Identifying Molecular Changes in Giardia lamblia Stages Using Hyperspectral Raman Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Instruments
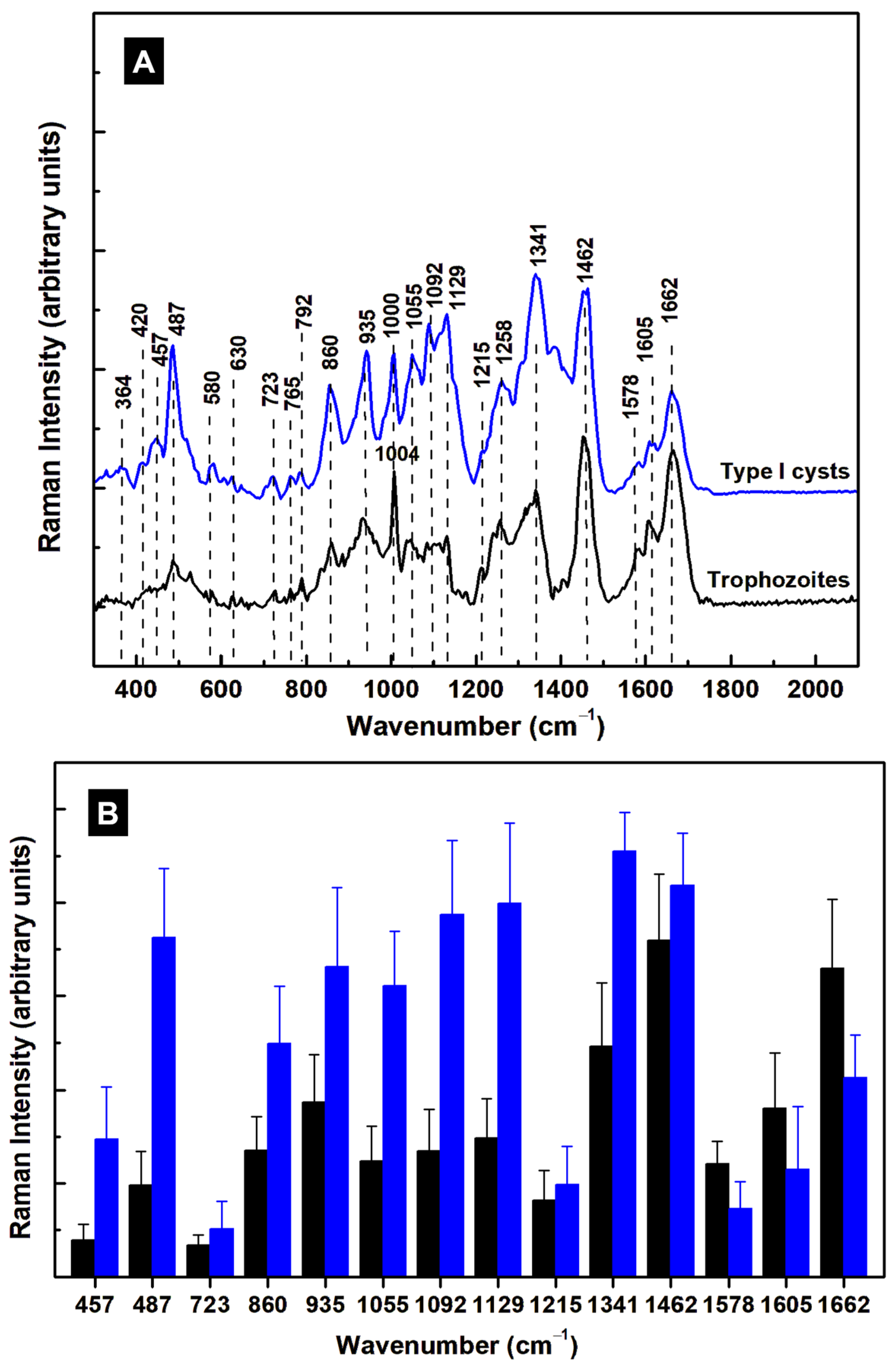
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajare, S.T.; Chekol, Y.; Chauhan, N.M. Assessment of Prevalence of Giardia Lamblia Infection and Its Associated Factors among Government Elementary School Children from Sidama Zone, SNNPR, Ethiopia. PLoS ONE 2022, 17, e0264812. [Google Scholar] [CrossRef]
- CDC Giardiasis NNDSS Summary Report for 2019. Available online: https://www.cdc.gov/healthy-water-data/documentation/giardiasis-nndss-summary-report-for-2019.html (accessed on 31 March 2025).
