Diagnostic Imaging and Clinical Implications of Heterotopic Ossification After Total Ankle Arthroplasty: A Systematic Review for Surgical Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
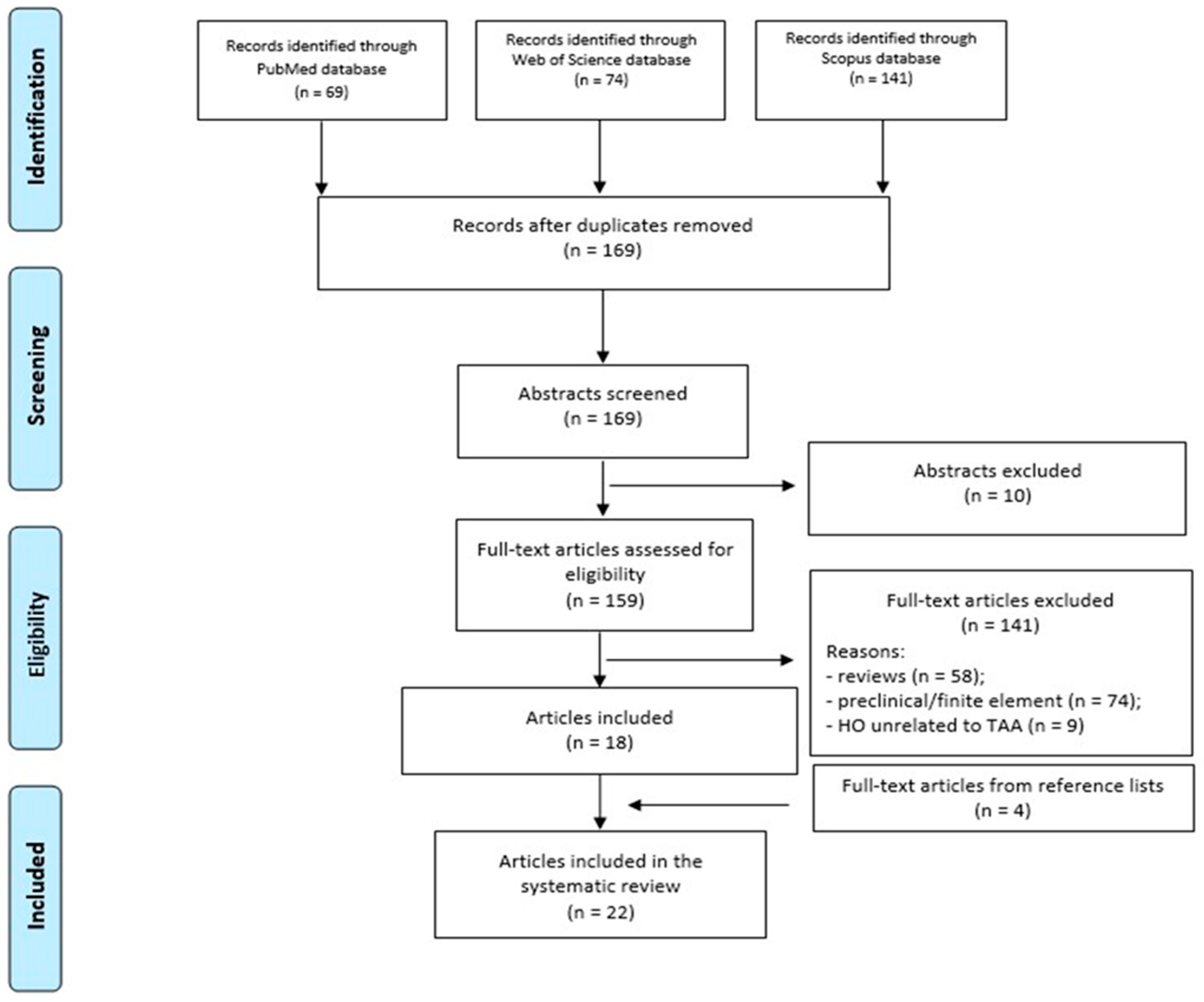
2.2. Study Selection
2.3. Data Extraction
- Reference (Ref.), study type, and patient treatment period (yrs);
- Number of patients (pts) and joints treated, including patient sex and age;
- Indications for surgery and their respective percentages;
- Type of prosthetic implant and mean follow-up duration in months (mo);
- Incidence of complications, HO rate, HO localization, and HO severity.
2.4. Risk of Bias Assessment
- Downs and Black checklist [20], consisting of 26 items across five domains:
- Reporting (9 items);
- External validity (3 items);
- Bias (7 items);
- Confounding (6 items);
- Power (1 item);
- The maximum score is 31 points.
- Modified Coleman Methodology Score (mCMS) [21], comprising 11 criteria:
- Study size;
- Mean follow-up duration;
- Number of surgical procedures per outcome;
- Study type;
- Surgical technique description;
- Postoperative rehabilitation details;
- Use of MRI outcomes;
- Inclusion of histological outcomes;
- Outcome measures;
- Clinical outcome assessment methods;
- Description of subject selection process.
2.5. Statistical Analysis
- Simple linear regression was applied to assess:
- The relationship between HO incidence and change in ROM, using pre- and post-operative ROM data from four studies.
- The association between HO incidence and radiographic follow-up duration. The Durbin–Watson test was performed to check for autocorrelation in residuals.
- Multiple linear regression models were developed to:
- Analyze whether the distribution of Brooker grades (I–III) predicted the likelihood of HO resection. Grade IV was excluded due to insufficient data.
- Explore whether overall reoperation rates were influenced by a combination of factors, including HO incidence, HO resection rate, infection, implant subsidence, and loosening.
