Abstract
Background/Objectives: Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease with limited epidemiological data from Central–Eastern Europe. This study characterized a Hungarian PSC cohort, comparing patients with and without inflammatory bowel disease (IBD), and longitudinally evaluated the predictive efficacy of established prognostic scores (Mayo Risk Score, Amsterdam-Oxford Model [AOM], UK-PSC short/long). Methods: Data from 135 PSC patients (median diagnosis age 31 years, 57.7% male) were collected yearly at two Hungarian centers, with a median follow-up of 8.8 years. Outcomes included liver transplantation (LT) and liver-related death. Prognostic value of baseline clinical scores was assessed for 2-, 5-, 8-, and 10-year composite outcome. Results: PSC-IBD patients (54.1%) were younger with higher baseline Mayo and AOM scores, and had increased rates of colorectal carcinoma (8.22% vs. 0.00%) and liver transplantation (26.03% vs. 9.68%) within 10 years than PSC-only patients. There were no differences in liver-related mortality or composite outcomes between the groups. All prognostic scores showed good short-term predictive ability for poor outcomes (AUROC at 2 years: 0.858–0.958), which diminished over time (AUROC at 10 years: 0.708–0.756). The AOM demonstrated the most consistent performance. Persistent alkaline phosphatase (ALP) elevation (≥2.2×ULN) 2 years post-diagnosis, despite ursodeoxycholic acid therapy, strongly predicted 10-year adverse outcomes (HR: 3.927, p < 0.001), outperforming formal scoring systems (HR: 2.688–1.522). Conclusions: While PSC-IBD patients had more CRC and liver transplantation, overall transplantation-free survival was similar to PSC-only patients. Prognostic utility of current scores declines with longer follow-up; AOM was most stable. Sustained ALP elevation is a robust long-term prognostic indicator.
1. Introduction
Primary sclerosing cholangitis (PSC) is a rare, chronic cholestatic liver disease characterized by progressive inflammation and fibrotic obliteration of the intra- and/or extrahepatic bile ducts. Over time, this destructive cholangiopathy leads to biliary cirrhosis, portal hypertension, and ultimately liver failure in many patients [1,2]. PSC is also strongly associated with an increased risk of hepatobiliary and colorectal malignancies, particularly cholangiocarcinoma (CCA) and colorectal cancer (CRC), the latter primarily among those with concomitant inflammatory bowel disease (IBD) [3,4]. Despite its rarity, PSC remains a leading indication for liver transplantation in Europe and North America [5,6,7]. The epidemiology of PSC varies geographically. The highest reported incidence rates (1.0–1.5 per 100,000 person-years) are seen in Northern Europe and North America [4,8,9] while Southern and Central–Eastern European countries report lower incidence and prevalence figures [10,11,12,13]. However, these findings are primarily based on selected patient cohorts from Poland and Austria, rather than population-based epidemiological studies. The current literature is heavily weighted toward populations in Northwestern Europe and the United States, with a notable scarcity of epidemiological and clinical data from Central–Eastern Europe. Given the potential for regional variation in disease expression, comorbidities, and outcomes, additional studies in underrepresented populations are essential.
Risk stratification is also crucial in PSC to identify high-risk patients and guide surveillance, therapy, and transplantation decisions. However, there is currently no universally accepted method for estimating prognosis at the time of PSC diagnosis. The EASL Clinical Practice Guidelines (CPG) outline favorable and unfavorable phenotypic, laboratory, and imaging features that may assist non-invasive risk stratification, although their predictive value varies [8]. Trivedi et al. proposed a risk classification framework based on clinical symptoms, biochemical parameters, liver stiffness measurements, and biliary abnormalities on MRI/MRCP, enabling early identification of patients at significant risk for hepatic decompensation, liver transplantation, or liver-related death [14,15].
Alkaline phosphatase (ALP), due to its accessibility and reproducibility, has been extensively studied as a prognostic biomarker [16,17,18]. Although persistently elevated ALP levels are associated with poorer outcomes, the enzyme’s natural variability and inconsistent cut-off thresholds across studies limit its standalone utility. Consequently, current guidelines do not support ALP as a singular prognostic tool [8].
Historically, risk stratification in PSC has relied on general liver disease models such as the Child–Pugh score and the Model for End-Stage Liver Disease (MELD), both of which are limited by their focus on end-stage liver dysfunction and poor performance in early disease stages.
To meet this clinical need, several PSC-specific prognostic models have been developed in the last three decades (Wiesner-1989 [19], Farrant-1991 [20], Broome-1996 [21], revised Mayo Risk Score-2000 [22], Boberg-2002 [23], Ponsioen-2002 [24], Tischendorf-2007 [25]).
The Revised Mayo Risk Score (rMRS) was designed to predict short-term mortality using simple clinical and biochemical parameters. More recently, the Amsterdam-Oxford Model (AOM) [26], the UK-PSC risk scores (for short-term and long-term) [27], and the PSC Risk Estimate Tool (PREsTo) [28] have expanded the range of predictors and time horizons covered. Prognostic systems fundamentally aim to estimate transplant-free survival—except for PREsTo that predicts decompensation—but the clinical and laboratory parameters used show some differences (Table 1). Both the UK-PSC and AOM have proven to be reliable [29,30], and were also shown to be prognostic in recurrent cases [31]. However, only a limited number of comparative studies have been conducted.

Table 1.
PSC-specific prognostic scoring systems contain similar parameters.
This study provides epidemiological data from the Central–Eastern European region, while also evaluating and comparing the performance of major PSC-specific risk scores over time, and explores the prognostic utility of ALP in a Hungarian bicenter cohort.
