Rotational Thromboelastometry (ROTEM) Hemostasis Profile in Pregnant Women with Preeclampsia and Their Offspring: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
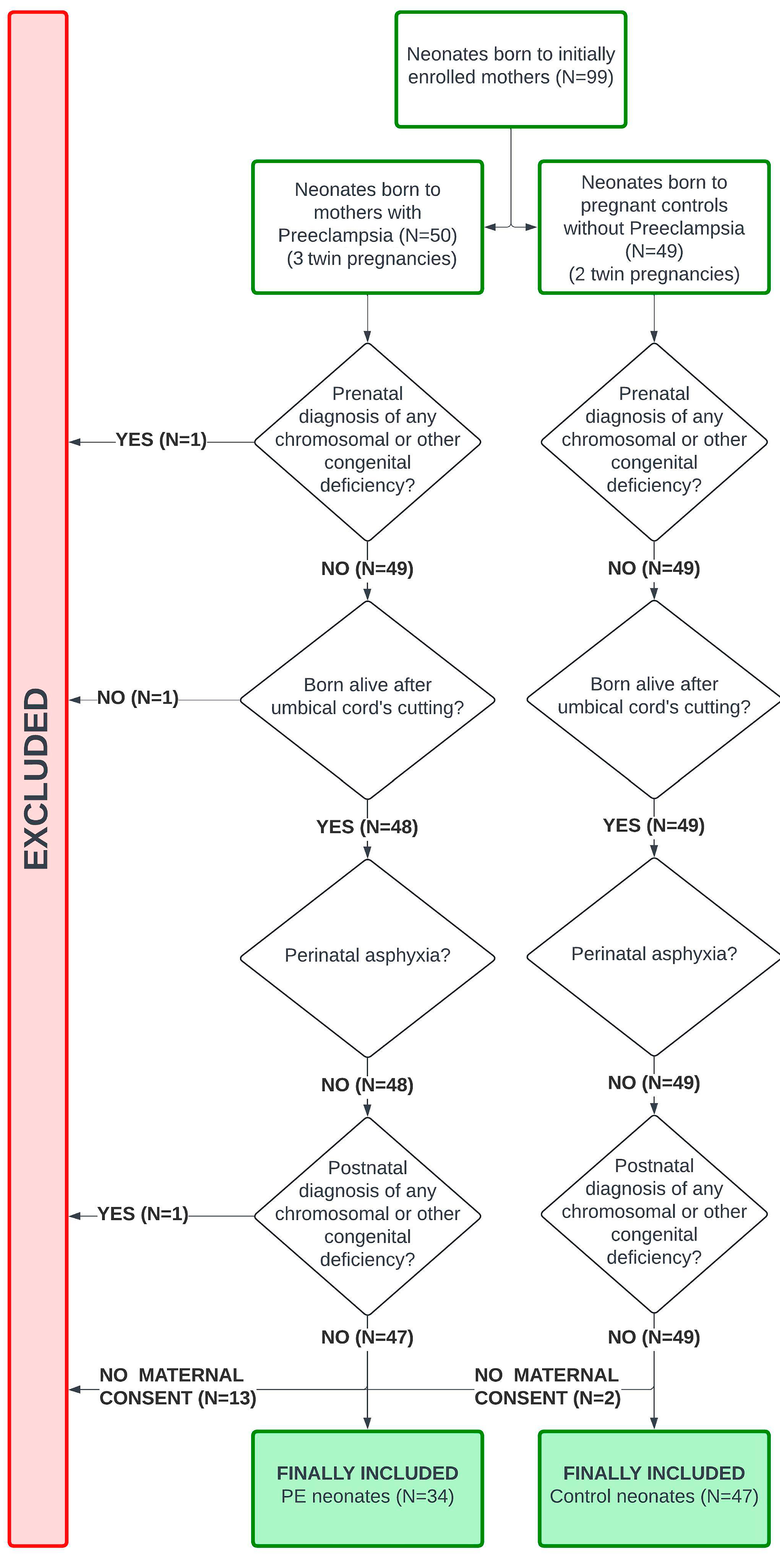
2.1. Study Design, Setting, and Participants
2.2. Definitions
2.3. Samples and Outcomes
