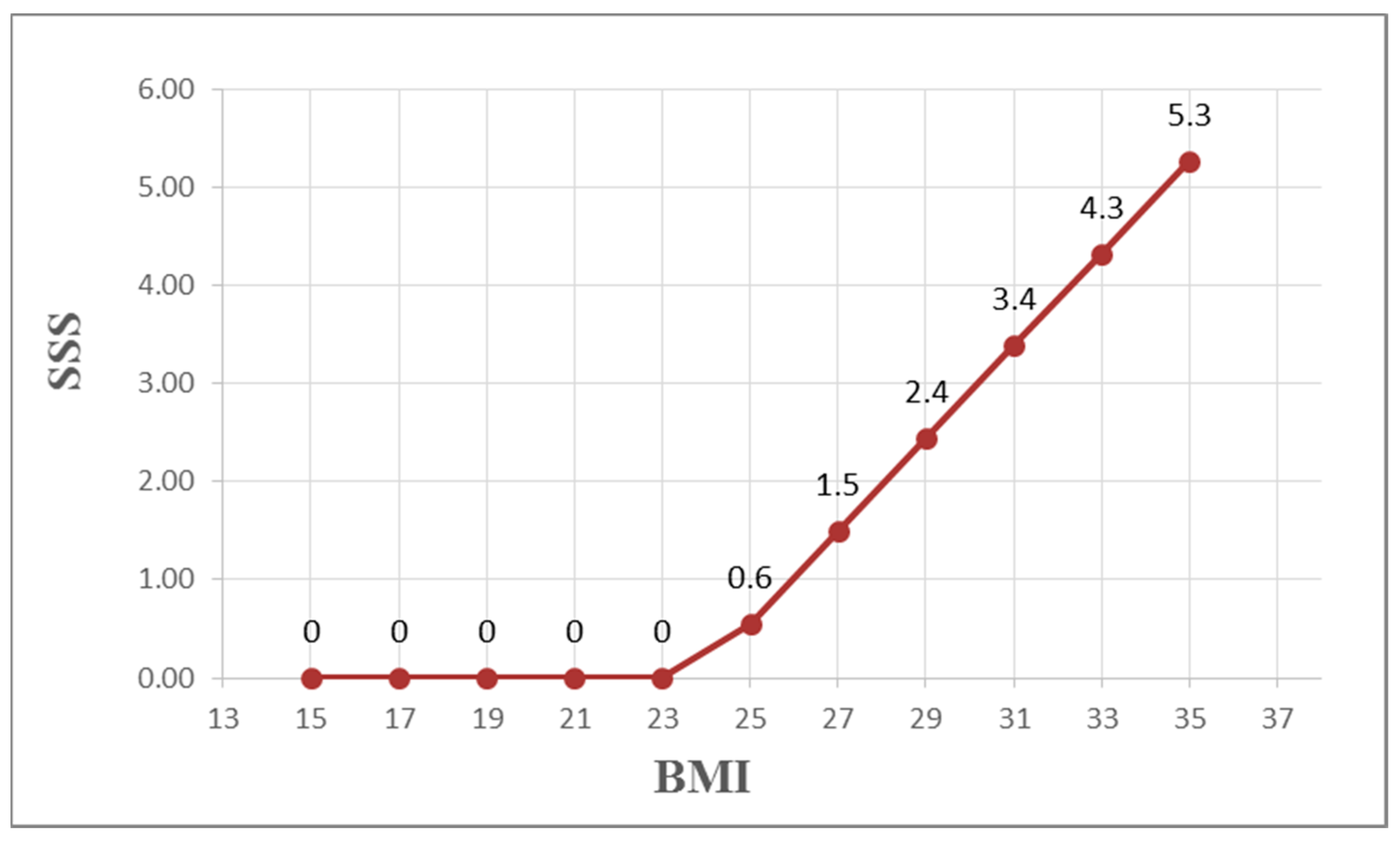
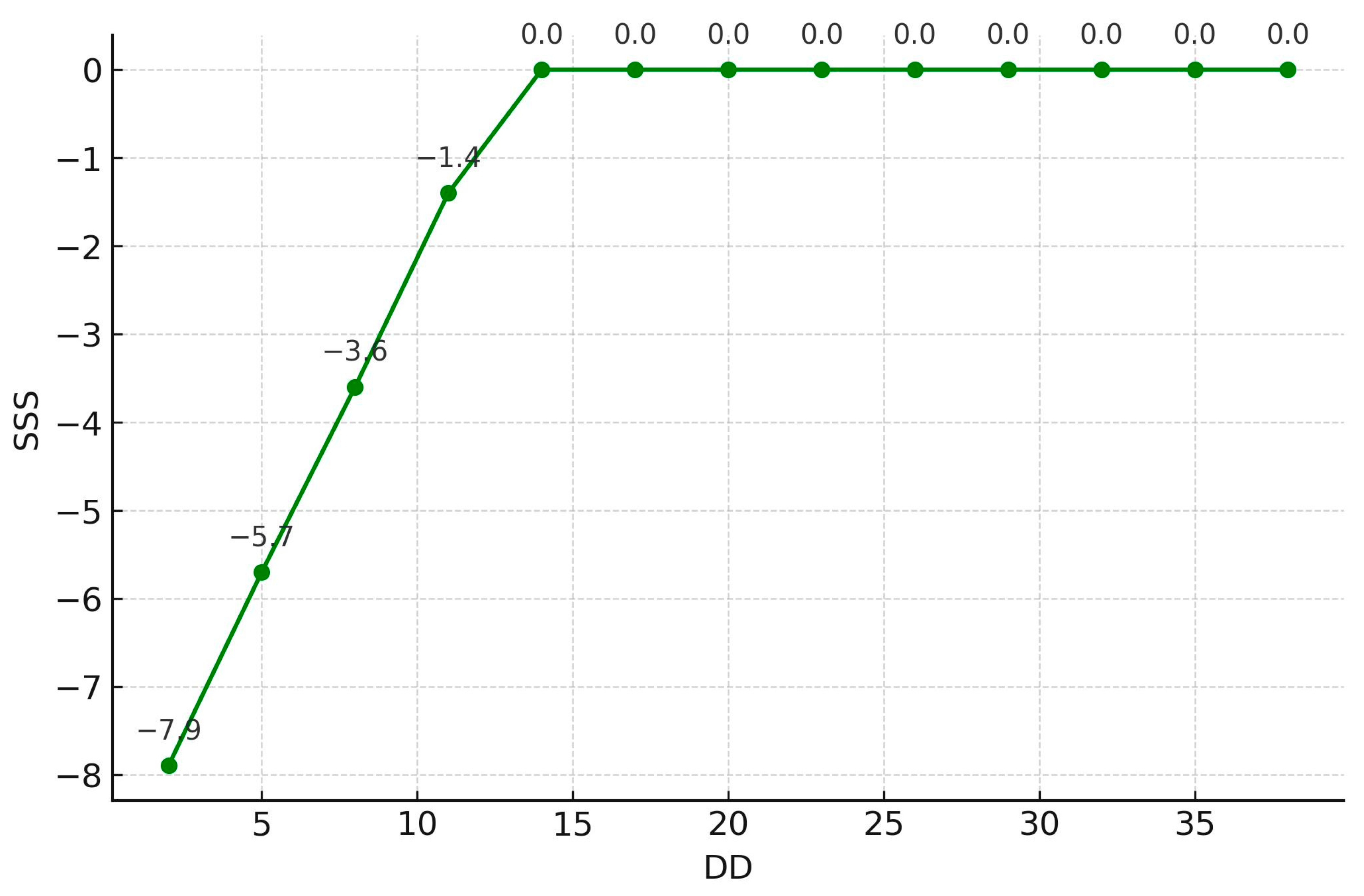
| SSS | −0.007 | 0.261 ** | −0.001 | −0.027 | −0.117 | −0.054 | −0.019 | 0.032 |
| Variable | HDL-C | LDL-C | ALT | Cr | MCR | HOMA-IR | HOMA-β | |
| SSS | −0.144 * | 0.008 | −0.012 | 0.032 | 0.121 * | 0.117 | −0.007 |
| RAE | RRSE | RMSE | |
|---|---|---|---|
| MARS | 1.0965 | 1.1883 | 8.0443 |
| MLR | 1.2073 | 1.2611 | 8.5376 |
| A | B | C | |
|---|---|---|---|
| 1 | Type BMI | =MAX(0, A1 − 23.83) | =0.472 × B1 |
| 2 | Type diabetic duration | =MAX(0, 12 − A2) | =−0.718 × B2 |
| 3 | Type hemoglobin A1c | =MAX(6.7 − A3) | =9.88 × B3 |
| 4 | |||
| 5 | SSS | ||
| 6 | =3.064 + C1 + C2 + C3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.-H.; Lee, P.-C.; Wu, S.-T.; Yang, C.-C.; Chu, T.-W.; Yeih, D.-F. Using Multivariate Adaptive Regression Splines to Estimate Summed Stress Score on Myocardial Perfusion Scintigraphy in Chinese Women with Type 2 Diabetes: A Comparative Study with Multiple Linear Regression. Diagnostics 2025, 15, 2270. https://doi.org/10.3390/diagnostics15172270