2. Patients and Methods
2.1. Patient Population
The study was conducted retrospectively in two Hungarian tertiary gastroenterology centers of University of Debrecen and University of Pécs in accordance with the Declaration of Helsinki and approved by the Regional and Institutional Research Ethics Committees of University of Debrecen and University of Pécs (12759-6/2019/EÜIG, 9335-PTE2022—9 October 2022).
The diagnosis of PSC was established by MRCP in cases of elevated cholestatic enzymes, in accordance with the EASL recommendation [8]. In the absence of radiological abnormalities and suspected small bile duct disease, histological sampling was performed to establish the diagnosis. The diagnosis of autoimmune hepatitis (AIH) variant syndrome was made in patients meeting the diagnostic criteria for both PSC and AIH [32]. Diagnosis of coexisting IBD is based on clinical, endoscopic and histological findings as recommended by the ECCO-ESGAR guideline [33]. Laboratory results were always compared to the reference value of the center at the time. The clinical, radiological or endoscopic appearance of varices, ascites or hepatic encephalopathy was evaluated as decompensated cirrhosis.
2.2. Study Design
Between 1 January 2001, and 30 April 2019, a total of 135 patients were diagnosed with PSC at the two participating centers and included in the study. The follow-up period for the study concluded on 31 December 2023. The recording of patients’ basic demographic data and laboratory and imaging results started at the time of diagnosis and continued until liver transplantation, death, or the end of follow-up. The date of diagnosis was considered to be the date of MRCP or histological findings showing PSC-specific pathological abnormalities. The primary endpoint was time to liver-related death or liver transplantation. Demographic data included age, sex, height, and weight. Coexisting IBD and its subtype, AIH variant syndrome, and comorbidities were also determined. Biochemical, serological, imaging (ultrasound, CT, MRCP, ERCP, transient liver elastography) and histological results were collected. ALP cut-offs were defined based on the study of Goode et al. [27]. Regular outpatient visits were performed on an annual basis, with an increased frequency in case of progression and therapy adjustment. Laboratory tests were performed at least once a year. Data on IBD, presence, development or decompensation of cirrhosis (ascites, hepatic encephalopathy, variceal bleeding), and presence or development of various malignancies were collected during follow-up along with data on medical treatment, transplantation, and mortality. The rMRS, the MELD score, the UK-PSC score, AOM, and the PREsTo were determined at diagnosis and annually during follow-up. Considering the prognostic periods covered by risk stratification systems, in our study, we compared the systems based on the cohort results at follow-up intervals of 2, 5, 8, and 10 years.
2.3. Statistical Methods
Continuous patient characteristics were summarized as median and interquartile range (IQR). For categorical variables, frequencies were examined and presented as n (%). Variables were tested for normality using Shapiro–Wilk W test. Mann–Whitney U test was used to compare continuous variable levels between 2 groups. Kruskal–Wallis test or one-way ANOVA with a post hoc test for multiple comparisons was used to compare 3 groups as appropriate according to the distribution of the variables. The efficacy of different parameters to discriminate between outcomes was estimated by the ROC curve analysis. AUROC with corresponding 95% confidence intervals (CIs) and p values were calculated. The association between clinical variables and outcomes during follow-up was assessed with univariable Cox regression. Associations are provided as HR with 95% CIs. A 2-sided p value < 0.05 was considered statistically significant. For statistical analyses and graphical presentation, SPSS 29.0 (IBM, Armonk, NY, USA) and GraphPad Prism 10.4.1 (GraphPad Software, Boston, MA, USA) programs were used.
3. Results
3.1. Clinical Characteristics
The cohort included 135 patients (57.7% male), with a median age of 31 years at diagnosis (IQR: 20–47). The median follow-up duration was 105 months (IQR: 61–162). A total of 92 patients were diagnosed with large bile duct disease, 13 with small bile duct disease, and 30 with both large and small bile duct involvement. Coexisting IBD was present in 54.1% (43% ulcerative colitis, 10.4% Crohn’s disease, 0.7% unclassified), of which 80.8% were diagnosed prior to the PSC diagnosis. PSC-AIH overlap was present in 12.5%. At diagnosis, 17.7% were asymptomatic; others presented with abdominal pain (20.7%), pruritus (13.3%), weight loss (8.9%), and fatigue (8.1%). Ursodeoxycholic acid (UDCA) therapy was initiated in 85% and increased to 95.5% during follow-up. Patients’ clinical characteristics and admission laboratory results and scores are summarized in Table 2 and Table 3.

Table 2.
Baseline characteristics of the entire cohort.

Table 3.
Laboratory parameters and prognostic scores in PSC-only and PSC-IBD patients.
3.2. Follow-Up Outcomes
The median time to study endpoints (death, liver transplant, or end of the study period) was 105 months. Liver cirrhosis was present at baseline in 14.1% and developed in 22.9% during follow-up. Hepatic decompensation occurred in 32 patients (23.7%). During follow-up, 20 patients (14.8%) experienced bacterial infections requiring hospitalization. Osteoporosis was diagnosed in 12 patients (8.9%). A spectrum of malignancies was also observed, including cholangiocarcinoma in 6 patients (4.4%), ampullary (Vater’s papilla) carcinoma in 2, colorectal carcinoma in 6 (4.4%), and hepatocellular carcinoma (HCC) in 3 (2.2%). Additionally, isolated cases of various extrahepatic malignancies were identified, including endometrial adenocarcinoma, prostate cancer, adrenocortical carcinoma, papillary thyroid carcinoma, minimally invasive lung adenocarcinoma, ovarian carcinoma, and glioblastoma.