3. Results
| Ref. | Type of Study (yrs) | Pts/N° of Joints (F/M and Age) | Indications for Surgery (%) | Type of Implant (%) Mean F-Up (mo) | Complications (%) | HO (%) Localization, Severity |
|---|---|---|---|---|---|---|
| Deleu, 2015 [22] | Retrospective case series (2008–2012) | 50/50 (25/25; 55 ± 12 yrs) | Post-traumatic OA (68%); Inflammatory OA (18%); Primary OA (14%) | HINTEGRA (100%). 45 mo | Osteolysis (48%); Additional surgery (18%); Lucency (14%); Revision (10%) | Total: 48%. Posterior: 100%. Grade I (42%), Grade II (29%), Grade III (17%), Grade IV (12%) |
| Manegold, 2017 [12] | Retrospective case series (2005–2009) | 84/88 (38/46; 55 ± 14 yrs) | Post-traumatic OA (70%); Primary OA (22%); Secondary OA (8%) | HINTEGRA (100%). 36 mo | n.r. | Total: 99%. Anterior (26%), posterior (100%), medial (23%), lateral (55%) |
| Clifton, 2021 [23] | Retrospective case serie (2010–2014) | 70/70 (16/54; mean 69 yrs) | Primary OA (64%); Post-traumatic OA (21%); Unknown (10%) Inflammatory OA (3%); Others, AVN (2%) | HINTEGRA (100%). 77 mo | Cysts (40%); Revision (16%); Re-operation (13%); Minor (13%); Major (4%); Intermediate (3%); i.o. fracture (1%) | Total: 1% |
| Lee, 2018 [7] | Prospective case series (2005–2012) | 144/144 (58/86; Mean 61 yrs) | Post-traumatic OA (59%); Primary OA (41%) | HINTEGRA (100%). 88 mo | Major (18%); Revision (17%); Minor (7%) | Total: 7% |
| Lee, 2019 [24] | Prospective case series (2005–2015) | 123/123 (54/69; Mean 56 yrs) | Post-traumtaic OA (64%); Primary OA (30%); RA (6%) | HINTEGRA (100%). 78 mo | Major (28%); Revision (12%); Minor (11%) | Total: 8%. |
| Haytmanek, 2015 [25] | Retrospective case series (1998–2007) | 76/79 (n.r.; 62 ± 11 yrs) | Post-traumatic OA (53%); Primary OA (33%); RA (13%); Hemochromatosis (1%) | STAR (100%). 96 mo | Lucency (56%); Revision (28%); Required secondary procedures (4%) | Total: 100%. Grade I (14%), Grade II (32%), Grande III (52%), Grade IV (2%) |
| Jastifer, 2015 [26] | Retrospective case series (1998–2003) | 18/18 (8/10; Mean 61 yrs) | Post-traumatic OA (77%); Primary OA (17%); RA (6%) | STAR (100%). 151 mo | Swelling (55%); Revision (39%); Talar subsidence 2–5 mm (11%); Talar subsidence > 5 mm (6%) | Total: 78%. |
| Palanca, 2018 [27] | Retrospective case series (1998–2000) | 24/24 (n.r.; Mean 74 yrs) | n.r. | STAR (100%). 188 mo | Cysts (14%); Osteolysis (14%); Revision (13%); Talar subsidence < 5 mm (10%); Talar subsidence > 5 mm (5%) | Total: 54%. Medial malleolus (54%), posterior tibia (69%). Grade III (23%) |
| Kerkhoff, 2016 [28] | Prospective case series (1999–2008) | 134/134 (84/50; 59 ± 13 yrs) | RA (43%); Post-traumatic OA (33%); Primary OA (25%); Hemochromatosis (1%) | STAR (100%). 90 mo | Cysts (60%); Nonrevision secondary procedures (16%); Prosthesis failure (15%); Revision (10%); Lucency (9%); Re-operation (5%) | Total: 98%. Grade III (>50%) |
| Jamjoom, 2022 [29] | Retrospective case series (2016–2019) | 28/29 (10/18; mean 68 yrs) | Revision TAA due to: Aseptic loosening of talar and tibial components (84%); Insert wear (10%); Talar malalignment (3%) | INBONE II (100%). 40 mo | Subsidence (31%); Loosening (21%); Osteolysis (17%); Re-operation (7%); i.o. deep peroneal nerve injury (3%) | Total: 31%. Tibia (45%), Talus (7%), Both (10%). Grade I (44%), Grade II (12%), Grade III (44%) |
| Rushing, 2021 [30] | Retrospective case series (2010–2014) | 15/15 (8/7; Mean 63 yrs) | n.r. | INBONE II (100%). 85 mo | Re-operation (33%); Lucency (27%); Non–implant-related revision (27%); Minor (20%); Major (7%); Intermediate (7%) | Total: 67%. Anterior (7%), Posterior (40%), Anterior and posterior 20%. Grade I (30%), Grade II (20%), Grade III (30%), Grade IV (20%) |
