EGFR Amplification in Diffuse Glioma and Its Correlation to Language Tract Integrity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Molecular Pathology
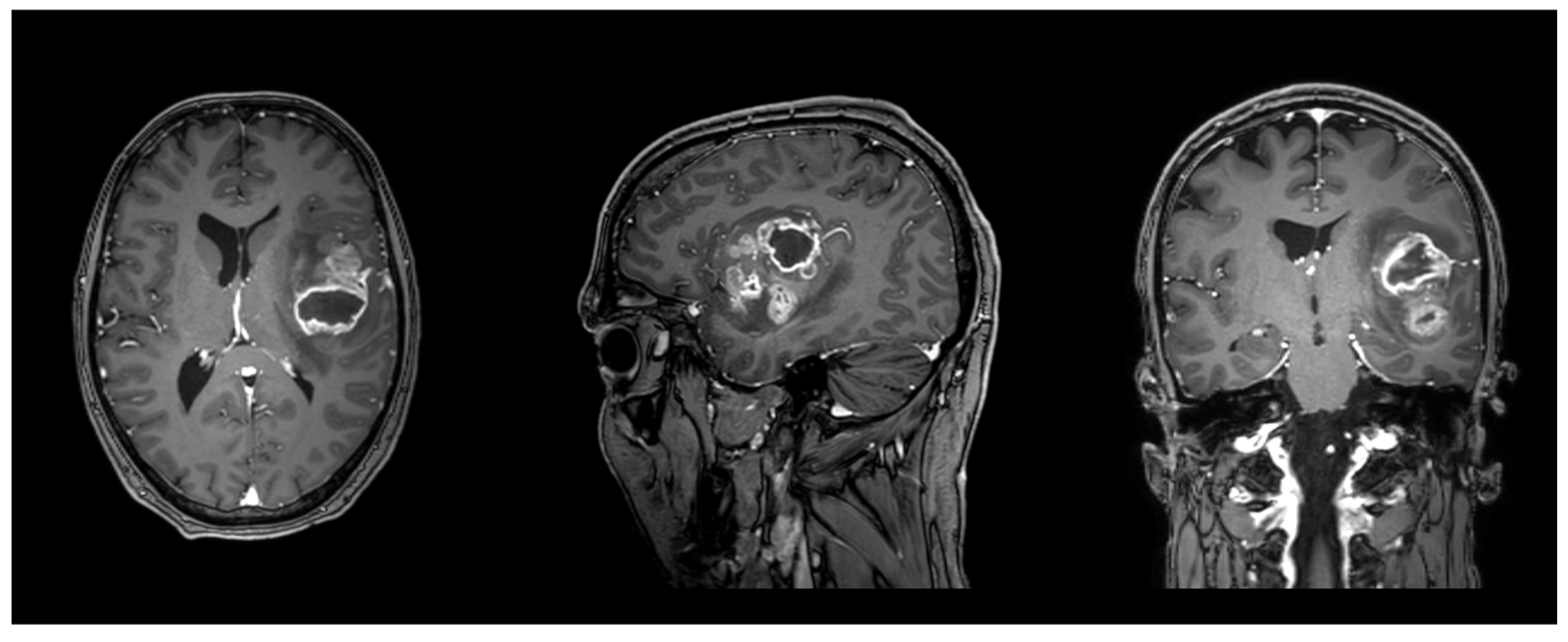
2.3. Imaging
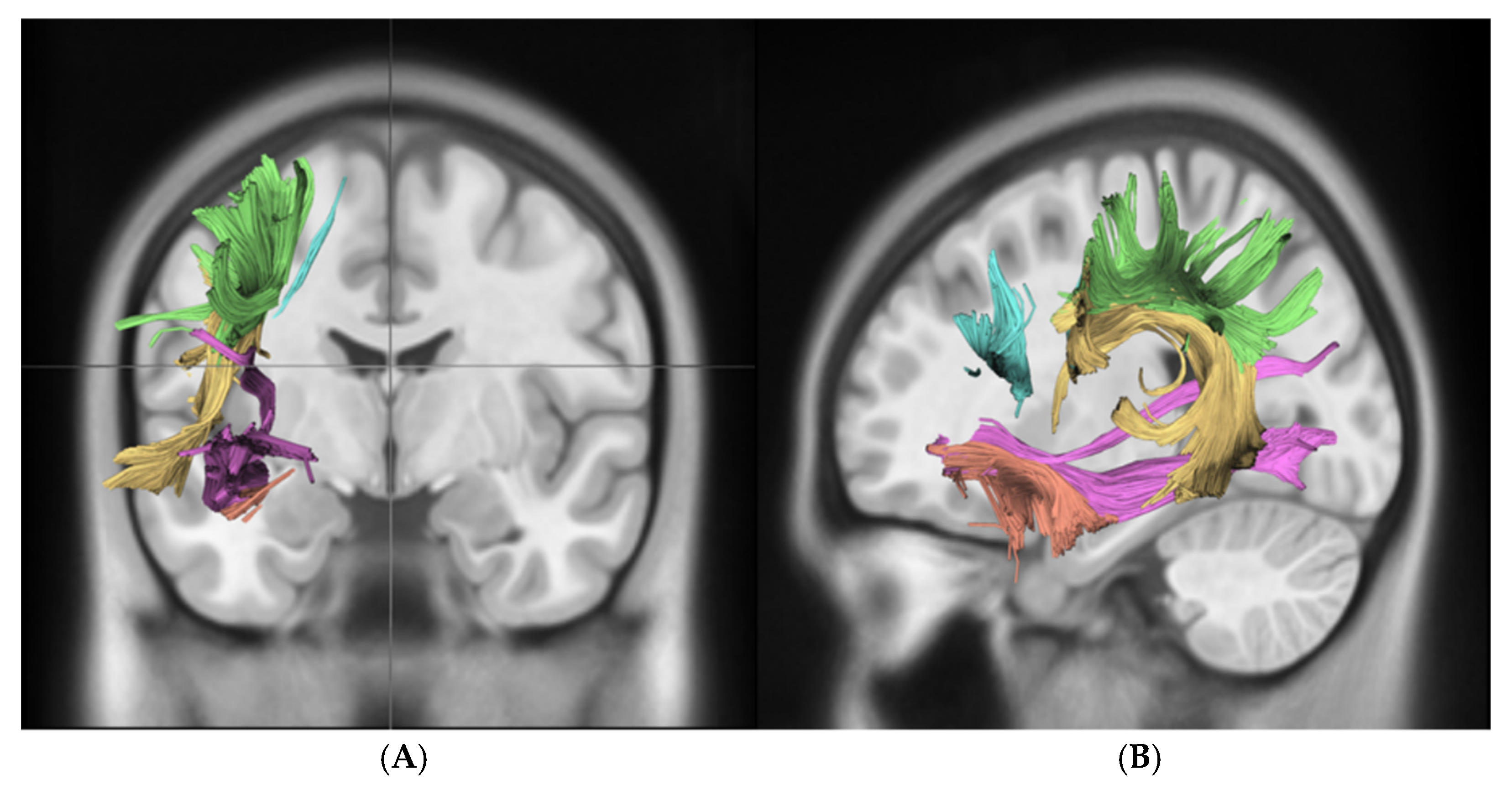
2.4. Data Analysis
3. Results
3.1. Patient Cohort
| Patients (n) | 27 | |
| Age | 56 ± 12.9 | |
| Sex | ||
| Male | 9/27 (33.3%) | |
| Female | 18/27 (66.7%) | |
| WHO Grade | ||
| 2 | 1/27 (3.8%) | |
| 3 | 4/27 (15.4%) | |
| 4 | 22/27 (81.5%) | |
| Lesion laterality (exclusive) | ||
| Left only | 9/27 (33.3%) | |
| Right only | 8/27 (29.6%) | |
| Bilateral | 10/27 (37.0%) | |
| Neuroanatomical Tumor Spread (non-exclusive counts *) | ||
| Left hemisphere | Σ19 | |
| Left frontal | 3 | |
| Left temporal | 9 | |
| Left parietal | 3 | |
| Left occipital | 0 | |
| Left cerebellum | 0 | |
| Left central | 4 | |
| Left thalamus | 0 | |
| Right hemisphere | Σ18 | |
| Right frontal | 6 | |
| Right temporal | 5 | |
| Right parietal | 2 | |
| Right occipital | 2 | |
| Right cerebellum | 0 | |
| Right central | 1 | |
| Right thalamus | 2 | |
| Corpus callosum | 1 | |
| EGFR amplified | ||
| No | 19 (70.4%) | |
| Yes | 8 (29.6%) | |
| IDH status | EGFR amplified | EGFR not amplified |
| Mutated | 1/27 (3.7%) | 8/27 (29.6%) |
| Wildtype | 7/27 (25.9%) | 11/27 (40.7%) |
| MGMT methylation status | EGFR amplified | EGFR not amplified |
| Unmethylated | 7/27 (25.9%) | 13/27 (48.1%) |
| Methylated | 1/27 (3.7%) | 6/27 (22.2%) |
3.2. White Matter Integrity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Herbet, G.; Duffau, H.; Mandonnet, E. Predictors of cognition after glioma surgery: Connectotomy, structure-function phenotype, plasticity. Brain 2024, 147, 2621–2635. [Google Scholar] [CrossRef]
- Young, J.S.; Morshed, R.A.; Hervey-Jumper, S.L.; Berger, M.S. The surgical management of diffuse gliomas: Current state of neurosurgical management and future directions. Neuro Oncol. 2023, 25, 2117–2133. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Liu, J.; Chen, W.; Guo, X.; Wang, Y.; Wang, Y.; Xing, H.; Liang, T.; Shi, Y.; et al. Clinical roles of EGFR amplification in diffuse gliomas: A real-world study using the 2021 WHO classification of CNS tumors. Front. Neurosci. 2024, 18, 1308627. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-P.; Guo, Z.-Q.; Wang, B.-F.; Zhao, M. EGFR alterations in glioblastoma play a role in antitumor immunity regulation. Front. Oncol. 2023, 13, 1236246. [Google Scholar] [CrossRef] [PubMed]