A total of 25 patients (18.5%) underwent liver transplantation, with 24 cases attributed to end-stage liver disease and 1 to hepatocellular carcinoma. Overall, 21 deaths (15.5%) were recorded during the study period. Of these, 15 (71.4%) were directly related to progression of hepatobiliary disease, including 3 associated with hepatobiliary malignancies. A total of 3 patients died due to colorectal cancer, while the remaining 3 deaths were attributed to unrelated causes. Additionally, 3 post-transplant deaths occurred, all due to sepsis.
At diagnosis, relatively little difference was observed between the PSC-only and PSC-IBD groups. Members of the PSC-IBD group were younger (26 yrs [IQR: 16–26] vs. 40 yrs [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]) and had significantly lower Mayo Risk Score (−1.82 [−2.54–−1.82] vs. −2.07 [−2.8–−2.07], p = 0.0187) and Amsterdam-Oxford Model values (1.13 [0.57–1.13] vs. 0.92 [0.13–0.92], p = 0.0151). However, there was no significant difference in composite outcome between the two groups (p = 0.3331). Outcome events in PSC-only and PSC-IBD groups are summarized in Table 4.

Table 4.
Primary outcomes in PSC-only and PSC-IBD groups.
We next evaluated the prognostic value of ALP levels measured at diagnosis, and at 1-year and 2-year follow-up visits, in relation to the 10-year composite endpoint. Despite the known intra-individual variability of ALP, ROC curve analysis demonstrated statistically significant associations at all time points (Figure 1). Notably, ALP measured at the 2-year mark—both as an absolute value and when normalized to the upper limit of normal (ULN)—consistently yielded the highest AUROC values, outperforming earlier measurements.
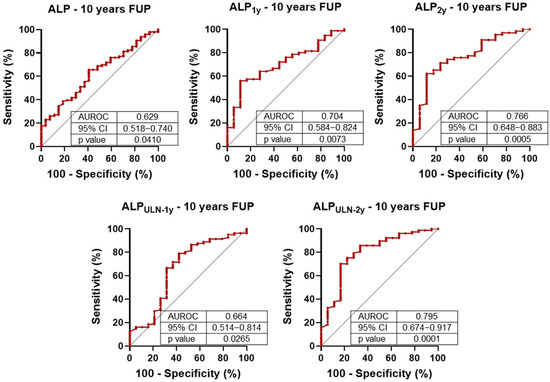
Figure 1.
Receiver operating characteristic (ROC) analysis of alkaline phosphatase (ALP) levels for 10-year composite outcome (liver-related death and liver transplantation). ALP concentrations were assessed at baseline, 1 year (1y), and 2 years (2y). The discriminatory performance of ALP at 1 and 2 years was also evaluated relative to the upper limit of normal (ULN). AUROC: area under the ROC curve; CI: confidence interval.
This trend was corroborated by univariate Cox regression analysis (Table 5): while ALP at diagnosis and at 1 year did not reach statistical significance, 2-year ALP levels were significantly associated with 10-year outcomes. Specifically, ALP levels exceeding 2.4 times the ULN at 1 year and 2.2 times the ULN at 2 years were associated with marked increases in hazard ratios, indicating improved predictive discrimination when using these cut-offs [27].

Table 5.
Univariate Cox proportional hazard regression analysis of 10-year composite endpoint.
Next, we longitudinally analyzed the prognostic value of currently used risk assessment systems (rMRS, AOM, UK-short, and UK-long) for 2-, 5-, 8-, and 10-year composite outcome. The results of the ROC analysis are presented in Figure 2. All scores demonstrated good AUROC values (>0.7) in all examined years. The highest result was observed in every case in the 2nd year, followed by declining AUROC values over time.
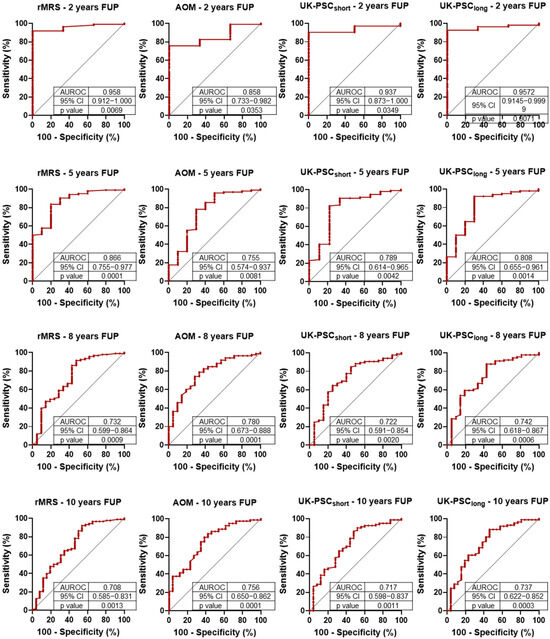
Figure 2.
Receiver operating characteristic (ROC) analysis of primary sclerosing cholangitis (PSC)-related prognostic scores for 2-, 5-, 8- and 10-year composite outcomes (liver-related death and liver transplantation). rMRS: Revised Mayo Risk Score; AOM: Amsterdam-Oxford Model; FUP: follow-up; AUROC: area under the ROC curve; CI: confidence interval.
The Cox proportional hazard regression (Table 6) provided similar results to the ROC analyses. All risk assessment scoring systems predicted the composite outcome for every investigated year. The greatest HR values were observed in the 2nd year, followed by declining trends during further follow-up. The UK-PSC-short score demonstrated the poorest predictive power while the AOM showed the most consistent results over time.

Table 6.
Univariate Cox proportional hazard regression analysis of liver transplantation or death.