| Bianchi, 2021 [31] | Retrospective case series (2004–2009) | 34/34 (17/17; 54 ± 12 yrs) | Post-traumatic OA (73%); Primary OA (21%); RA (6%) | BOX (100%). 143 mo | Lucency (94%); Revision (59%); Re-operation (37%); Cysts (6%) | Total: 91%. Grade III-IV |
| Wan, 2018 [32] | Retrospective case series (2012–2015) | 59/59 (28/31; Mean 64 yrs) | Post-traumatic OA (85%); Degenerative OA (8%); RA (5%); Tuberculous arthritis (2%) | Salto mobile-bearing. 36 mo | Osteolysis (46%); Re-operation (12%); Revision (5%); Progressive subtalar arthritis (2%) | Total: 22%. Grade I (62%), Grade II (38%) |
| Van Es, 2022 [33] | Retrospective case series (2004–2012) | 237/254 (117/120; 59 ± 11 yrs) | Post-traumatic OA (59%); RA (25%); Idiopathic OA (9%); Hemochromatosis (2%); AVN (2%); Juvenile chronic arthritis (1%); Arthritic psoriasis (<1%); Ankylosing arthritis (<1%); Postinfectious OA (<1%); Ehlers-Danlos syndrome (<1%) | CCI evolution implant (100%). 83 mo | Re-operation (53%); Revision (22%) | Total: 13% |
| Penner, 2018 [34] | Prospective, case series (2013–2015) | 67/67 (30/37; Mean 62 yrs) | Post-traumatic OA (70%); Inflammatory OA (6%); Primary OA (16%); Secondary OA (8%) | INFINITY (100%). 35 mo | Re-operation (9%); Revision (3%) | Total: 3% |
| Rushing, 2021 [35] | Prospective case series (2016–2018) | 32/32 (14/18; Mean 61 yrs) | n.r. | CADENCE (100%). 24 mo | Lucency (41%); Minor (22%); Re-operation (19%); Major (13%); Intermediate (6%); Revision (6%) | Total: 31% All posterior |
| COMPARATIVE | ||||||
| Jung, 2015 [36] | Retrospective comparative case series (2004–2012) | 52/54 (24/28; Mean 65 yrs) | Post-traumatic OA (66%); Primary OA (26%); RA (8%) | Group (1): HINTEGRA (40%); Group (2): MOBILITY (60%); 31 mo | Group (1): Ankle impingement (38%) *; Osteolysis (19%); Neuralgia (14%); Revision (10%). Group (2): Osteolysis (12%); Neuralgia (15%); Revision (9%); Ankle impingement (9%) | Group (1): 33%; Group (2): 15%. |
| Jung, 2016 [37] | Retrospective comparative case series (2004–2012) | 52/54 (24/28; Mean 65 yrs) | Post-traumatic OA (67%); Primary OA (26%); RA (6%); Post-infection sequelae (1%) | Group (1): HINTEGRA (39%); Group (2): MOBILITY (61%). 31 mo | n.r. | Group (1): total (33%). Posterior (86%), Anterior (14%). Group (2): total (15%). Anterior (83%), Anterior–posterior (17%) |
| Rushing, 2022 [38] | Retrospective comparative case series (2015–2018) | 90/90 (48/42; Mean 60 yrs) | Post-traumatic OA (71%); Primary OA (18%); Inflammatory arthritis (3%); Recurrent instability (6%) | Group (1): INFINITY (69%); Group (2): CADENCE (31%). 24 mo | Re-operation (2%) | Group (1): total (56%). Anterior (19%), Posterior (23%), Anterior–Posterior (15%). Grade I (37%), Grade II (40%), Grade III (17%), Grade IV (6%). Group (2): total (54%). Anterior (14%), Posterior (39%). Grade I (40%), Grade II (40%), Grade III (20%) |
| Doyle, 2022 [39] | Retrospective comparative case series (2017–2020) | 71/71 (n.r.) | n.r. | Group (1): INFINITY (57%); Group (2): CADENCE (23%); Group 3): VANTAGE (20%). 20 mo | n.r. | Group (1): total (81%) *. Grade I (58%), Grade II (15%), Grade III (24%), Grade IV (3%). Group (2): total (50%). Grade I (50%), Grade II (25%), Grade III (25%). Group 3): total (57%). Grade I (100%) |
| Nunley, 2018 [40] | Prospective randomized (2011–2014) | 84/84 (n.r.; Mean 65 yrs) | n.r. | Group (1): STAR (49%); Group (2): Salto Talaris (51%). 54 mo | Group (1): Lucency/cyst (51%), Subsidence (29%), Re-operation (20%); Group (2): Lucency/cyst (23%), Subsidence (2%), Re-operation (7%) | Group (1): total (61%) **. Group (2): total (30%) |