- Rudà, R.; Bruno, F.; Ius, T.; Silvani, A.; Minniti, G.; Pace, A.; Lombardi, G.; Bertero, L.; Pizzolitto, S.; Pollo, B.; et al. IDH wild-type grade 2 diffuse astrocytomas: Prognostic factors and impact of treatments within molecular subgroups. Neuro Oncol. 2022, 24, 809–820. [Google Scholar] [CrossRef]
- Lan, Z.; Li, X.; Zhang, X. Glioblastoma: An Update in Pathology, Molecular Mechanisms and Biomarkers. Int. J. Mol. Sci. 2024, 25, 3040. [Google Scholar] [CrossRef]
- Jeuken, J.; Sijben, A.; Alenda, C.; Rijntjes, J.; Dekkers, M.; Boots-Sprenger, S.; McLendon, R.; Wesseling, P. Robust detection of EGFR copy number changes and EGFR variant III: Technical aspects and relevance for glioma diagnostics. Brain Pathol. 2009, 19, 661–671. [Google Scholar] [CrossRef]
- Wijtenburg, S.; McGuire, S.; Rowland, L.; Sherman, P.; Lancaster, J.; Tate, D.; Hardies, L.; Patel, B.; Glahn, D.; Hong, L.; et al. Relationship between fractional anisotropy of cerebral white matter and metabolite concentrations measured using (1)H magnetic resonance spectroscopy in healthy adults. Neuroimage 2013, 66, 161–168. [Google Scholar] [CrossRef]
- Inano, S.; Takao, H.; Hayashi, N.; Abe, O.; Ohtomo, K. Effects of age and gender on white matter integrity. AJNR Am. J. Neuroradiol. 2011, 32, 2103–2109. [Google Scholar] [CrossRef]
- El Ouadih, Y.; Pereira, B.; Biau, J.; Claise, B.; Chaix, R.; Verrelle, P.; Khalil, T.; Durando, X.; Lemaire, J.-J. DTI Abnormalities Related to Glioblastoma: A Prospective Comparative Study with Metastasis and Healthy Subjects. Curr. Oncol. 2022, 29, 2823–2834. [Google Scholar] [CrossRef]
- Miloushev, V.; Chow, D.; Filippi, C. Meta-analysis of diffusion metrics for the prediction of tumor grade in gliomas. AJNR Am. J. Neuroradiol. 2015, 36, 302–308. [Google Scholar] [CrossRef]
- Yuan, J.; Siakallis, L.; Li, H.B.; Brandner, S.; Zhang, J.; Li, C.; Mancini, L.; Bisdas, S. Structural- and DTI-MRI enable automated prediction of IDH Mutation Status in CNS WHO Grade 2-4 glioma patients: A deep Radiomics Approach. BMC Med. Imaging 2024, 24, 104. [Google Scholar] [CrossRef]
- Ghaderi, S.; Mohammadi, S.; Fatehi, F. Diffusion Tensor Imaging (DTI) Biomarker Alterations in Brain Metastases and Comparable Tumors: A Systematic Review of DTI and Tractography Findings. World Neurosurg. 2024, 190, 113–129. [Google Scholar] [CrossRef]