4. Discussion
Over the past several decades, numerous population-based studies have characterized the epidemiology and natural history of PSC in North America and Northwestern Europe [21,34,35,36,37,38,39,40,41,42,43]. In contrast, data from other global regions—including Central and Eastern Europe—remain limited. Our study addresses this regional data gap by providing the first population-based clinical and prognostic insights into PSC from a Hungarian cohort. Among Central European countries, most previously available epidemiologic insights originate from Austria and Poland, with findings largely consistent across studies regarding sex distribution, mean age at diagnosis, and the prevalence of IBD and cirrhosis [10,11,12,44]. However, compared to certain Polish cohorts, our study observed a lower prevalence of IBD and fewer patients undergoing liver transplantation [10,11,44]. These discrepancies may stem from differences in study design and selection criteria, as prior investigations were not explicitly epidemiological in nature. In our cohort, 54.1% of patients had concurrent IBD, a lower rate than the >60% typically reported in Western European cohorts [45]. Nonetheless, IBD preceded the diagnosis of PSC in over 80% of these patients, consistent with existing literature [6]. The higher transplantation rate in PSC-IBD patients may reflect earlier recognition and/or more proactive management rather than intrinsically worse liver disease, as cirrhosis development rates were similar between groups. While a few studies reported worse outcomes in patients with PSC-IBD [46] or specifically PSC-UC [47], most studies demonstrate comparable outcome rates regardless of IBD status [48,49,50]. Consistently, our study found similar composite outcome rates between groups, supporting the role of timely transplantation.
Cholangiocarcinoma (CCA) was identified in 4.4% of patients, which is at the lower end of the 8–13% range reported in large cohorts. However, this rate aligns with findings from several European studies reporting similarly low CCA incidences, such as those from Austria (3.3%), France (4.0%), Italy (2.2%), and Finland (4.5%) [12,36,51,52]. In contrast, studies from other Western European and Scandinavian countries tend to report higher CCA rates among patients with PSC. Notably, a large international study involving 37 centers across 17 countries found a CCA incidence of only 7.48%, further supporting the presence of regional variation [47]. This geographic discrepancy suggests that underlying genetic, environmental, lifestyle, or healthcare system-related factors may influence the detected incidence of CCA in PSC patients. Further studies are warranted to clarify the determinants of these regional differences, including the potential impact of varying screening practices, diagnostic approaches, and biological predispositions. There were no significant differences in CCA occurrence between PSC-only and PSC-IBD patients. However, colorectal cancer occurred exclusively in the PSC-IBD group, reinforcing its strong association with long-standing colonic inflammation in this population [53]. PSC-AIH overlap syndrome occurs in 7-14% of cases [8], which is in line with our data (12.5%). Only 2 (11%) of the PSC-AIH patients were confirmed to have small duct PSC, which is significantly lower than expected [8].
ALP, despite its high variability, is well-suited for prognostic purposes when interpreted in the appropriate context. In addition to the development of the UK-PSC scoring system, Goode and colleagues also formulated the independent predictive function of ALP in their study. Improved transplant-free survival results were found, when ALP decreased to <2.4 × ULN and <2.2 × ULN at 1 and 2 years following diagnosis, respectively [27]. Our study validates these findings, demonstrating that persistent ALP elevation despite UDCA treatment —considering these cut-off values—can serve as a valuable marker for transplantation and mortality risk assessment tools. Moreover, in our cohort, this single biomarker outperformed composite scores in predicting 10-year composite outcome. Notably, new therapies are being investigated for reducing ALP levels in PSC including fenofibrate [54], elafibranor (a dual peroxisome proliferator-activated receptor-α/δ agonist) [55], and cenicriviroc (a dual antagonist of C-C chemokine receptor types 2 and 5) [56]; however their long-term impact on survival still needs to be evaluated.
To date, few studies have compared PSC-specific prognostic scoring systems directly within a single cohort. A major strength of our study is the head-to-head, longitudinal evaluation of the most widely used models: the revised rMRS, the AOM, and the UK-PSC score. This allowed us to assess and contrast their short- and long-term predictive capacities over time. Validation studies of the newer prognostic scoring systems showed excellent predictive value for the UK-PSC score (AUROC: 0.81 and 0.80 for short- and long-term, respectively), while the Amsterdam-Oxford Model (AUROC: 0.67 (95% CI 0.64–0.70) also demonstrated reliable performance [27,28,57,58]. In a study investigating soluble macrophage markers, the rMRS and the AOM performed similarly for predicting 8-year transplant-free survival [59]. In our study, the rMRS had the highest early AUROC but declined more sharply over time. The AOM model showed the most stable performance and may be particularly well-suited for long-term stratification. In our cohort, the UK-PSC short-term risk score demonstrated lower predictive performance compared to other prognostic models and to its originally published validation metrics. A decline in the accuracy of all scoring systems over extended follow-up was also observed. This temporal decrease in prognostic discrimination is likely multifactorial. In early disease stages—characterized by biochemical fluctuations and heterogeneous progression—it remains challenging to reliably estimate disease trajectory, even with sophisticated composite scoring systems. In contrast, once advanced liver disease manifests, clinical endpoints become more predictable and more easily captured by risk algorithms. These findings underscore the limitations of current models in forecasting long-term outcomes from early disease data and highlight the need for dynamic or longitudinally adaptive scoring approaches. Our results confirm the conclusion already suggested by the literature: despite their differences, all three scoring systems (RMS, UK-PSC, AOM) can be reliably used for risk assessment. In the ROC analysis, we did not observe significant differences between them; however, the similarity of the results may be influenced by the sample size of our cohort, which limits the granularity of the assessment.