- World Health Organization; Food and Agriculture Organization of the United Nations. Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites: Report of a Joint FAO/WHO Expert Meeting, 3–7 September 2012, FAO Headquarters, Rome, Italy; Organisation des Nations Unies Pour L’alimentation et L’agriculture, Organisation Mondiale de la Santé, Eds.; Microbiological Risk Assessment Series; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2014; ISBN 978-92-4-156470-0. [Google Scholar]
- Cedillo-Rivera, R.; Leal, Y.A.; Yépez-Mulia, L.; Gómez-Delgado, A.; Ortega-Pierres, G.; Tapia-Conyer, R.; Muñoz, O. Seroepidemiology of Giardiasis in Mexico. Am. J. Trop. Med. Hyg. 2009, 80, 6–10. [Google Scholar] [CrossRef]
- Currie, S.L.; Stephenson, N.; Palmer, A.S.; Jones, B.L.; Alexander, C.L. Under-Reporting Giardiasis: Time to Consider the Public Health Implications. Epidemiol. Infect. 2017, 145, 3007–3011. [Google Scholar] [CrossRef]
- Lalle, M.; Hanevik, K. Treatment-Refractory Giardiasis: Challenges and Solutions. Infect. Drug Resist. 2018, 11, 1921–1933. [Google Scholar] [CrossRef]
- Benchimol, M.; de Souza, W. Giardia Intestinalis and Its Endomembrane System*. J. Eukaryot. Microbiol. 2022, 69, e12893. [Google Scholar] [CrossRef]
- Adam, R.D. Giardia Duodenalis: Biology and Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00024-19. [Google Scholar] [CrossRef]
- Krtková, J.; Thomas, E.B.; Alas, G.C.M.; Schraner, E.M.; Behjatnia, H.R.; Hehl, A.B.; Paredez, A.R. Rac Regulates Giardia Lamblia Encystation by Coordinating Cyst Wall Protein Trafficking and Secretion. mBio 2016, 7, e01003-16. [Google Scholar] [CrossRef]
- Ankarklev, J.; Jerlström-Hultqvist, J.; Ringqvist, E.; Troell, K.; Svärd, S.G. Behind the Smile: Cell Biology and Disease Mechanisms of Giardia Species. Nat. Rev. Microbiol. 2010, 8, 413–422. [Google Scholar] [CrossRef]