- Jütten, K.; Mainz, V.; Gauggel, S.; Patel, H.J.; Binkofski, F.; Wiesmann, M.; Clusmann, H.; Na, C.-H. Diffusion Tensor Imaging Reveals Microstructural Heterogeneity of Normal-Appearing White Matter and Related Cognitive Dysfunction in Glioma Patients. Front. Oncol. 2019, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Mabray, M.C.; Barajas, R.F.; Cha, S. Modern Brain Tumor Imaging. Brain Tumor Res. Treat. 2015, 3, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lu, C.; Li, G.; Li, Z.; Sun, S.; Zhang, Y.; Hou, Z.; Xie, J. Prediction of Lower Grade Insular Glioma Molecular Pathology Using Diffusion Tensor Imaging Metric-Based Histogram Parameters. Front. Oncol. 2021, 11, 627202. [Google Scholar] [CrossRef] [PubMed]
- Kankara, S.R.; Sandeep, S.; Kumaran, S.P.; Kankara, S.R.; Kancharla, M. Utilizing Diffusion Tensor Imaging to Differentiate High-Grade Gliomas and Solitary Brain Metastases. Asian J. Neurosurg. 2025, 20, 278–284. [Google Scholar] [CrossRef]
- Mandonnet, E.; Duffau, H. An attempt to conceptualize the individual onco-functional balance: Why a standardized treatment is an illusion for diffuse low-grade glioma patients. Crit. Rev. Oncol. Hematol. 2018, 122, 83–91. [Google Scholar] [CrossRef]
- Duffau, H.; Mandonnet, E. The “onco-functional balance” in surgery for diffuse low-grade glioma: Integrating the extent of resection with quality of life. Acta Neurochir. 2013, 155, 951–957. [Google Scholar] [CrossRef]
- Dubey, A.; Kataria, R.; Sinha, V.D. Role of Diffusion Tensor Imaging in Brain Tumor Surgery. Asian J. Neurosurg. 2018, 13, 302–306. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Zhang, K.; Wei, H.; Fan, J.; Yu, S.; Li, T.; Yang, X. Diffusion tensor imaging versus intraoperative subcortical mapping for glioma resection: A systematic review and meta-analysis. Neurosurg. Rev. 2023, 46, 154. [Google Scholar] [CrossRef]
- Verburg, N.; Hamer, P.C.d.W. State-of-the-art imaging for glioma surgery. Neurosurg. Rev. 2021, 44, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Steward, C.; Desmond, P. Diffusion tensor imaging in glioblastoma multiforme and brain metastases: The role of p, q, L, and fractional anisotropy. AJNR Am. J. Neuroradiol. 2009, 30, 203–208. [Google Scholar] [CrossRef]