| Togher, 2023 [41] | Retrospective comparative case series (2016–2021) | 22/22 (14/8; Mean 64 yrs) | n.r. | Group (1): Smooth-stemmed INBONE II (50%); Group (2): Fully porous-coated stemmed INBONE II (50%). 28 mo | Group (1): Revision (45%); Re-operation (9%). Group (2): Periprosthetic tibial cyst (55%); Re-operation (9%) | Group (1): total (55%). Group (2): total (46%) |
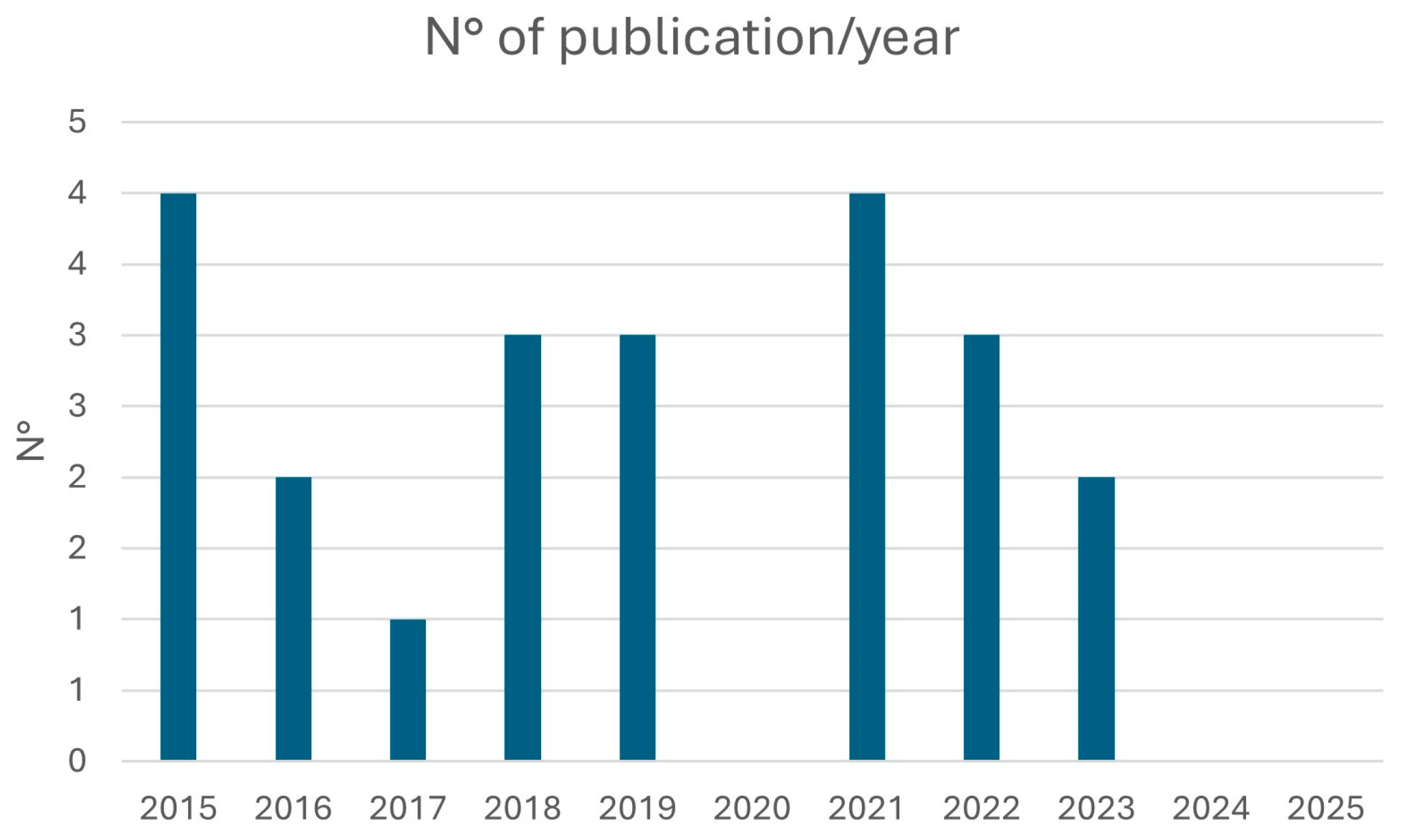
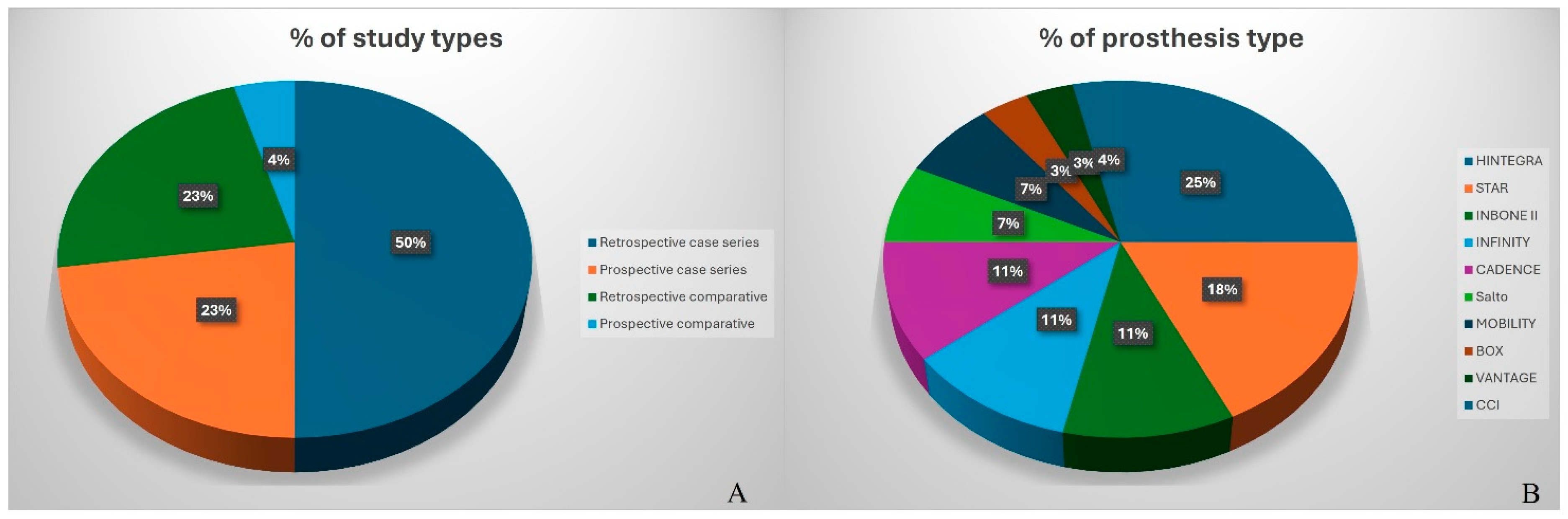
3.1. Study Characteristics
3.2. Surgical Indications
3.3. Surgical Approach
3.4. HO and Coronal Alignment or ROM
3.5. Implant Design
3.5.1. Case Series by Implant Design
3.5.2. Comparative Studies
3.6. HO Incidence and Radiographic Follow-Up
3.7. HO and Reoperation
3.8. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HO | Heterotopic ossification |
| TAA | Total ankle arthroplasty |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| ROM | Range of motion |
| mCMS | Modified Coleman Methodology Score |
| AVN | Avascular necrosis |
| CCI | Ceramic coated implant |
| F | Females |
| f-up | Follow-up |
| i.o. | Intra-operative |
| M | Males |
| mo | Months |
| n.r. | Not reported |
| OA | Osteoarthritis |
| PE | Polyethylene |
| Pts | Patients |
| RA | Rheumatoid arthritis |
| Ref. | Reference |
| yrs | Years |
References
- Giannini, S.; Romagnoli, M.; O’Connor, J.J.; Catani, F.; Nogarin, L.; Magnan, B.; Malerba, F.; Massari, L.; Guelfi, M.; Milano, L.; et al. Early Clinical Results of the BOX Ankle Replacement Are Satisfactory: A Multicenter Feasibility Study of 158 Ankles. J. Foot Ankle Surg. 2011, 50, 641–647. [Google Scholar] [CrossRef]
- Barg, A.; Wimmer, M.D.; Wiewiorski, M.; Wirtz, D.C.; Pagenstert, G.I.; Valderrabano, V. Total ankle replacement-indications, implant designs, and results. Dtsch. Arztebl. Int. 2015, 112, 177–184. [Google Scholar]
- Mosca, M.; Caravelli, S.; Vocale, E.; Maitan, N.; Grassi, A.; Massimi, S.; Fuiano, M.; Zaffagnini, S. Clinical-radiological outcomes and complications after total ankle replacement through a lateral transfibular approach: A retrospective evaluation at a midterm follow-up. Int. Orthop. 2021, 45, 437–443. [Google Scholar] [CrossRef]
- Raikin, S.M.; Sandrowski, K.; Kane, J.M.; Beck, D.; Winters, B.S. Midterm Outcome of the Agility Total Ankle Arthroplasty. Foot Ankle Int. 2017, 38, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Koivu, H.; Kohonen, I.; Mattila, K.; Loyttyniemi, E.; Tiusanen, H. Long-term results of Scandinavian Total Ankle Replacement. Foot Ankle Int. 2017, 38, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Krishnapillai, S.; Joling, B.; Sierevelt, I.N.; Kerkhoffs, G.M.M.J.; Haverkamp, D.; Hoornenborg, D. Long-term Follow-up Results of Buchel-Pappas Ankle Arthroplasty. Foot Ankle Int. 2019, 40, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Wang, S.H.; Lee, K.B. Comparison of intermediate to long-term outcomes of total ankle arthroplasty in ankles with preoperative varus, valgus, and neutral alignment. J. Bone Jt. Surg. Am. 2018, 100, 835–842. [Google Scholar] [CrossRef]
- Cody, E.A.; Scott, D.J.; Easley, M.E. Total ankle arthroplasty. A critical analysis review. JBJS Rev. 2018, 6, e8. [Google Scholar] [CrossRef]
- Malherbe, C.; Deleu, P.A.; Bevernage, B.D.; Birch, I.; Maldague, P.; Gombault, V.; Putzeys, P.; Leemrijse, T. Early-Term Results of the Cadence Total Ankle Prosthesis: A European Noninventor Study. Foot Ankle Int. 2023, 44, 1–12. [Google Scholar] [CrossRef]
- Bemenderfer, T.B.; Davis, W.H.; Anderson, R.B.; Wing, K.; Escudero, M.I.; Waly, F.; Penner, M. Heterotopic ossification in total ankle arthroplasty: Case series and systematic review. J. Foot Ankle Surg. 2020, 59, 716–721. [Google Scholar] [CrossRef]