- Townson, S.M.; Boreham, P.F.; Upcroft, P.; Upcroft, J.A. Resistance to the Nitroheterocyclic Drugs. Acta Trop. 1994, 56, 173–194. [Google Scholar] [CrossRef]
- Freeman, C.D.; Klutman, N.E.; Lamp, K.C. Metronidazole. A Therapeutic Review and Update. Drugs 1997, 54, 679–708. [Google Scholar] [CrossRef]
- Nabarro, L.E.B.; Lever, R.A.; Armstrong, M.; Chiodini, P.L. Increased Incidence of Nitroimidazole-Refractory Giardiasis at the Hospital for Tropical Diseases, London: 2008–2013. Clin. Microbiol. Infect. 2015, 21, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Krakovka, S.; Ribacke, U.; Miyamoto, Y.; Eckmann, L.; Svärd, S. Characterization of Metronidazole-Resistant Giardia Intestinalis Lines by Comparative Transcriptomics and Proteomics. Front. Microbiol. 2022, 13, 834008. [Google Scholar] [CrossRef]
- Barat, L.M.; Bloland, P.B. Drug Resistance among Malaria and Other Parasites. Infect. Dis. Clin. North Am. 1997, 11, 969–987. [Google Scholar] [CrossRef]
- De Chatterjee, A.; Mendez, T.L.; Roychowdhury, S.; Das, S. The Assembly of GM1 Glycolipid- and Cholesterol-Enriched Raft-Like Membrane Microdomains Is Important for Giardial Encystation. Infect. Immun. 2015, 83, 2030–2042. [Google Scholar] [CrossRef]
- Jarroll, E.L.; Macechko, P.T.; Steimle, P.A.; Bulik, D.; Karr, C.D.; van Keulen, H.; Paget, T.A.; Gerwig, C.; Kamerling, J.; Vliegenthart, J.; et al. Regulation of Carbohydrate Metabolism During Giardia Encystment. Eukaryot. Microbiol. 2005, 48, 22–26. [Google Scholar] [CrossRef]
- Pech-Santiago, E.O.; Argüello-García, R.; Vázquez, C.; Saavedra, E.; González-Hernández, I.; Jung-Cook, H.; Rafferty, S.P.; Ortega-Pierres, M.G. Giardia Duodenalis: Flavohemoglobin Is Involved in Drug Biotransformation and Resistance to Albendazole. PLOS Pathog. 2022, 18, e1010840. [Google Scholar] [CrossRef]