- Álvarez-Vázquez, A.; San-Segundo, L.; Cerveró-García, P.; Flores-Hernández, R.; Ollauri-Ibáñez, C.; Segura-Collar, B.; Hubert, C.G.; Morrison, G.; Pollard, S.M.; Lathia, J.D.; et al. EGFR amplification and EGFRvIII predict and participate in TAT-Cx43266-283 antitumor response in preclinical glioblastoma models. Neuro Oncol. 2024, 26, 1230–1246. [Google Scholar] [CrossRef]
- Tan, W.L.; Huang, W.Y.; Yin, B.; Xiong, J.; Wu, J.S.; Geng, D.Y. Can diffusion tensor imaging noninvasively detect IDH1 gene mutations in astrogliomas? A retrospective study of 112 cases. AJNR Am. J. Neuroradiol. 2014, 35, 920–927. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Chen, Y.; Wang, W.; Duan, W.; Wang, L.; Zhang, S.; Ding, T.; Liu, L.; Sun, Q.; et al. Deep learning features from diffusion tensor imaging improve glioma stratification and identify risk groups with distinct molecular pathway activities. EBioMedicine 2021, 72, 103583. [Google Scholar] [CrossRef]
- Eichinger, P.; Alberts, E.; Delbridge, C.; Trebeschi, S.; Valentinitsch, A.; Bette, S.; Huber, T.; Gempt, J.; Meyer, B.; Schlegel, J.; et al. Diffusion tensor image features predict IDH genotype in newly diagnosed WHO grade II/III gliomas. Sci. Rep. 2017, 7, 13396. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Stichel, D.; Sahm, F.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Schmid, S.; Hovestadt, V.; Reuss, D.E.; Koelsche, C.; et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: The Heidelberg experience. Acta Neuropathol. 2018, 136, 181–210. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Lee, S.M.; Goldberg, D.; Spix, N.J.; Hinoue, T.; Li, H.-T.; Dwaraka, V.B.; Smith, R.; Shen, H.; Liang, G.; et al. Comprehensive Evaluation of The Infinium Human MethylationEPIC v2 BeadChip. Epigenetics Commun. 2023, 3, 6. [Google Scholar] [CrossRef]
- Yeh, F.-C. DSI Studio: An integrated tractography platform and fiber data hub for accelerating brain research. Nat. Methods 2025, 22, 1617–1619. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C. Population-based tract-to-region connectome of the human brain and its hierarchical topology. Nat. Commun. 2022, 13, 4933. [Google Scholar] [CrossRef]