- Seavey, J.G.; Wheatley, B.M.; Pavey, G.J.; Tomasino, A.M.; Hanson, M.A.; Sanders, E.M.; Dey, D.; Moss, K.L.; Potter, B.K.; Forsberg, J.A.; et al. Early local delivery of vancomycin suppresses ectopic bone formation in a rat model of trauma-induced heterotopic ossification. J. Orthop. Res. 2017, 35, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Loder, S.; Agarwal, S.; Wong, V.W.; Forsberg, J.; Davis, T.A.; Wang, S.; James, A.W.; Levi, B. Heterotopic Ossification: Basic-Science Principles and Clinical Correlates. J. Bone Joint Surg. Am. 2015, 97, 1101–1111. [Google Scholar] [CrossRef]
- Shehab, D.; Elgazzar, A.H.; Collier, B.D. Heterotopic ossification. J. Nucl. Med. 2002, 43, 346–353. [Google Scholar]
- Pavey, G.J.; Qureshi, A.T.; Hope, D.N.; Pavlicek, R.L.; Potter, B.K.; Forsberg, J.A.; Davis, T.A. Bioburden increases heterotopic ossification formation in an established rat model. Clin. Orthop. Relat. Res. 2015, 473, 2840–2847. [Google Scholar] [CrossRef]
- Mercurio, M.; Cofano, E.; Kennedy, J.G.; Butler, J.J.; Zanini, A.; Galasso, O.; Gasparini, G.; Marangon, A. Indications, Functional Outcomes, Return to Sport and Complications of Anterior and Lateral Approaches for Total Ankle Arthroplasty: A Comprehensive Review. Healthcare 2025, 13, 841. [Google Scholar] [CrossRef]
- Manegold, S.; Springer, A.; Landvoigt, K.; Tsitsilonis, S. Heterotopic ossification after total ankle replacement: The role of prosthesis alignment. Foot Ankle Surg. 2017, 23, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Healy, W.L. Heterotopic ossification after hip and knee arthroplasty: Risk factors, prevention, and treatment. J. Am. Acad. Orthop. Surg. 2002, 10, 409–416. [Google Scholar] [CrossRef]
- Brooker, A.F.; Bowerman, J.W.; Robinson, R.A.; Riley, L.H., Jr. Ectopic ossification following total hip replacement. Incidence and a method of classification. J. Bone Joint Surg. Am. 1973, 55, 1629–1632. [Google Scholar] [CrossRef]
- Lee, K.B.; Cho, Y.J.; Park, J.K.; Song, E.K.; Yoon, T.R.; Seon, J.K. Heterotopic ossification after primary total ankle arthroplasty. J. Bone Joint Surg. Am. 2011, 93, 751–758. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Verdonk, P.; Condello, V.; Delcogliano, M.; Dhollander, A.; Filardo, G.; Pignotti, E.; Marcacci, M. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: Systematic clinical data review and study quality analysis. Am. J. Sports Med. 2009, 37, 156S–166S. [Google Scholar] [CrossRef]
- Deleu, P.A.; Bevernage, B.D.; Gombault, V.; Maldague, P.; Leemrijse, T. Intermediate-term Results of Mobile-bearing Total Ankle Replacement. Foot Ankle Int. 2015, 36, 518–530. [Google Scholar] [CrossRef]
- Clifton, L.J.; Kingman, A.; Rushton, P.R.P.; Murty, A.; Kakwani, R.; Coorsh, J.; Townshend, D.N. The Hintegra total ankle replacement: Survivorship, failure modes and patient reported outcomes in seventy consecutive cases with a minimum five year follow-up. Int. Orthop. 2021, 45, 2331–2336. [Google Scholar] [CrossRef]
- Lee, G.W.; Seon, J.K.; Kim, N.S.; Lee, K.B. Comparison of Intermediate-Term Outcomes of Total Ankle Arthroplasty in Patients Younger and Older Than 55 Years. Foot Ankle Int. 2019, 40, 762–768. [Google Scholar] [CrossRef]
- Haytmanek, C.T.; Gross, C.; Easley, M.E.; Nunley, J.A. Radiographic Outcomes of a Mobile-Bearing Total Ankle Replacement. Foot Ankle Int. 2015, 36, 1038–1044. [Google Scholar] [CrossRef]
- Jastifer, J.R.; Coughlin, M.J. Long-term follow-up of mobile bearing total ankle arthroplasty in the United States. Foot Ankle Int. 2015, 36, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Palanca, A.; Mann, R.A.; Mann, J.A.; Haskell, A. Scandinavian Total Ankle Replacement: 15-Year Follow-up. Foot Ankle Int. 2018, 39, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, Y.R.; Kosse, N.M.; Metsaars, W.P.; Louwerens, J.W.K. Long-term Functional and Radiographic Outcome of a Mobile Bearing Ankle Prosthesis. Foot Ankle Int. 2016, 37, 1292–1302. [Google Scholar] [CrossRef]