- Vicente, B.; Freitas, A.D.; Freitas, M.; Midlej, V. Systematic Review of Diagnostic Approaches for Human Giardiasis: Unveiling Optimal Strategies. Diagnostics 2024, 14, 364. [Google Scholar] [CrossRef]
- Hooshyar, H.; Rostamkhani, P.; Arbabi, M.; Delavari, M. Giardia Lamblia Infection: Review of Current Diagnostic Strategies. Gastroenterol. Hepatol. Bed Bench 2019, 12, 3–12. [Google Scholar]
- Keister, D.B. Axenic Culture of Giardia Lamblia in TYI-S-33 Medium Supplemented with Bile. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 487–488. [Google Scholar] [CrossRef]
- Gillin, F.D.; Boucher, S.E.; Rossi, S.S.; Reiner, D.S. Giardia Lamblia: The Roles of Bile, Lactic Acid, and pH in the Completion of the Life Cycle in Vitro. Exp. Parasitol. 1989, 69, 164–174. [Google Scholar] [CrossRef]
- Einarsson, E.; Troell, K.; Hoeppner, M.P.; Grabherr, M.; Ribacke, U.; Svärd, S.G. Coordinated Changes in Gene Expression Throughout Encystation of Giardia Intestinalis. PLoS Neglected Trop. Dis. 2016, 10, e0004571. [Google Scholar] [CrossRef]
- Manciu, F.S.; Guerrero, J.; Pence, B.C.; Martinez Lopez, L.V.; Das, S. Assessment of Drug Activities against Giardia Using Hyperspectral Raman Microscopy. Pathogens 2024, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G. Raman Spectroscopy in Cell Biology and Microbiology. J. Raman Spectrosc. 2021, 52, 2348–2443. [Google Scholar] [CrossRef]
- Alam, S.; Yee, J.; Couture, M.; Takayama, S.J.; Tseng, W.-H.; Mauk, A.G.; Rafferty, S. Cytochrome B5 from Giardia Lamblia. Metallomics 2012, 4, 1255–1261. [Google Scholar] [CrossRef]