- Waskom, M.L. seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Karschnia, P.; Young, J.S.; Dono, A.; Häni, L.; Sciortino, T.; Bruno, F.; Juenger, S.T.; Teske, N.; Morshed, R.A.; Haddad, A.F.; et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro Oncol. 2023, 25, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Heiland, D.H.; Simon-Gabriel, C.P.; Demerath, T.; Haaker, G.; Pfeifer, D.; Kellner, E.; Kiselev, V.G.; Staszewski, O.; Urbach, H.; Weyerbrock, A.; et al. Integrative Diffusion-Weighted Imaging and Radiogenomic Network Analysis of Glioblastoma multiforme. Sci. Rep. 2017, 7, 43523. [Google Scholar] [CrossRef]
- Vidyadharan, S.; Rao, B.V.V.S.N.P.; Yogeeswari, P.; Kesavadas, C.; Rajagopalan, V. Accurate low and high grade glioma classification using free water eliminated diffusion tensor metrics and ensemble machine learning. Sci. Rep. 2024, 14, 19844. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Sahm, F.; Jeibmann, A.; Habel, A.; Paulus, W.; Troost, D.; von Deimling, A. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch. Neurol. 2012, 69, 523–526. [Google Scholar] [CrossRef]
- Park, K.Y.; Snyder, A.Z.; Olufawo, M.; Trevino, G.; Luckett, P.H.; Lamichhane, B.; Xie, T.; Lee, J.J.; Shimony, J.S.; Leuthardt, E.C. Glioblastoma induces whole-brain spectral change in resting state fMRI: Associations with clinical comorbidities and overall survival. Neuroimage Clin. 2023, 39, 103476. [Google Scholar] [CrossRef] [PubMed]
- Seker-Polat, F.; Degirmenci, N.P.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef]
- Senhaji, N.; Louati, S.; Chbani, L.; El Fatemi, H.; Hammas, N.; Mikou, K.; Maaroufi, M.; Benzagmout, M.; Boujraf, S.; El Bardai, S.; et al. EGFR Amplification and IDH Mutations in Glioblastoma Patients of the Northeast of Morocco. Biomed. Res. Int. 2017, 2017, 8045859. [Google Scholar] [CrossRef]
- Hu, L.S.; D’aNgelo, F.; Weiskittel, T.M.; Caruso, F.P.; Ensign, S.P.F.; Blomquist, M.R.; Flick, M.J.; Wang, L.; Sereduk, C.P.; Meng-Lin, K.; et al. Integrated molecular and multiparametric MRI mapping of high-grade glioma identifies regional biologic signatures. Nat. Commun. 2023, 14, 6066. [Google Scholar] [CrossRef]
- Brito, C.; Azevedo, A.; Esteves, S.; Marques, A.R.; Martins, C.; Costa, I.; Mafra, M.; Marques, J.M.B.; Roque, L.; Pojo, M. Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation. BMC Cancer 2019, 19, 968. [Google Scholar] [CrossRef]
- Li, J.; Liang, R.; Song, C.; Xiang, Y.; Liu, Y. Prognostic significance of epidermal growth factor receptor expression in glioma patients. OncoTargets Ther. 2018, 11, 731–742. [Google Scholar] [CrossRef]