In summary, our study provides data on the clinical profile and prognosis of PSC in Hungary addressing a key regional data gap in Central–Eastern Europe, and offers the first direct, longitudinal comparison of major PSC-specific prognostic models within the same cohort. It also confirms the utility of ALP, particularly persistent elevation above 2.2 × ULN, as a robust predictor of long-term outcomes. Despite its strengths, our study has some limitations including the retrospective design and the relatively small sample size. However, the population-based nature of the data might mitigate these limitations and enhance its real-world relevance.
5. Conclusions
The 10-year liver-related mortality or composite adverse outcomes of PSC were found to be independent of coexisting IBD, despite the higher number of transplanted patients in the PSC-IBD group in our large Hungarian cohort. However, colorectal carcinoma occurred exclusively in the IBD group. Our findings demonstrate that the prognostic accuracy of established PSC-specific scoring systems, including the rMRS, AOM, and UK-PSC models, diminishes over extended follow-up periods. Although the overall performance of these scores was comparable, the UK-PSC short model exhibited the lowest prognostic ability, whereas the AOM provided the most consistent predictive performance over time. Importantly, a persistent elevation in serum ALP levels two years after diagnosis, despite UDCA therapy, emerged as a more robust predictor of poor 10-year composite outcomes (liver transplantation or liver-related death) than any of the evaluated formal prognostic scores. These results underscore the importance of monitoring dynamic markers like ALP for long-term risk stratification in PSC patients and highlight the need for further improvement in current PSC-specific prognostic tools.
Author Contributions
Conceptualization, M.P. and D.T.; methodology, D.T.; formal analysis, D.T.; investigation, P.L.V., D.T., B.T., Z.V., I.T., T.T., G.P. and M.P.; data curation, P.L.V. and D.T.; writing—original draft preparation, P.L.V. and D.T.; writing—review and editing, M.P. and D.T.; supervision, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, No. 138041. DT was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the University Research Fellowship Program (EKÖP-24) of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund through the University of Debrecen.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional and Institutional Research Ethics Committees of University of Debrecen and University of Pécs (12759-6/2019/EÜIG, 9335-PTE2022—9 October 2022).
Informed Consent Statement
Patient consent was waived due to the retrospective, non-interventional design of the study, which involved only analysis of existing data presented in anonymized, aggregate format.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data is not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AIH | Autoimmune hepatitis |
| ALP | Alkaline phosphatase |
| AOM | Amsterdam-Oxford Model |
| AST | Aspartate aminotransferase |
| AUROC | Area under the receiver operating characteristic curve |
| CCA | Cholangiocarcinoma |
| CI | Confidence interval |
| CRC | Colorectal cancer |
| CT | Computed tomography |
| EASL | European Association for the Study of the Liver |
| CPG | Clinical Practice Guideline |
| ECCO | European Crohn’s and Colitis Organisation |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| ESGAR | European Society of Gastrointestinal and Abdominal Radiology |
| FUP | Follow-up |
| Hb | Hemoglobin |
| HCC | Hepatocellular carcinoma |
| HR | Hazard ratio |
| INR | International normalized ratio |
| IQR | Interquartile range |
| IBD | Inflammatory bowel disease |
| LT | Liver transplantation |
| MELD | Model for End-Stage Liver Disease |
| MRI | Magnetic resonance imaging |
| MRCP | Magnetic resonance cholangiopancreatography |
| PREsTo | PSC Risk Estimate Tool |
| PSC | Primary sclerosing cholangitis |
| ROC | Receiver operating characteristic |
| rMRS | Revised Mayo Risk Score |
| UDCA | Ursodeoxycholic acid |
| ULN | Upper limit of normal |
References
- Chapman, R.W.; Arborgh, B.A.; Rhodes, J.M.; Summerfield, J.A.; Dick, R.; Scheuer, P.J.; Sherlock, S. Primary Sclerosing Cholangitis: A Review of Its Clinical Features, Cholangiography, and Hepatic Histology. Gut 1980, 21, 870–877. [Google Scholar] [CrossRef]
- Ueno, Y.; LaRusso, N.F. Primary Sclerosing Cholangitis. J. Gastroenterol. 1994, 29, 531–543. [Google Scholar] [CrossRef]
- Barberio, B.; Massimi, D.; Cazzagon, N.; Zingone, F.; Ford, A.C.; Savarino, E.V. Prevalence of Primary Sclerosing Cholangitis in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2021, 161, 1865–1877. [Google Scholar] [CrossRef]