- Rule, K.L.; Vikesland, P.J. Surface-Enhanced Resonance Raman Spectroscopy for the Rapid Detection of Cryptosporidium Parvum and Giardia Lamblia. Environ. Sci. Technol. 2009, 43, 1147–1152. [Google Scholar] [CrossRef]
- Cui, D.; Kong, L.; Wang, Y.; Zhu, Y.; Zhang, C. In Situ Identification of Environmental Microorganisms with Raman Spectroscopy. Environ. Sci. Ecotechnology 2022, 11, 100187. [Google Scholar] [CrossRef]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S.; et al. Near Real-Time Intraoperative Brain Tumor Diagnosis Using Stimulated Raman Histology and Deep Neural Networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef]
- Manciu, F.S.; Guerrero, J.; Bennet, K.E.; Chang, S.-Y.; Rahman, M.; Martinez Lopez, L.V.; Chantigian, S.; Castellanos, M.; Manciu, M. Assessing Nordihydroguaiaretic Acid Therapeutic Effect for Glioblastoma Multiforme. Sensors 2022, 22, 2643. [Google Scholar] [CrossRef]
- Manciu, F.S.; Ciubuc, J.D.; Parra, K.; Manciu, M.; Bennet, K.E.; Valenzuela, P.; Sundin, E.M.; Durrer, W.G.; Reza, L.; Francia, G. Label-Free Raman Imaging to Monitor Breast Tumor Signatures. Technol. Cancer Res. Treat. 2017, 16, 461–469. [Google Scholar] [CrossRef]
- Spiro, T.G. Resonance Raman Spectroscopy as a Probe of Heme Protein Structure and Dynamics. Adv. Protein Chem. 1985, 37, 111–159. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman Spectroscopy of Proteins: A Review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Kitagawa, T.; Kyogoku, Y.; Iizuka, T.; Ikeda-Saito, M.; Yamanaka, T. Resonance Raman Scattering from Hemoproteins: Effects of Ligands upon the Raman Spectra of Various C-Type Cytochromes. J. Biochem. 1975, 78, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicz, B.; McColl, E.; Yee, J.; Rafferty, S.; Couture, M. Resonance Raman Studies on the Flavohemoglobin of the Protist Giardia Intestinalis: Evidence of a Type I/II-Peroxidase-like Heme Environment and Roles of the Active Site Distal Residues. JBIC J. Biol. Inorg. Chem. 2017, 22, 1099–1108. [Google Scholar] [CrossRef]