- Jamjoom, B.A.; Siddiqui, B.M.; Salem, H.; Raglan, M.; Dhar, S. Clinical and Radiographic Outcomes of Revision Total Ankle Arthroplasty Using the INBONE II Prosthesis. J. Bone Joint Surg. Am. 2022, 104, 1554–1562. [Google Scholar] [CrossRef]
- Rushing, C.J.; Mckenna, B.J.; Zulauf, E.A.; Hyer, C.F.; Berlet, G.C. Intermediate-Term Outcomes of a Third-Generation, 2-Component Total Ankle Prosthesis. Foot Ankle Int. 2021, 42, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Martinelli, N.; Caboni, E.; Raggi, G.; Manfroni, F.; Sansone, V. Long-term follow-up of Bologna-Oxford (BOX) total ankle arthroplasty. Int. Orthop. 2021, 45, 1223–1231. [Google Scholar] [CrossRef]
- Wan, D.D.; Choi, W.J.; Shim, D.W.; Hwang, Y.; Park, Y.J.; Lee, J.W. Short-term Clinical and Radiographic Results of the Salto Mobile Total Ankle Prosthesis. Foot Ankle Int. 2018, 39, 155–165. [Google Scholar] [CrossRef]
- van Es, L.J.M.; van der Plaat, L.W.; Sierevelt, I.N.; Hoornenborg, D.; Haverkamp, D. Long-term Follow-up of 254 Ceramic Coated Implant (CCI) Evolution Total Ankle Replacements. Foot Ankle Int. 2022, 43, 1285–1294. [Google Scholar] [CrossRef]
- Penner, M.; Davis, W.H.; Wing, K.; Bemenderfer, T.; Waly, F.; Anderson, R.B. The Infinity Total Ankle System: Early Clinical Results With 2- to 4-Year Follow-up. Foot Ankle Spec. 2019, 12, 159–166. [Google Scholar] [CrossRef]
- Rushing, C.J.; Law, R.; Hyer, C.F. Early Experience With the CADENCE Total Ankle Prosthesis. J. Foot Ankle Surg. 2021, 60, 67–73. [Google Scholar] [CrossRef]
- Jung, H.G.; Shin, M.H.; Lee, S.H.; Eom, J.S.; Lee, D.O. Comparison of the outcomes between two 3-component total ankle implants. Foot Ankle Int. 2015, 36, 656–663. [Google Scholar] [CrossRef]
- Jung, H.G.; Lee, S.H.; Shin, M.H.; Lee, D.O.; Eom, J.S.; Lee, J.S. Anterior Heterotopic Ossification at the Talar Neck After Total Ankle Arthroplasty. Foot Ankle Int. 2016, 37, 703–708. [Google Scholar] [CrossRef]
- Rushing, C.J.; Steriovski, J.; Hyer, C.F.; Berlet, G.C. Heterotopic Ossification Following Total Ankle Arthroplasty With Fourth-Generation Prostheses. Foot Ankle Spec. 2022, 15, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.D.; Mitchell, L.H.; Ishibashi, M.A.; Castellucci-Garza, F.M.; Sherick, R.M.; Rao, N.M. Radiographic Comparison of Heterotopic Ossification After Primary Total Ankle Arthroplasty in Fourth-Generation Implants. Foot Ankle Spec. 2023, 16, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Nunley, J.A.; Adams, S.B.; Easley, M.E.; DeOrio, J.K. Prospective Randomized Trial Comparing Mobile-Bearing and Fixed-Bearing Total Ankle Replacement. Foot Ankle Int. 2019, 40, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Togher, C.J.; Thompson, J.M.; Perkins, J.M.; Berlet, G.C.; Hyer, C.F. A Study of Tibial Cyst Formation in Modular Stemmed Total Ankle Arthroplasty: Exploring a Possible Relationship to Smooth and Porous Coating on the Stem Segments. J. Foot Ankle Surg. 2023, 62, 756–763. [Google Scholar] [CrossRef]
- Longo, U.G.; Papalia, R.; Bonifacini, C.; Martinelli, N.; Candela, V.; Ruzzini, L.; De Salvatore, S.; Piergentili, I.; Denaro, V. Emerging National Trends in Ankle Prosthesis: A 15-Year Analysis of the Italian National Hospital Discharge Records. J. Foot Ankle Surg. 2022, 61, 695–699. [Google Scholar] [CrossRef]
- Karzon, A.L.; Bariteau, J.T.; Coleman, M.M.; Kadakia, R.J.; Jacobson, J.E.; Labib, S.A. The Fall and Rise of Total Ankle Arthroplasty: A Database Analysis of Procedural Incidence Between 2009 and 2019. Foot Ankle Orthop. 2022, 7, 2473011421S00719. [Google Scholar] [CrossRef]