- Mehta, T.I.; Weissman, S.; Fung, B.M.; Sotiriadis, J.; Lindor, K.D.; Tabibian, J.H. Global Incidence, Prevalence and Features of Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis. Liver Int. 2021, 41, 2418–2426. [Google Scholar] [CrossRef]
- Bjøro, K.; Brandsærter, B.; Foss, A.; Schrumpf, E. Liver Transplantation in Primary Sclerosing Cholangitis. Semin. Liver Dis. 2006, 26, 69–79. [Google Scholar] [CrossRef]
- Tabibian, J.H.; Bowlus, C.L. Primary Sclerosing Cholangitis: A Review and Update. Liver Res. 2017, 1, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.H.; Boberg, K.M. Update on Primary Sclerosing Cholangitis. J. Hepatol. 2013, 59, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Chazouilleres, O.; Beuers, U.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Samyn, M.; Schramm, C.; Trauner, M. EASL Clinical Practice Guidelines on Sclerosing Cholangitis. J. Hepatol. 2022, 77, 761–806. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD Practice Guidance on Primary Sclerosing Cholangitis and Cholangiocarcinoma. Hepatology 2023, 77, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, E.; Krawczyk, M.; Milkiewicz, M.; Trottier, J.; Barbier, O.; Neurath, M.F.; Lammert, F.; Kremer, A.E.; Milkiewicz, P. Serum Autotaxin Is a Marker of the Severity of Liver Injury and Overall Survival in Patients with Cholestatic Liver Diseases. Sci. Rep. 2016, 6, 30847. [Google Scholar] [CrossRef]
- Kruk, B.; Liebe, R.; Milkiewicz, M.; Wunsch, E.; Raszeja-Wyszomirska, J.; Lammert, F.; Milkiewicz, P.; Krawczyk, M. PNPLA3 p.I148M and TM6SF2 p.E167K Variants Do Not Predispose to Liver Injury in Cholestatic Liver Diseases: A Prospective Analysis of 178 Patients with PSC. PLoS ONE 2018, 13, e0202942. [Google Scholar] [CrossRef] [PubMed]
- Poetter-Lang, S.; Ba-Ssalamah, A.; Messner, A.; Bastati, N.; Ambros, R.; Kristic, A.; Kittinger, J.; Pochepnia, S.; Ba-Ssalamah, S.A.; Hodge, J.C.; et al. Disease Severity Prognostication in Primary Sclerosing Cholangitis: A Validation of the Anali Scores and Comparison with the Potential Functional Stricture. Eur. Radiol. 2024, 34, 7632–7644. [Google Scholar] [CrossRef]
- Kruk, B.; Raszeja-Wyszomirska, J.; Krawczyk, M.; Milkiewicz, P. Fatigue and Itch Severity in Patients with PBC and PSC: Prospective Analysis of Two Large Cohorts. Eur. J. Clin. Investig. 2025, 55, e70041. [Google Scholar] [CrossRef]
- Trivedi, P.J. Risk Stratification in Primary Sclerosing Cholangitis: It’s Time to Move on from Replicating Imperfection and Break the Glass Ceiling. J. Hepatol. 2019, 71, 867–870. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Corpechot, C.; Pares, A.; Hirschfield, G.M. Risk Stratification in Autoimmune Cholestatic Liver Diseases: Opportunities for Clinicians and Trialists. Hepatology 2016, 63, 644–659. [Google Scholar] [CrossRef]
- Lindor, K.D.; Kowdley, K.V.; Luketic, V.A.C.; Harrison, M.E.; McCashland, T.; Befeler, A.S.; Harnois, D.; Jorgensen, R.; Petz, J.; Keach, J.; et al. High-Dose Ursodeoxycholic Acid for the Treatment of Primary Sclerosing Cholangitis. Hepatology 2009, 50, 808–814. [Google Scholar] [CrossRef]
- de Vries, E.M.G.; Wang, J.; Leeflang, M.M.G.; Boonstra, K.; Weersma, R.K.; Beuers, U.H.; Geskus, R.B.; Ponsioen, C.Y. Alkaline Phosphatase at Diagnosis of Primary Sclerosing Cholangitis and 1 Year Later: Evaluation of Prognostic Value. Liver Int. 2016, 36, 1867–1875. [Google Scholar] [CrossRef]
- Lindström, L.; Hultcrantz, R.; Boberg, K.M.; Friis-Liby, I.; Bergquist, A. Association Between Reduced Levels of Alkaline Phosphatase and Survival Times of Patients With Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2013, 11, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.H.; Grambsch, P.M.; Dickson, E.R.; Ludwig, J.; Maccarty, R.L.; Hunter, E.B.; Fleming, T.R.; Fisher, L.D.; Beaver, S.J.; Larusso, N.F. Primary Sclerosing Cholangitis: Natural History, Prognostic Factors and Survival Analysis. Hepatology 1989, 10, 430–436. [Google Scholar] [CrossRef]
- Farrant, J.M.; Hayllar, K.M.; Wilkinson, M.L.; Karani, J.; Portmann, B.C.; Westaby, D.; Williams, R. Natural History and Prognostic Variables in Primary Sclerosing Cholangitis. Gastroenterology 1991, 100, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Broomé, U.; Olsson, R.; Lööf, L.; Bodemar, G.; Hultcrantz, R.; Danielsson, Å.; Prytz, H.; Sandberg-Gertzén, H.; Wallerstedt, S.; Lindberg, G. Natural History and Prognostic Factors in 305 Swedish Patients with Primary Sclerosing Cholangitis. Gut 1996, 38, 610. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Therneau, T.M.; Wiesner, R.H.; Poterucha, J.J.; Benson, J.T.; Malinchoc, M.; Larusso, N.F.; Lindor, K.D.; Dickson, E.R. A Revised Natural History Model for Primary Sclerosing Cholangitis. Mayo Clin. Proc. 2000, 75, 688–694. [Google Scholar] [CrossRef]