- Voleman, L.; Najdrová, V.; Ástvaldsson, Á.; Tůmová, P.; Einarsson, E.; Švindrych, Z.; Hagen, G.M.; Tachezy, J.; Svärd, S.G.; Doležal, P. Giardia Intestinalis Mitosomes Undergo Synchronized Fission but Not Fusion and Are Constitutively Associated with the Endoplasmic Reticulum. BMC Biol. 2017, 15, 27. [Google Scholar] [CrossRef]
- Ashton, L.; Pudney, P.D.A.; Blanch, E.W.; Yakubov, G.E. Understanding Glycoprotein Behaviours Using Raman and Raman Optical Activity Spectroscopies: Characterising the Entanglement Induced Conformational Changes in Oligosaccharide Chains of Mucin. Adv. Colloid Interface Sci. 2013, 199–200, 66–77. [Google Scholar] [CrossRef]
- Schmidt, C.; Jahn, S. Raman Spectra of Oxidized Sulfur Species in Hydrothermal Fluids. J. Volcanol. Geotherm. Res. 2024, 454, 108146. [Google Scholar] [CrossRef]
- Das, S.; Gillin, F.D. Giardia Lamblia: Increased UDP-N-Acetyl-D-Glucosamine and N-Acetyl-D-Galactosamine Transferase Activities during Encystation. Exp. Parasitol. 1996, 83, 19–29. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman Spectroscopy of Lipids: A Review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Gerwig, G.J.; van Kuik, J.A.; Leeflang, B.R.; Kamerling, J.P.; Vliegenthart, J.F.G.; Karr, C.D.; Jaroll, E.L. The Giardia Intestinalis Filamentous Cyst Wall Contains a Novel b(1-3)-N-Acetyl-D-Galactosamine Polymer: A Structural and Conformational Study. Glycobiology 2002, 12, 499–505. [Google Scholar] [CrossRef]
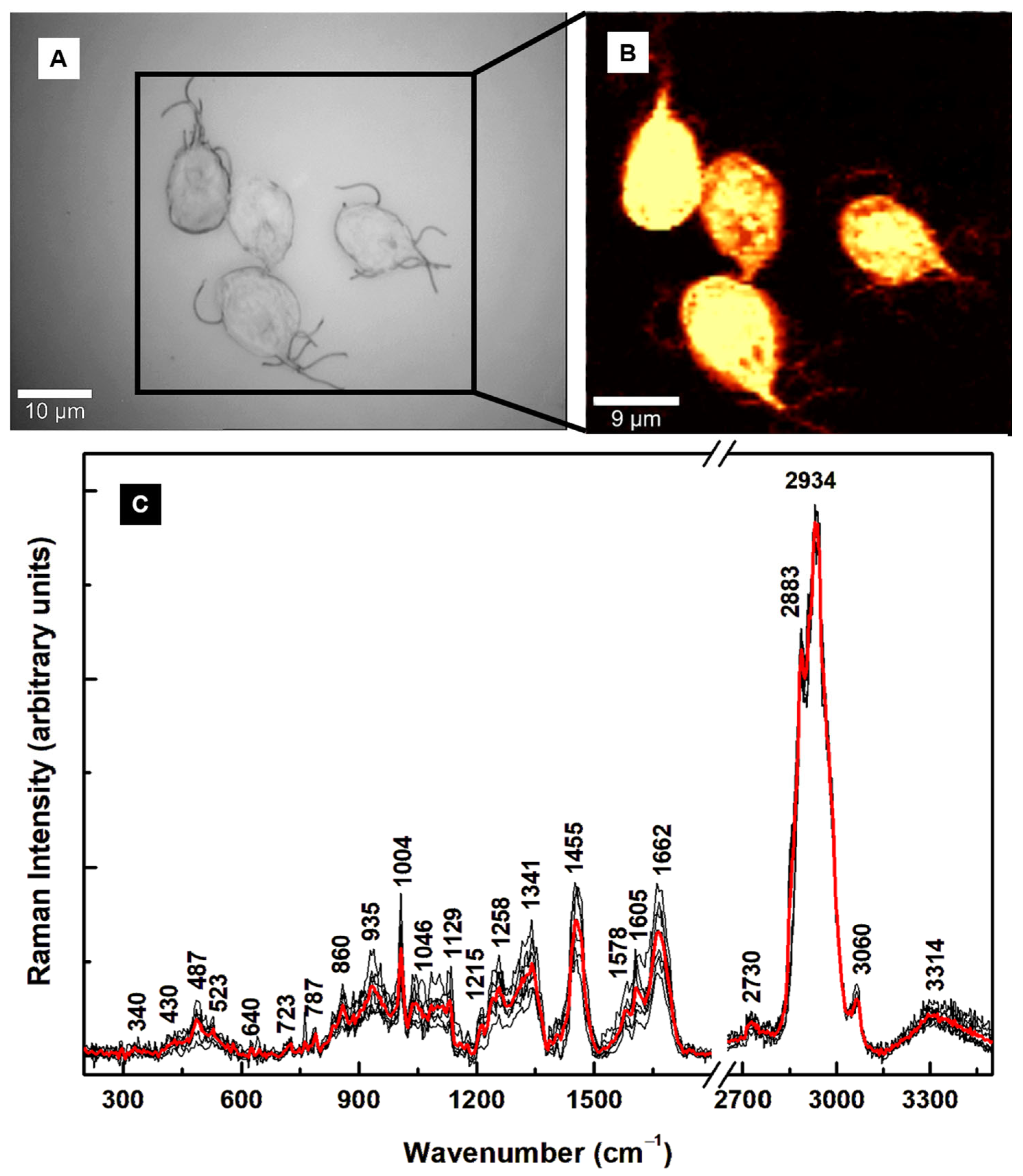

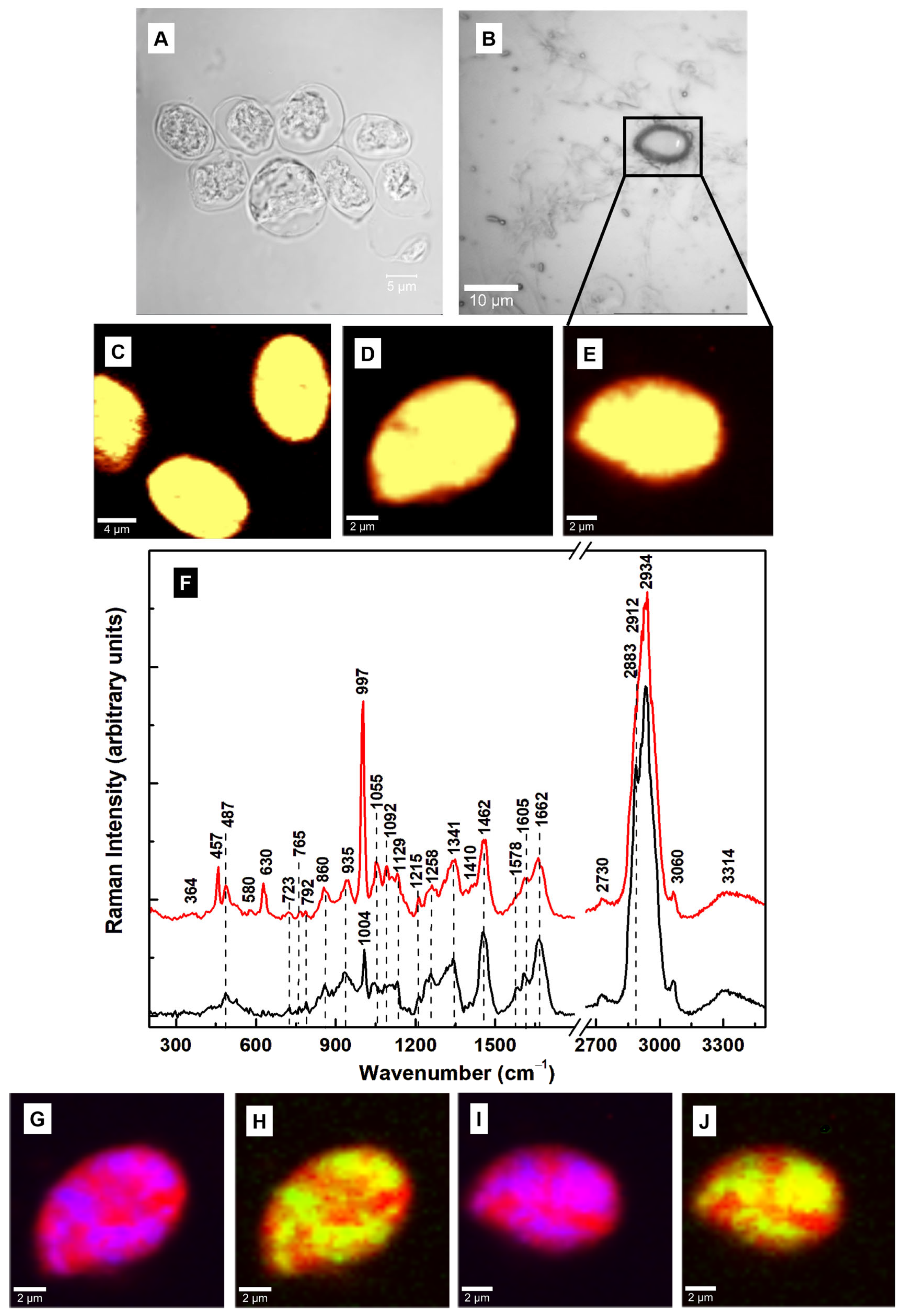
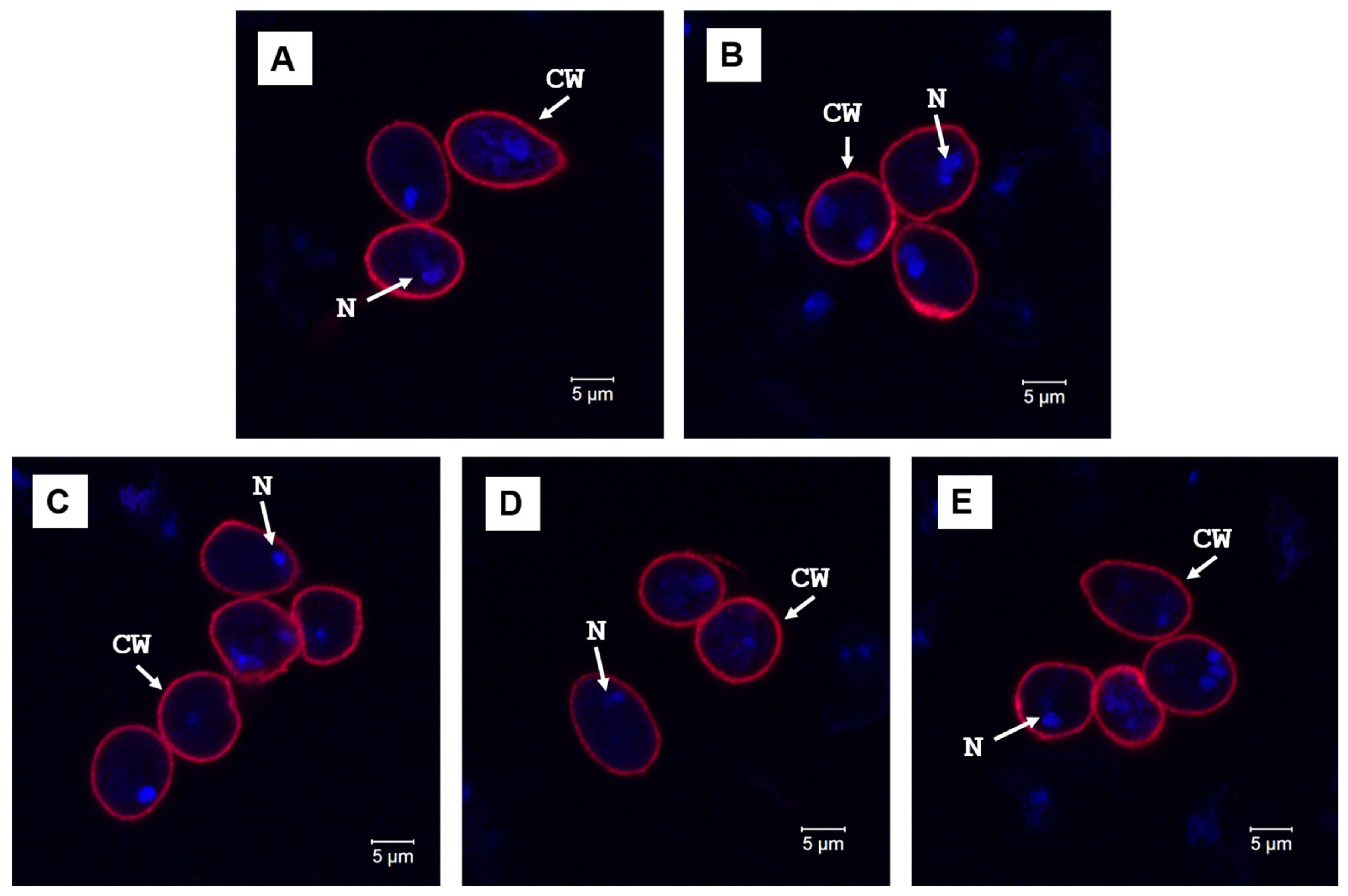