Yuan C-H, Lee P-C, Wu S-T, Yang C-C, Chu T-W, Yeih D-F. Using Multivariate Adaptive Regression Splines to Estimate Summed Stress Score on Myocardial Perfusion Scintigraphy in Chinese Women with Type 2 Diabetes: A Comparative Study with Multiple Linear Regression. Diagnostics. 2025; 15(17):2270. https://doi.org/10.3390/diagnostics15172270
Chicago/Turabian StyleYuan, Chien-Han, Po-Chun Lee, Sheng-Tang Wu, Chung-Chi Yang, Ta-Wei Chu, and Dong-Feng Yeih. 2025. "Using Multivariate Adaptive Regression Splines to Estimate Summed Stress Score on Myocardial Perfusion Scintigraphy in Chinese Women with Type 2 Diabetes: A Comparative Study with Multiple Linear Regression" Diagnostics 15, no. 17: 2270. https://doi.org/10.3390/diagnostics15172270
APA StyleYuan, C.-H., Lee, P.-C., Wu, S.-T., Yang, C.-C., Chu, T.-W., & Yeih, D.-F. (2025). Using Multivariate Adaptive Regression Splines to Estimate Summed Stress Score on Myocardial Perfusion Scintigraphy in Chinese Women with Type 2 Diabetes: A Comparative Study with Multiple Linear Regression. Diagnostics, 15(17), 2270. https://doi.org/10.3390/diagnostics15172270