- Bjorland, L.S.; Mahesparan, R.; Fluge, Ø.; Gilje, B.; Kurz, K.D.; Farbu, E. Impact of extent of resection on outcome from glioblastoma using the RANO resect group classification system: A retrospective, population-based cohort study. Neurooncol. Adv. 2023, 5, vdad126. [Google Scholar] [CrossRef]
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. N. Engl. J. Med. 2024, 390, 1290–1298. [Google Scholar] [CrossRef]
- Kasper, J.; Wach, J.; Vychopen, M.; Arlt, F.; Güresir, E.; Wende, T.; Wilhelmy, F. Unplanned 30-Day Readmission in Glioblastoma Patients: Implications for the Extent of Resection and Adjuvant Therapy. Cancers 2023, 15, 3907. [Google Scholar] [CrossRef]
- Kasper, J.; Frydrychowicz, C.; Jähne, K.; Wende, T.; Wilhelmy, F.; Arlt, F.; Seidel, C.; Hoffmann, K.-T.; Meixensberger, J. The Role of Delayed Radiotherapy Initiation in Patients with Newly Diagnosed Glioblastoma with Residual Tumor Mass. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2022, 83, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Blobner, J.; Dengler, L.; Blobner, S.; Eberle, C.; Weller, J.; Teske, N.; Karschnia, P.; Rühlmann, K.; Heinrich, K.; Ziemann, F.; et al. Significance of molecular diagnostics for therapeutic decision-making in recurrent glioma. Neurooncol. Adv. 2023, 5, vdad060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, F.; Ni, W.; Qi, W.; Cao, W.; Xu, C.; Chen, J.; Gao, Y. Survival impact of delaying postoperative chemoradiotherapy in newly-diagnosed glioblastoma patients. Transl. Cancer Res. 2020, 9, 5450–5458. [Google Scholar] [CrossRef] [PubMed]
- Katsigiannis, S.; Krischek, B.; Barleanu, S.; Grau, S.; Galldiks, N.; Timmer, M.; Kabbasch, C.; Goldbrunner, R.; Stavrinou, P. Impact of time to initiation of radiotherapy on survival after resection of newly diagnosed glioblastoma. Radiat. Oncol. 2019, 14, 73. [Google Scholar] [CrossRef]
- Buszek, S.M.; Al Feghali, K.A.; Elhalawani, H.; Chevli, N.; Allen, P.K.; Chung, C. Optimal Timing of Radiotherapy Following Gross Total or Subtotal Resection of Glioblastoma: A Real-World Assessment using the National Cancer Database. Sci. Rep. 2020, 10, 4926. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.; Tang, W.; Baronik, Z.F.; Ragdale, H.S.; Oria, R.; Volteras, D.; White, I.J.; Beattie, G.; Uddin, I.; Lenn, T.; et al. Axonal injury is a targetable driver of glioblastoma progression. Nature, 2025; advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef]
