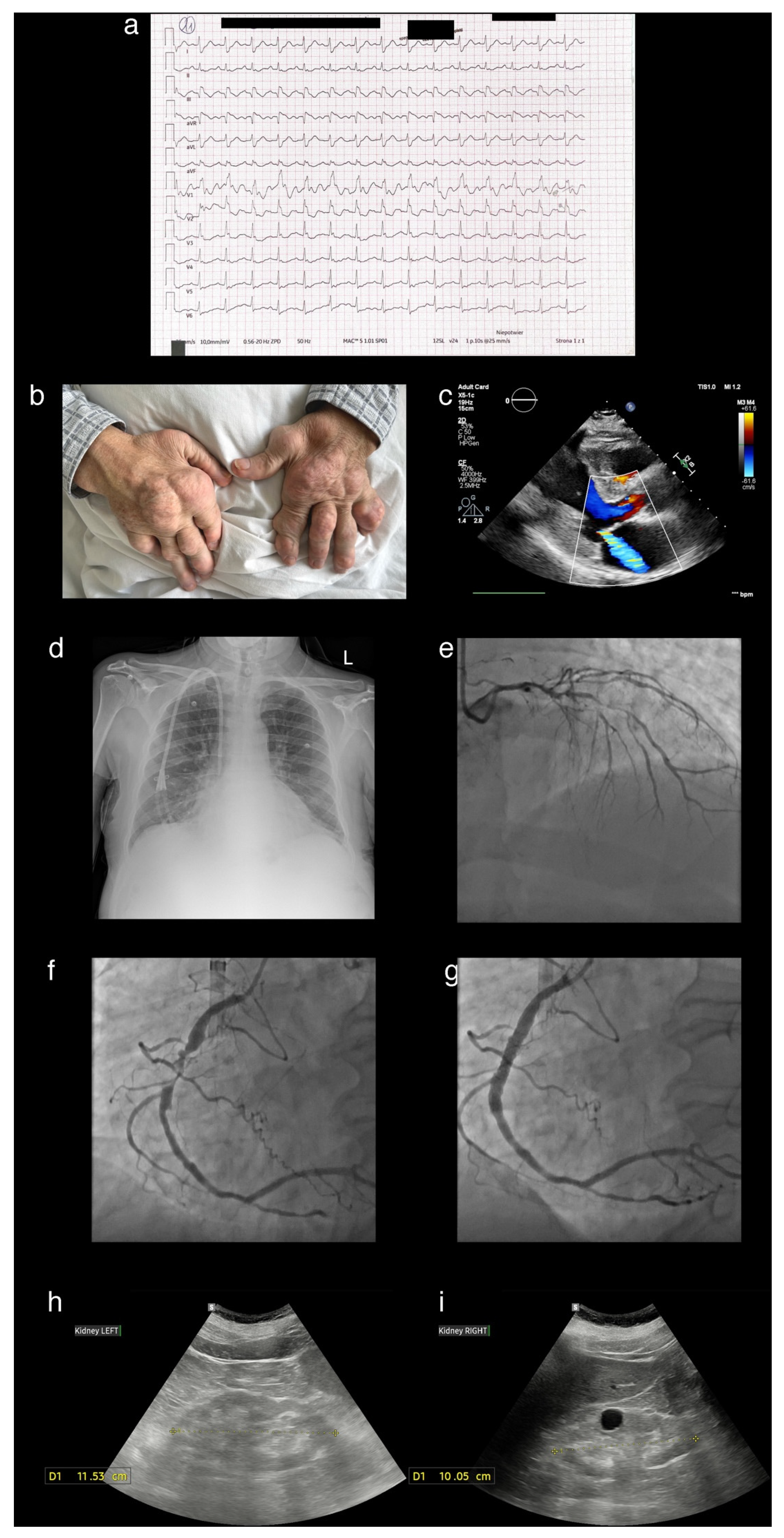
A Patient Presenting with Joint Deformities and ST-Elevation Myocardial Infarction
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414, Erratum in Eur. Heart J. 2025, ehaf306. https://doi.org/10.1093/eurheartj/ehaf306. [Google Scholar] [CrossRef]
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Lawrence Edwards, N.; Singh, J.A.; Troum, O.; Yeo, A.E.; Lipsky, P.E. Characterization of patients with chronic refractory gout who do and do not have clinically apparent tophi and their response to pegloticase. Rheumatology 2019, 6, kez017. [Google Scholar] [CrossRef] [PubMed]
- Stack, J.; McCarthy, G. Calcium pyrophosphate deposition (CPPD) disease—Treatment options. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101720. [Google Scholar] [CrossRef] [PubMed]
- Kralisz, P.; Dąbrowski, E.J.; Dobrzycki, S.; Kozłowska, W.U.; Lipska, P.O.; Nowak, K.; Gugała, K.; Prokopczuk, P.; Mężyński, G.; Święczkowski, M.; et al. Long-term impact of diabetes on mortality in patients undergoing unprotected left main PCI: A propensity score-matched analysis from the BIA-LM registry. Cardiovasc. Diabetol. 2025, 24, 175. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, K.; Yao, Q.; Shui, X.; Zheng, J.; He, Y.; Lei, W. The potential relationship of coronary artery disease and hyperuricemia: A cardiometabolic risk factor. Heliyon 2023, 9, e16097. [Google Scholar] [CrossRef] [PubMed]
- Maloberti, A.; Biolcati, M.; Ruzzenenti, G.; Giani, V.; Leidi, F.; Monticelli, M.; Algeri, M.; Scarpellini, S.; Nava, S.; Soriano, F.; et al. The Role of Uric Acid in Acute and Chronic Coronary Syndromes. J. Clin. Med. 2021, 10, 4750. [Google Scholar] [CrossRef] [PubMed]
- Disveld, I.J.M.; Zoakman, S.; Jansen, T.L.T.A.; Rongen, G.A.; Kienhorst, L.B.E.; Janssens, H.J.E.M.; Fransen, J. Crystal-proven gout patients have an increased mortality due to cardiovascular diseases, cancer, and infectious diseases especially when having tophi and/or high serum uric acid levels: A prospective cohort study. Clin. Rheumatol. 2019, 38, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castañeda-Sanabria, J.; Coyfish, M.; Guillo, S.; Jansen, T.L.; Janssens, H.; et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann. Rheum. Dis. 2017, 76, 29–42. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowski, E.J.; Kozłowska, W.U.; Lipska, P.O.; Kożuch, M.; Dobrzycki, S. A Patient Presenting with Joint Deformities and ST-Elevation Myocardial Infarction. Diagnostics 2025, 15, 2254. https://doi.org/10.3390/diagnostics15172254
Dąbrowski EJ, Kozłowska WU, Lipska PO, Kożuch M, Dobrzycki S. A Patient Presenting with Joint Deformities and ST-Elevation Myocardial Infarction. Diagnostics. 2025; 15(17):2254. https://doi.org/10.3390/diagnostics15172254
Chicago/Turabian StyleDąbrowski, Emil J., Wiktoria U. Kozłowska, Patrycja O. Lipska, Marcin Kożuch, and Sławomir Dobrzycki. 2025. "A Patient Presenting with Joint Deformities and ST-Elevation Myocardial Infarction" Diagnostics 15, no. 17: 2254. https://doi.org/10.3390/diagnostics15172254
APA StyleDąbrowski, E. J., Kozłowska, W. U., Lipska, P. O., Kożuch, M., & Dobrzycki, S. (2025). A Patient Presenting with Joint Deformities and ST-Elevation Myocardial Infarction. Diagnostics, 15(17), 2254. https://doi.org/10.3390/diagnostics15172254