- Karzon, A.L.; Kadakia, R.J.; Coleman, M.M.; Bariteau, J.T.; Labib, S.A. The Rise of Total Ankle Arthroplasty Use: A Database Analysis Describing Case Volumes and Incidence Trends in the United States Between 2009 and 2019. Foot Ankle Int. 2022, 43, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Raikin, S.M.; Rasouli, M.R.; Espandar, R.; Maltenfort, M.G. Trends in treatment of advanced ankle arthropathy by total ankle replacement or ankle fusion. Foot Ankle Int. 2014, 35, 216–224. [Google Scholar] [CrossRef]
- Coester, L.M.; Saltzman, C.L.; Leupold, J.; Pontarelli, W. Long term results following ankle arthrodesis for post-traumatic arthritis. J. Bone Jt. Surg. Am. 2001, 82, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Herman, Z.J.; Edelman, D.G.; Ilyas, A.M. Heterotopic Ossification After Elbow Fractures. Orthopedics 2021, 44, 10–16. [Google Scholar] [CrossRef]
- Liu, E.Y.; Hildebrand, A.; Horner, N.S.; Athwal, G.S.; Khan, M.; Alolabi, B. Heterotopic ossification after total elbow arthroplasty: A systematic review. J. Shoulder Elbow Surg. 2019, 28, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, R.; Tracey, O.C.; Tahvilian, S.; Baksh, N.; Zikria, B.; Naziri, Q. Incidence of heterotopic ossification following total hip arthroplasty by approach: A systematic review. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 2089–2098. [Google Scholar] [CrossRef]
- Bernasconi, A.; Izzo, A.; Sgadari, A.; D’Agostino, M.; Mariconda, M.; Goldberg, A.J. Median age of patients undergoing total ankle replacement has not significantly changed between 1999 and 2023: A systematic review of prospective studies. Foot Ankle Surg. 2025, 31, 3–9. [Google Scholar] [CrossRef]
- Butler, J.J.; Healy, H.; Anil, U.; Habibi, A.; Azam, M.T.; Walls, R.J.; Kennedy, J.G. The significance of heterotopic ossification following total ankle arthroplasty: A systematic review and meta-analysis. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 1945–1956. [Google Scholar] [CrossRef]
- Cenni, F.; Leardini, A.; Cheli, A.; Catani, F.; Belvedere, C.; Romagnoli, M.; Giannini, S. Position of the prosthesis components in total ankle replacement and the effect on motion at the replaced joint. Int. Orthop. 2012, 36, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Braito, M.; Dammerer, D.; Reinthaler, A.; Kaufmann, G.; Huber, D.; Biedermann, R. Effect of Coronal and Sagittal Alignment on Outcome After Mobile-Bearing Total Ankle Replacement. Foot Ankle Int. 2015, 36, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.T.; Singh, J.A. Biomarkers in arthroplasty: A systematic review. Open Orthop. J. 2011, 5, 92–105. [Google Scholar] [CrossRef] [PubMed]
| Class | Criteria |
|---|---|
| 0 | No heterotopic ossification |
| I | Islands of bone within the soft tissue about the ankle |
| II | Bone spurs from the tibia or talus, reducing the posterior joint space by <50% |
| III | Bone spurs from the tibia or talus, reducing the posterior joint space by ≥50% |
| IV | Bridging bone continuous between the tibia and the talus |
| Study | Alignment | Post-op ROM | Comment |
|---|---|---|---|
| Lee, 2018 [7] | Varus: dorsiflexion 9° → 10°, plantarflexion 22° → 26° Valgus: dorsiflexion 9° → 11°, plantarflexion 20° → 25° Neutral: dorsiflexion 8° → 10°, plantarflexion 20° → 27° | ROM improvements observed across all groups, with no statistically significant differences | Coronal plane alignment does not appear to significantly influence ROM |
| Clifton, 2021 [23] | Valgus: ROM 17° pre-op → 8° post-opVarus: ROM 11° pre-op → 6° post-op | Decreased ROM postoperatively, but more neutral component positioning | Slight trend toward improved outcomes with more neutral alignment; weak correlation with ROM |