- Okita, Y.; Takano, K.; Tateishi, S.; Hayashi, M.; Sakai, M.; Kinoshita, M.; Kishima, H.; Nakanishi, K. Neurite orientation dispersion and density imaging and diffusion tensor imaging to facilitate distinction between infiltrating tumors and edemas in glioblastoma. Magn. Reson. Imaging 2023, 100, 18–25. [Google Scholar] [CrossRef]
- Sexton, C.E.; Kalu, U.G.; Filippini, N.; Mackay, C.E.; Ebmeier, K.P. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2011, 32, e5–e18. [Google Scholar] [CrossRef]
- Bergamino, M.; Walsh, R.R.; Stokes, A.M. Free-water diffusion tensor imaging improves the accuracy and sensitivity of white matter analysis in Alzheimer’s disease. Sci. Rep. 2021, 11, 6990. [Google Scholar] [CrossRef] [PubMed]
- Caranova, M.; Soares, J.F.; Batista, S.; Castelo-Branco, M.; Duarte, J.V. A systematic review of microstructural abnormalities in multiple sclerosis detected with NODDI and DTI models of diffusion-weighted magnetic resonance imaging. Magn. Reson. Imaging 2023, 104, 61–71. [Google Scholar] [CrossRef]
- Bao, J.; Tu, H.; Li, Y.; Sun, J.; Hu, Z.; Zhang, F.; Li, J. Diffusion Tensor Imaging Revealed Microstructural Changes in Normal-Appearing White Matter Regions in Relapsing-Remitting Multiple Sclerosis. Front. Neurosci. 2022, 16, 837452. [Google Scholar] [CrossRef]
- Ma, J.; Yang, X.; Xu, F.; Li, H. Application of Diffusion Tensor Imaging (DTI) in the Diagnosis of HIV-Associated Neurocognitive Disorder (HAND): A Meta-Analysis and a System Review. Front. Neurol. 2022, 13, 898191. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, J.; Fang, H.; Fu, D.; Su, D.; Zhang, W. Diffusion tensor magnetic resonance imaging of white matter integrity in patients with HIV-associated neurocognitive disorders. Ann. Transl. Med. 2020, 8, 1314. [Google Scholar] [CrossRef]
- Shahim, P.; Holleran, L.; Kim, J.H.; Brody, D.L. Test-retest reliability of high spatial resolution diffusion tensor and diffusion kurtosis imaging. Sci. Rep. 2017, 7, 11141. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Marques, P.; Alves, V.; Sousa, N. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef]