- Boberg, K.M.; Rocca, G.; Egeland, T.; Bergquist, A.; Broomé, U.; Caballeria, L.; Chapman, R.; Hultcrantz, R.; Mitchell, S.; Pares, A.; et al. Time-Dependent Cox Regression Model Is Superior in Prediction of Prognosis in Primary Sclerosing Cholangitis. Hepatology 2002, 35, 652–657. [Google Scholar] [CrossRef]
- Ponsioen, C.Y.; Vrouenraets, S.M.; Prawirodirdjo, W.; Rajaram, R.; Rauws, E.A.; Mulder, C.J.; Reitsma, J.B.; Heisterkamp, S.H.; Tytgat, G.N. Natural History of Primary Sclerosing Cholangitis and Prognostic Value of Cholangiography in a Dutch Population. Gut 2002, 51, 562–566. [Google Scholar] [CrossRef]
- Tischendorf, J.J.; Hecker, H.; Krüger, M.; Manns, M.P.; Meier, P.N. Characterization, Outcome, and Prognosis in 273 Patients with Primary Sclerosing Cholangitis: A Single Center Study. Am. J. Gastroenterol. 2007, 102, 107–114. [Google Scholar] [CrossRef]
- De Vries, E.M.; Wang, J.; Williamson, K.D.; Leeflang, M.M.; Boonstra, K.; Weersma, R.K.; Beuers, U.; Chapman, R.W.; Geskus, R.B.; Ponsioen, C.Y. A Novel Prognostic Model for Transplant-Free Survival in Primary Sclerosing Cholangitis. Gut 2018, 67, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Goode, E.C.; Clark, A.B.; Mells, G.F.; Srivastava, B.; Spiess, K.; Gelson, W.T.H.; Trivedi, P.J.; Lynch, K.D.; Castren, E.; Vesterhus, M.N.; et al. Factors Associated With Outcomes of Patients With Primary Sclerosing Cholangitis and Development and Validation of a Risk Scoring System. Hepatology 2019, 69, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.E.; Vesterhus, M.; McCauley, B.M.; Atkinson, E.J.; Schlicht, E.M.; Juran, B.D.; Gossard, A.A.; LaRusso, N.F.; Gores, G.J.; Karlsen, T.H.; et al. Primary Sclerosing Cholangitis Risk Estimate Tool (PREsTo) Predicts Outcomes of the Disease: A Derivation and Validation Study Using Machine Learning. Hepatology 2020, 71, 214–224. [Google Scholar] [CrossRef]
- Schmeltzer, P.A.; Russo, M.W. Systematic Review of Prognostic Models Compared to the Mayo Risk Score for Primary Sclerosing Cholangitis. J. Clin. Med. 2021, 10, 4476. [Google Scholar] [CrossRef]
- Tornai, D.; Ven, P.L.; Lakatos, P.L.; Papp, M. Serological Biomarkers for Management of Primary Sclerosing Cholangitis. World J. Gastroenterol. 2022, 28, 2291. [Google Scholar] [CrossRef]
- Lytvyak, E.; Wang, D.; Shreekumar, D.; Ebadi, M.; Alrifae, Y.; Mason, A.; Montano-Loza, A.J. PSC-Specific Prognostic Scores Associated with Graft Loss and Overall Mortality in Recurrent PSC after Liver Transplantation. Dig. Liver Dis. 2025, 57, 1238–1246. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune Hepatitis. J. Hepatol. 2015, 63, 971–1004. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Sørensen, J.Ø.; Nielsen, O.H.; Andersson, M.; Ainsworth, M.A.; Ytting, H.; Bélard, E.; Jess, T. Inflammatory Bowel Disease with Primary Sclerosing Cholangitis: A Danish Population-Based Cohort Study 1977-2011. Liver Int. 2018, 38, 532–541. [Google Scholar] [CrossRef]
- Lindkvist, B.; De Valle, M.B.; Gullberg, B.; Björnsson, E. Incidence and Prevalence of Primary Sclerosing Cholangitis in a Defined Adult Population in Sweden. Hepatology 2010, 52, 571–577. [Google Scholar] [CrossRef]
- Barner-Rasmussen, N.; Pukkala, E.; Jussila, A.; Färkkilä, M. Epidemiology, Risk of Malignancy and Patient Survival in Primary Sclerosing Cholangitis: A Population-Based Study in Finland. Scand. J. Gastroenterol. 2020, 55, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-Based Epidemiology, Malignancy Risk, and Outcome of Primary Sclerosing Cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Boberg, K.M.; Aadland, E.; Jahnsen, J.; Raknerud, N.; Stiris, M.; Bell, H. Incidence and Prevalence of Primary Biliary Cirrhosis, Primary Sclerosing Cholangitis, and Autoimmune Hepatitis in a Norwegian Population. Scand. J. Gastroenterol. 1998, 33, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, B.; Mells, G.F.; Cordell, H.J.; Muriithi, A.; Brown, M.; Ellinghaus, E.; Franke, A.; Karlsen, T.H.; Sandford, R.N.; Alexander, G.J.; et al. Fine Mapping and Replication of Genetic Risk Loci in Primary Sclerosing Cholangitis. Scand. J. Gastroenterol. 2012, 47, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Halliday, J.S.; Djordjevic, J.; Lust, M.; Culver, E.L.; Braden, B.; Travis, S.P.L.; Chapman, R.W.G. A Unique Clinical Phenotype of Primary Sclerosing Cholangitis Associated with Crohn’s Disease. J. Crohns Colitis 2012, 6, 174–181. [Google Scholar] [CrossRef]
- Toy, E.; Balasubramanian, S.; Selmi, C.; Li, C.S.; Bowlus, C.L. The Prevalence, Incidence and Natural History of Primary Sclerosing Cholangitis in an Ethnically Diverse Population. BMC Gastroenterol. 2011, 11, 83. [Google Scholar] [CrossRef]
- Angulo, P.; Grandison, G.A.; Fong, D.G.; Keach, J.C.; Lindor, K.D.; Bjornsson, E.; Koch, A. Bone Disease in Patients with Primary Sclerosing Cholangitis. Gastroenterology 2011, 140, 180–188. [Google Scholar] [CrossRef]