| Wan, 2018 [32] | Not specified | ROM improved (from 33° to 40°) | No association found between ROM and alignment |
| Bianchi, 2021 [31] | α, β, γ angles remained stable over time | ROM remained nearly unchanged | No significant correlation with implant orientation |
| Jung, 2015/2016 [36,37] | Some implants in varus; MOBILITY group in neutral alignment | ROM improvements noted particularly in MOBILITY-neutral group | Tendency toward better ROM with neutral alignment |
| Manegold, 2017 [12] | Varus alignment (>92°) | Not directly assessed for ROM | Weak correlation between varus alignment and heterotopic ossification (HO) in anterior/lateral recesses; excessive HO may contribute to joint stiffness |
| Downs and Black Checklist | Modified Coleman Methodology Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Article | Reporting | External Validity | Internal Validity Bias | Internal Validity Confounding | Power | Total Score | Part A | Part B | Total Score |
| Deleu, 2015 [22] | 8 | 3 | 3 | 2 | 0 | 16 | 40 | 30 | 10 |
| Manegold, 2017 [12] | 6 | 2 | 3 | 2 | 0 | 13 | 35 | 25 | 10 |
| Clifton, 2021 [23] | 6 | 2 | 2 | 3 | 0 | 13 | 40 | 30 | 10 |
| Lee, 2018 [7] | 9 | 3 | 4 | 3 | 0 | 19 | 54 | 44 | 10 |
| Lee, 2019 [24] | 8 | 3 | 3 | 2 | 0 | 16 | 54 | 44 | 10 |
| Haytmanek, 2015 [25] | 8 | 3 | 3 | 3 | 1 | 18 | 37 | 27 | 10 |
| Jastifer, 2015 [26] | 6 | 3 | 3 | 3 | 0 | 15 | 24 | 14 | 10 |
| Palanca, 2018 [27] | 6 | 3 | 3 | 3 | 0 | 15 | 28 | 18 | 10 |
| Kerkhoff, 2016 [28] | 9 | 3 | 3 | 3 | 0 | 18 | 60 | 50 | 10 |
| Jamjoom, 2022 [29] | 7 | 3 | 2 | 2 | 1 | 15 | 28 | 18 | 10 |
| Rushing, 2021 [30] | 8 | 2 | 3 | 2 | 0 | 15 | 29 | 19 | 10 |
| Bianchi, 2021 [31] | 7 | 3 | 3 | 3 | 0 | 16 | 28 | 18 | 10 |
| Wan, 2018 [32] | 8 | 2 | 3 | 3 | 0 | 16 | 29 | 19 | 10 |
| Van Es, 2022 [33] | 9 | 3 | 3 | 3 | 0 | 18 | 39 | 29 | 10 |
| Penner, 2019 [34] | 9 | 3 | 3 | 3 | 0 | 18 | 44 | 34 | 10 |
| Rushing, 2021 [35] | 7 | 2 | 3 | 2 | 0 | 14 | 38 | 28 | 10 |
| Jung, 2015 [36] | 7 | 3 | 3 | 2 | 0 | 15 | 42 | 32 | 10 |
| Jung, 2016 [37] | 8 | 3 | 3 | 3 | 0 | 17 | 44 | 34 | 10 |
| Rushing, 2022 [38] | 7 | 2 | 3 | 2 | 0 | 14 | 32 | 22 | 10 |
| Doyle, 2023 [39] | 5 | 3 | 2 | 2 | 0 | 12 | 50 | 40 | 10 |
| Nunley, 2019 [40] | 7 | 3 | 3 | 5 | 0 | 18 | 29 | 19 | 10 |
| Togher, 2023 [41] | 7 | 2 | 2 | 3 | 1 | 15 | 40 | 30 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielli, S.O.; Veronesi, F.; Sacchi, G.; Mazzotti, A.; Faldini, C.; Giavaresi, G. Diagnostic Imaging and Clinical Implications of Heterotopic Ossification After Total Ankle Arthroplasty: A Systematic Review for Surgical Strategy. Diagnostics 2025, 15, 2203. https://doi.org/10.3390/diagnostics15172203
Zielli SO, Veronesi F, Sacchi G, Mazzotti A, Faldini C, Giavaresi G. Diagnostic Imaging and Clinical Implications of Heterotopic Ossification After Total Ankle Arthroplasty: A Systematic Review for Surgical Strategy. Diagnostics. 2025; 15(17):2203. https://doi.org/10.3390/diagnostics15172203
Chicago/Turabian StyleZielli, Simone Ottavio, Francesca Veronesi, Giulia Sacchi, Antonio Mazzotti, Cesare Faldini, and Gianluca Giavaresi. 2025. "Diagnostic Imaging and Clinical Implications of Heterotopic Ossification After Total Ankle Arthroplasty: A Systematic Review for Surgical Strategy" Diagnostics 15, no. 17: 2203. https://doi.org/10.3390/diagnostics15172203
APA StyleZielli, S. O., Veronesi, F., Sacchi, G., Mazzotti, A., Faldini, C., & Giavaresi, G. (2025). Diagnostic Imaging and Clinical Implications of Heterotopic Ossification After Total Ankle Arthroplasty: A Systematic Review for Surgical Strategy. Diagnostics, 15(17), 2203. https://doi.org/10.3390/diagnostics15172203