- Aabedi, A.A.; Young, J.S.; Chang, E.F.; Berger, M.S.; Hervey-Jumper, S.L. Involvement of White Matter Language Tracts in Glioma: Clinical Implications, Operative Management, and Functional Recovery After Injury. Front. Neurosci. 2022, 16, 932478. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, P.; Nath, V.; Blaber, J.A.; Schilling, K.G.; Hainline, A.E.; Mojahed, E.; Anderson, A.W.; Landman, B.A. Empirical reproducibility, sensitivity, and optimization of acquisition protocol, for Neurite Orientation Dispersion and Density Imaging using AMICO. Magn. Reson. Imaging 2018, 50, 96–109. [Google Scholar] [CrossRef] [PubMed]

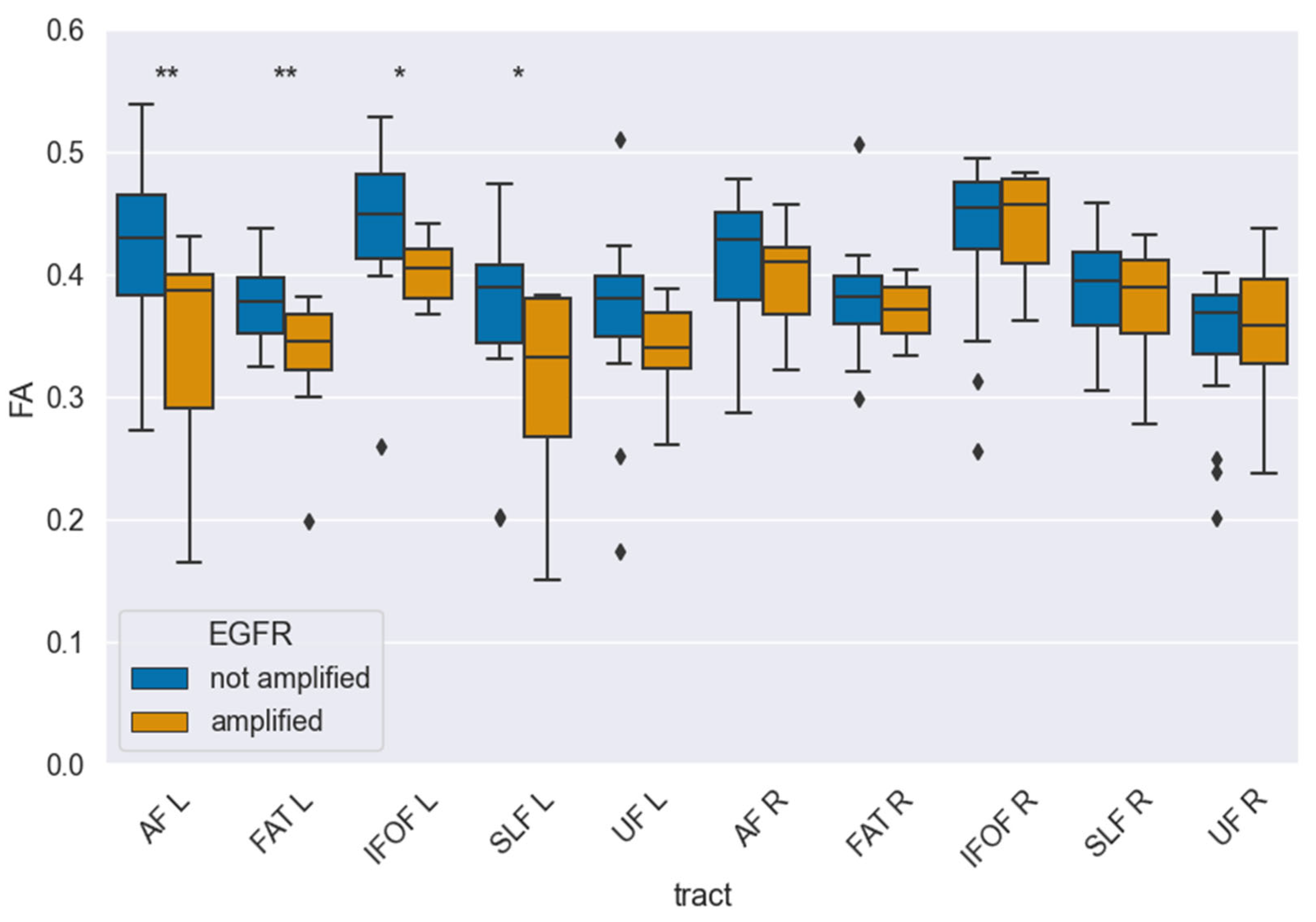
| Tract | FA Mean ± SEM (EGFR Amplified) | FA Mean ± SEM (EGFR Not Amplified) | p-Value (Unadjusted) | p-Value (Corrected) |
|---|---|---|---|---|
| AF-L | 0.34 ± 0.04 | 0.41 ± 0.02 | 0.030 | 0.025 |
| FAT-L | 0.33 ± 0.02 | 0.38 ± 0.01 | 0.008 | 0.020 |
| IFOF-L | 0.40 ± 0.01 | 0.44 ± 0.01 | 0.044 | 0.055 |
| SLF-II-L | 0.33 ± 0.03 | 0.39 ± 0.01 | 0.037 | 0.055 |
| UF-L | 0.34 ± 0.01 | 0.37 ± 0.02 | 0.127 | 0.127 |
| AF-R | 0.40 ± 0.02 | 0.42 ± 0.01 | 0.206 | 0.392 |
| FAT-R | 0.37 ± 0.01 | 0.38 ± 0.01 | 0.319 | 0.392 |
| IFOF-R | 0.44 ± 0.02 | 0.43 ± 0.02 | 0.392 | 0.392 |
| SLF-II-R | 0.39 ± 0.02 | 0.39 ± 0.01 | 0.294 | 0.392 |
| UF-R | 0.36 ± 0.02 | 0.35 ± 0.01 | 0.333 | 0.392 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basaran, A.E.; Barrantes-Freer, A.; Braune, M.; Prasse, G.; Jacobs, P.-P.; Wach, J.; Vychopen, M.; Güresir, E.; Wende, T. EGFR Amplification in Diffuse Glioma and Its Correlation to Language Tract Integrity. Diagnostics 2025, 15, 2266. https://doi.org/10.3390/diagnostics15172266
Basaran AE, Barrantes-Freer A, Braune M, Prasse G, Jacobs P-P, Wach J, Vychopen M, Güresir E, Wende T. EGFR Amplification in Diffuse Glioma and Its Correlation to Language Tract Integrity. Diagnostics. 2025; 15(17):2266. https://doi.org/10.3390/diagnostics15172266
Chicago/Turabian StyleBasaran, Alim Emre, Alonso Barrantes-Freer, Max Braune, Gordian Prasse, Paul-Philipp Jacobs, Johannes Wach, Martin Vychopen, Erdem Güresir, and Tim Wende. 2025. "EGFR Amplification in Diffuse Glioma and Its Correlation to Language Tract Integrity" Diagnostics 15, no. 17: 2266. https://doi.org/10.3390/diagnostics15172266
APA StyleBasaran, A. E., Barrantes-Freer, A., Braune, M., Prasse, G., Jacobs, P.-P., Wach, J., Vychopen, M., Güresir, E., & Wende, T. (2025). EGFR Amplification in Diffuse Glioma and Its Correlation to Language Tract Integrity. Diagnostics, 15(17), 2266. https://doi.org/10.3390/diagnostics15172266