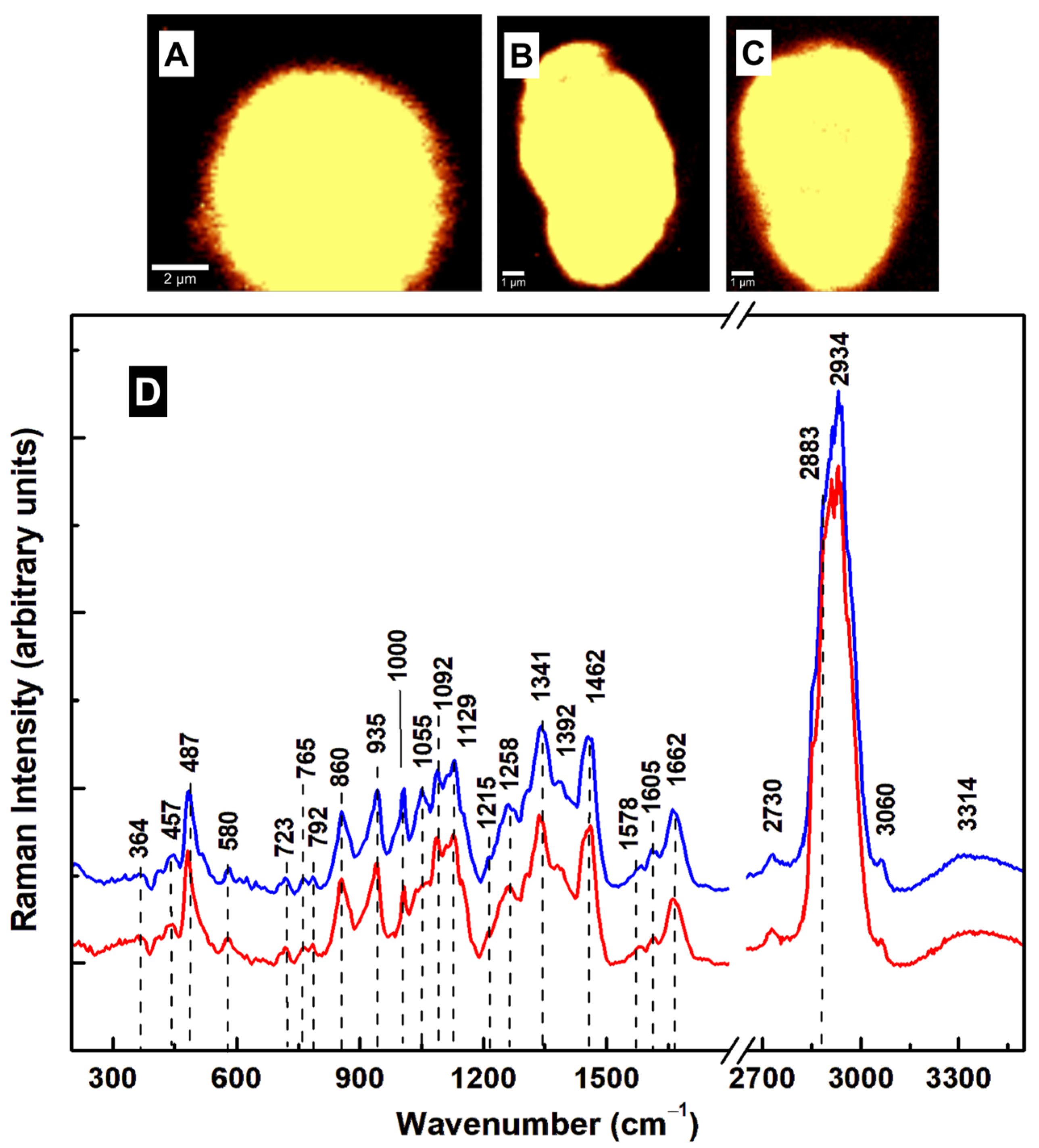
| Raman Shift (cm–1) | Assignment | Attribution |
|---|---|---|
| 340 | C–C–C pyrrole ring deformation | Heme-binding proteins, cytochrome b5 [25,26,32,35] |
| 430 | C–C–C pyrrole ring deformation | Heme-binding proteins [25,26,32,35] |
| 487 | Fe–CO, C–C stretching | Heme-binding proteins, glycogen [28,32,35] |
| 523 | Fe–CO, S–S stretching | Heme-binding proteins, cysteine [32,35] |
| 640 | C–N, C–C twisting | Heme-binding proteins, guanine, tyrosine [25,28,32,35] |
| 723 | C–N ring breathing | Phospholipid, adenine [25,28,32] |
| 787 | Pyrrole breathing | Cytosine, nucleic acids [25,26,28,32,35] |
| 860 | C–C stretching, –CH out of plane bending, C–N bending | Tryptophan, glycogen, polysaccharide, arginine [25,28] |
| 935 | –CH3 symmetric stretching, N–C–N symmetric stretching, C–N bending | Lipids, proteins, arginine [25,28] |
| 1004 | Benzene ring breathing | Phenylalanine, collagen [25] |
| 1046 | C–C, C–N, C–O, O–S–O stretching | Lipids, proteins, glycogen, oxidized sulfur species [25,37] |
| 1129 | C–C, O–S–O stretching | Lipids, oxidized sulfur species [25,37] |
| 1215 | N–H bending, C–N stretching, O–S–O | Amide III, oxidized sulfur species [25,28,37] |
| 1258 | –CH2 deformation, N–H bending, C–N stretching | Lipids, Amide III [25,28] |
| 1341 | C–N breathing, –CH2 deformation | Heme proteins, lipids, adenine, guanine [25,26,32,35] |
| 1455 | C–N bending, –CH3 out-of-plane deformation | Lipids, proteins [25] |
| 1578 | N–H bending, C–N stretching | Tryptophan, Amide II, NADH [25] |
| 1605 | C–O stretching, C=C bending | Phenylalanine, tyrosine [25] |
| 1662 | C=O stretching, out-of-plane C–N stretching | Amide I [25] |
| 1732 | C=O stretching | Lipids, phospholipids, triacylglycerides [25] |