- Yazdanfar, M.; Zepeda, J.; Dean, R.; Wu, J.; Levy, C.; Goldberg, D.; Lammert, C.; Prenner, S.; Reddy, K.R.; Pratt, D.; et al. African American Race Does Not Confer an Increased Risk of Clinical Events in Patients with Primary Sclerosing Cholangitis. Hepatol. Commun. 2024, 8, e0366. [Google Scholar] [CrossRef]
- Kruk, B.; Milkiewicz, M.; Raszeja-Wyszomirska, J.; Milkiewicz, P.; Krawczyk, M. A Common Variant in the Hepatobiliary Phospholipid Transporter ABCB4 Modulates Liver Injury in PBC but Not in PSC: Prospective Analysis in 867 Patients. Orphanet J. Rare Dis. 2022, 17, 419. [Google Scholar] [CrossRef]
- Boudewijn De Vries, A.; Janse, M.; Blokzijl, H.; Boudewijn De Vries, W.A.; Weersma, R.K. Distinctive Inflammatory Bowel Disease Phenotype in Primary Sclerosing Cholangitis. World J. Gastroenterol. 2015, 21, 1956–1971. [Google Scholar] [CrossRef] [PubMed]
- Ngu, J.H.; Gearry, R.B.; Wright, A.J.; Stedman, C.A.M. Inflammatory Bowel Disease Is Associated with Poor Outcomes of Patients with Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2011, 9, 1092–1097. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Strassburg, C.P.; Trivedi, P.J.; Hirschfield, G.M.; Trivedi, P.J.; Bergquist, A.; Said, K.; Imam, M.; Lazaridis, K.N.; Juran, B.D.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e8. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Bowlus, C.L.; Yimam, K.K.; Razavi, H.; Estes, C. Epidemiology, Natural History, and Outcomes of Primary Sclerosing Cholangitis: A Systematic Review of Population-Based Studies. Clin. Gastroenterol. Hepatol. 2022, 20, 1687–1700.e4. [Google Scholar] [CrossRef]
- Nardelli, M.J.; Bittencourt, P.L.; Cançado, G.G.L.; Faria, L.C.; Villela-Nogueira, C.A.; Rotman, V.; Silva De Abreu, E.; Maria Farage Osório, F.; Evangelista, A.S.; Sampaio Costa Mendes, L.; et al. Clinical Features and Outcomes of Primary Sclerosing Cholangitis in the Highly Admixed Brazilian Population. Can. J. Gastroenterol. Hepatol. 2021, 2021, 7746401. [Google Scholar] [CrossRef]
- Navaneethan, U.; Venkatesh, P.G.K.; Lashner, B.A.; Shen, B.; Kiran, R.P. The Impact of Ulcerative Colitis on the Long-Term Outcome of Patients with Primary Sclerosing Cholangitis. Aliment. Pharmacol. Ther. 2012, 35, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Garioud, A.; Seksik, P.; Chrétien, Y.; Corphechot, C.; Poupon, R.; Poupon, R.E.; Chazouillères, O. Characteristics and Clinical Course of Primary Sclerosing Cholangitis in France: A Prospective Cohort Study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Franceschet, I.; Cazzagon, N.; Del Ross, T.; D’Incà, R.; Buja, A.; Floreani, A. Primary Sclerosing Cholangitis Associated with Inflammatory Bowel Disease: An Observational Study in a Southern Europe Population Focusing on New Therapeutic Options. Eur. J. Gastroenterol. Hepatol. 2016, 28, 508–513. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Crothers, H.; Mytton, J.; Bosch, S.; Iqbal, T.; Ferguson, J.; Hirschfield, G.M. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People With Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology 2020, 159, 915–928. [Google Scholar] [CrossRef]
- Hatami, B.; Mosala, M.; Hassani, A.H.; Ardakani, M.J.E.; Gholami, S.; Zali, M.R. Fenofibrate in Primary Sclerosing Cholangitis; a Randomized, Double-Blind, Placebo-Controlled Trial. Pharmacol. Res. Perspect. 2022, 10, e00984. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Abouda, G.F.; Bilir, B.M.; Bonder, A.; Bowlus, C.L.; Campos-Varela, I.; Cazzagon, N.; Chandok, N.; Cheent, K.; Cortez-Pinto, H.; et al. Safety and Efficacy of Elafibranor in Primary Sclerosing Cholangitis: The ELMWOOD Phase II Randomized-Controlled Trial. J. Hepatol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Eksteen, B.; Bowlus, C.L.; Montano-Loza, A.J.; Lefebvre, E.; Fischer, L.; Vig, P.; Martins, E.B.; Ahmad, J.; Yimam, K.K.; Pockros, P.J.; et al. Efficacy and Safety of Cenicriviroc in Patients With Primary Sclerosing Cholangitis: PERSEUS Study. Hepatol. Commun. 2021, 5, 478–490. [Google Scholar] [CrossRef]
- Ciobanu, C.; Russo, M.W. Prognostic Modeling in Biliary Diseases. Curr. Opin. Gastroenterol. 2023, 39, 89–94. [Google Scholar] [CrossRef]
- Goet, J.C.; Floreani, A.; Verhelst, X.; Cazzagon, N.; Perini, L.; Lammers, W.J.; de Vries, A.C.; van der Meer, A.J.; van Buuren, H.R.; Hansen, B.E. Validation, Clinical Utility and Limitations of the Amsterdam-Oxford Model for Primary Sclerosing Cholangitis. J. Hepatol. 2019, 71, 992–999. [Google Scholar] [CrossRef]
- Bossen, L.; Vesterhus, M.; Hov, J.R.; Färkkilä, M.; Rosenberg, W.M.; Møller, H.J.; Boberg, K.M.; Karlsen, T.H.; Grønbæk, H. Circulating Macrophage Activation Markers Predict Transplant-Free Survival in Patients With Primary Sclerosing Cholangitis. Clin. Transl. Gastroenterol. 2021, 12, e00315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).