| 2730 | –CH stretching | Saturated fatty acids [25] |
| 2883 | –CH2 antisymmetric stretching | Lipids, raft-like ordered domains [25,28,33] |
| 2912 | –CH3 symmetric stretching | Lipids, proteins [25,28] |
| 2934 | –CH3 | Proteins, fat, cholesterol [25,28] |
| 3060 | =CH | Unsaturated fatty acids [25,28] |
| 3314 | –NH, –OH | Proteins, water band [25,28] |
| Raman Shift (cm–1) | p-Value |
|---|---|
| 457 | 1.0 × 10−3 |
| 487 | 1.4 × 10−5 |
| 723 | 0.14 |
| 860 | 1.5 × 10−3 |
| 935 | 6.0 × 10−3 |
| 1055 | 1.4 × 10−4 |
| 1092 | 1.3 × 10−5 |
| 1129 | 1.8 × 10−5 |
| 1215 | 0.59 |
| 1341 | 2.3 × 10−3 |
| 1462 | 0.29 |
| 1578 | 1.8 × 10−2 |
| 1605 | 0.16 |
| 1662 | 3.7 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manciu, F.S.; Pence, B.C.; Ibechenjo, B.A.; Manciu, M.; Bhattarai, S.; Das, S. Identifying Molecular Changes in Giardia lamblia Stages Using Hyperspectral Raman Microscopy. Diagnostics 2025, 15, 2161. https://doi.org/10.3390/diagnostics15172161
Manciu FS, Pence BC, Ibechenjo BA, Manciu M, Bhattarai S, Das S. Identifying Molecular Changes in Giardia lamblia Stages Using Hyperspectral Raman Microscopy. Diagnostics. 2025; 15(17):2161. https://doi.org/10.3390/diagnostics15172161
Chicago/Turabian StyleManciu, Felicia S., Breanna C. Pence, Blessing A. Ibechenjo, Marian Manciu, Sudhir Bhattarai, and Siddhartha Das. 2025. "Identifying Molecular Changes in Giardia lamblia Stages Using Hyperspectral Raman Microscopy" Diagnostics 15, no. 17: 2161. https://doi.org/10.3390/diagnostics15172161
APA StyleManciu, F. S., Pence, B. C., Ibechenjo, B. A., Manciu, M., Bhattarai, S., & Das, S. (2025). Identifying Molecular Changes in Giardia lamblia Stages Using Hyperspectral Raman Microscopy. Diagnostics, 15(17), 2161. https://doi.org/10.3390/diagnostics15172161