2.4. Addressing Bias
2.5. Sample Size
2.6. Statistical Analysis
3. Results
3.1. Analysis of PE Groups
3.2. Analysis of PE Severity
3.3. Onset Analysis
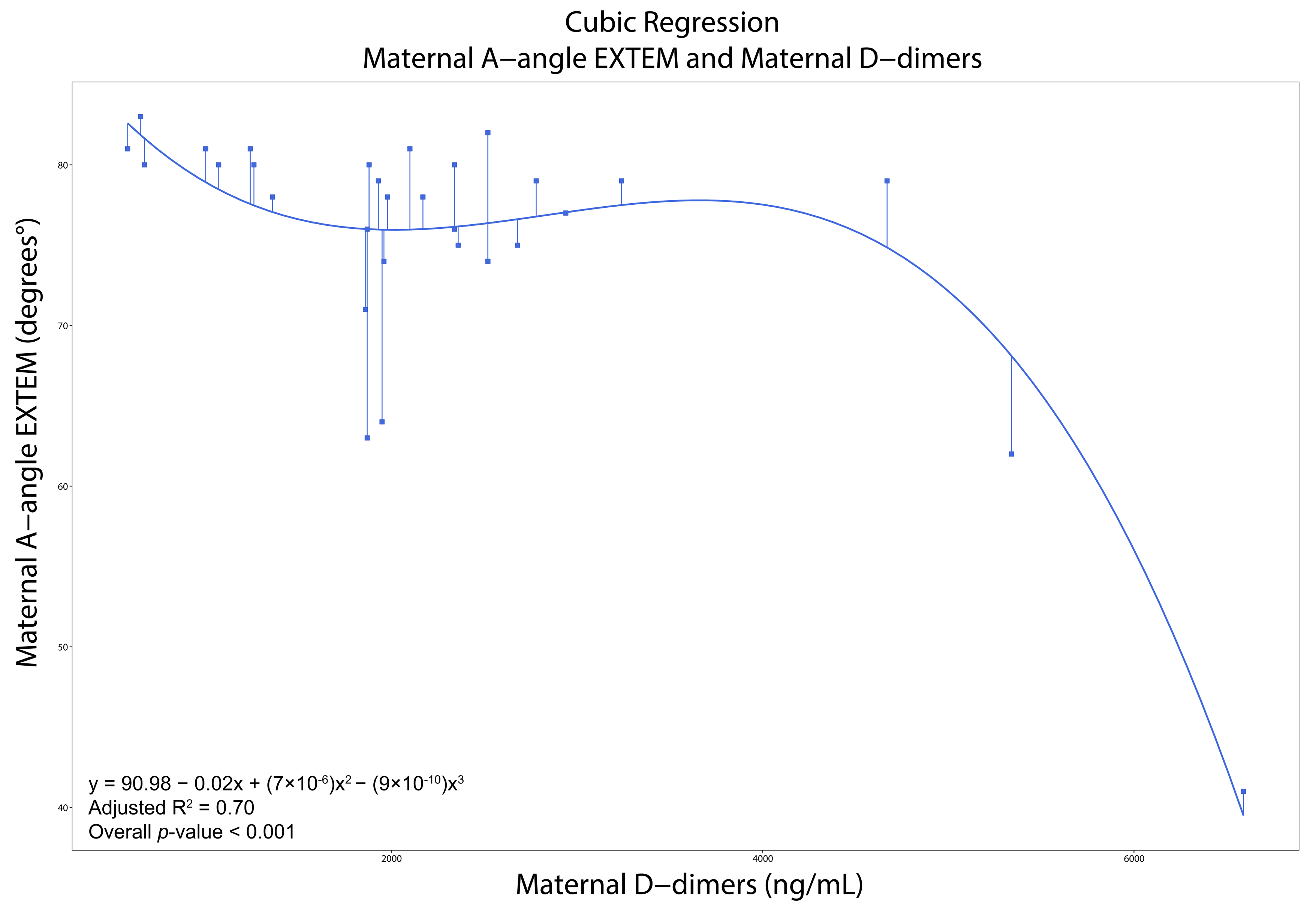
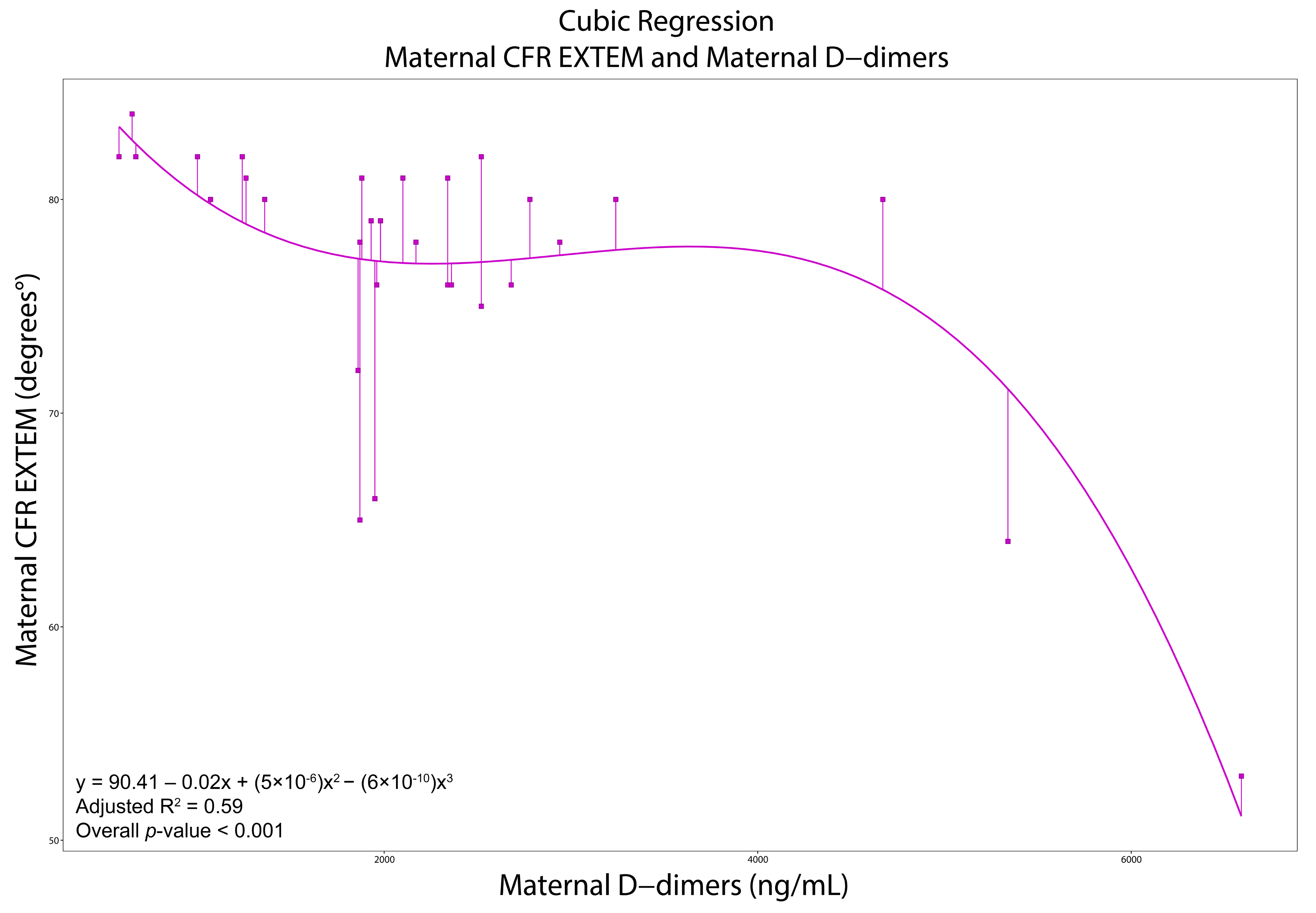
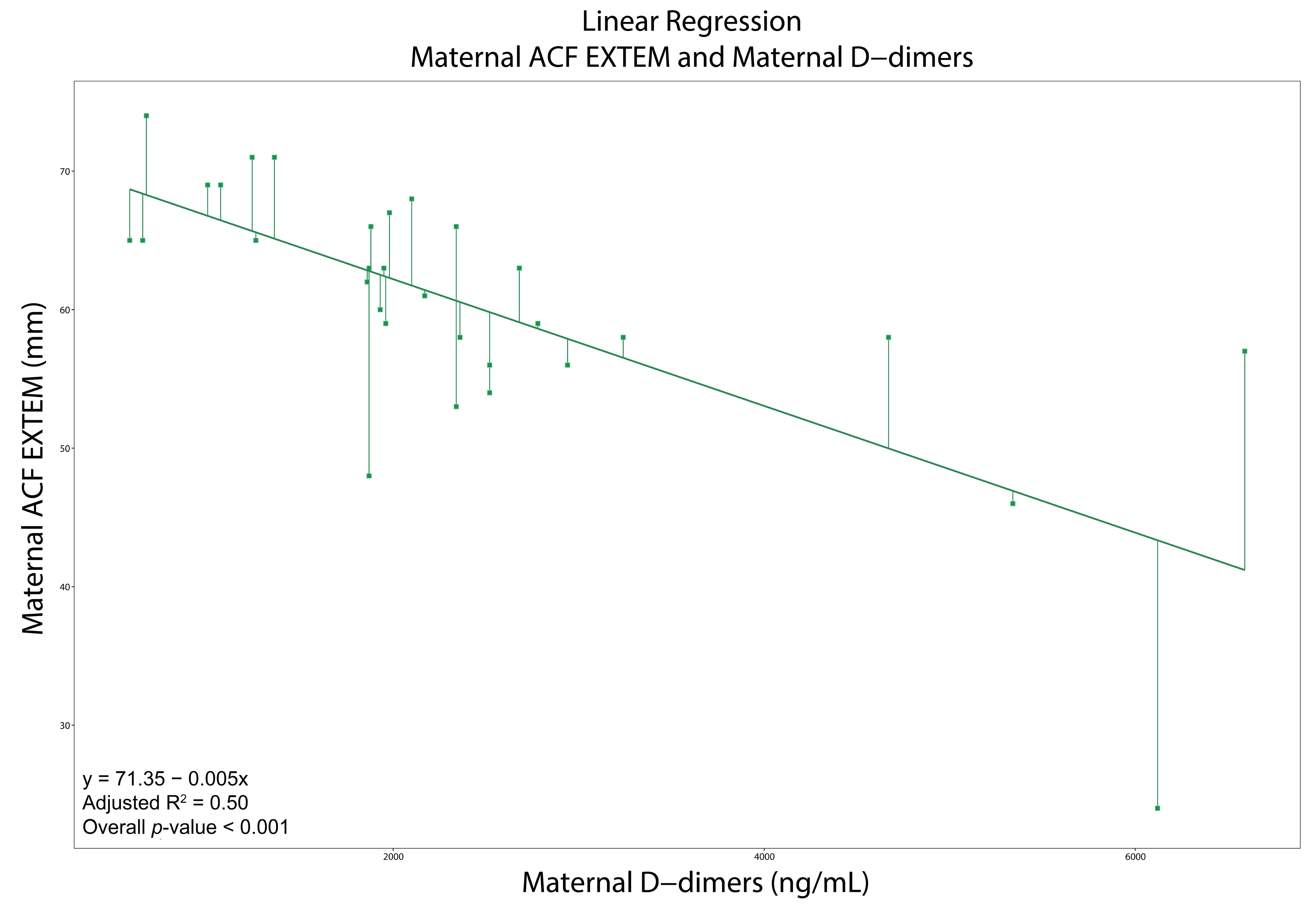
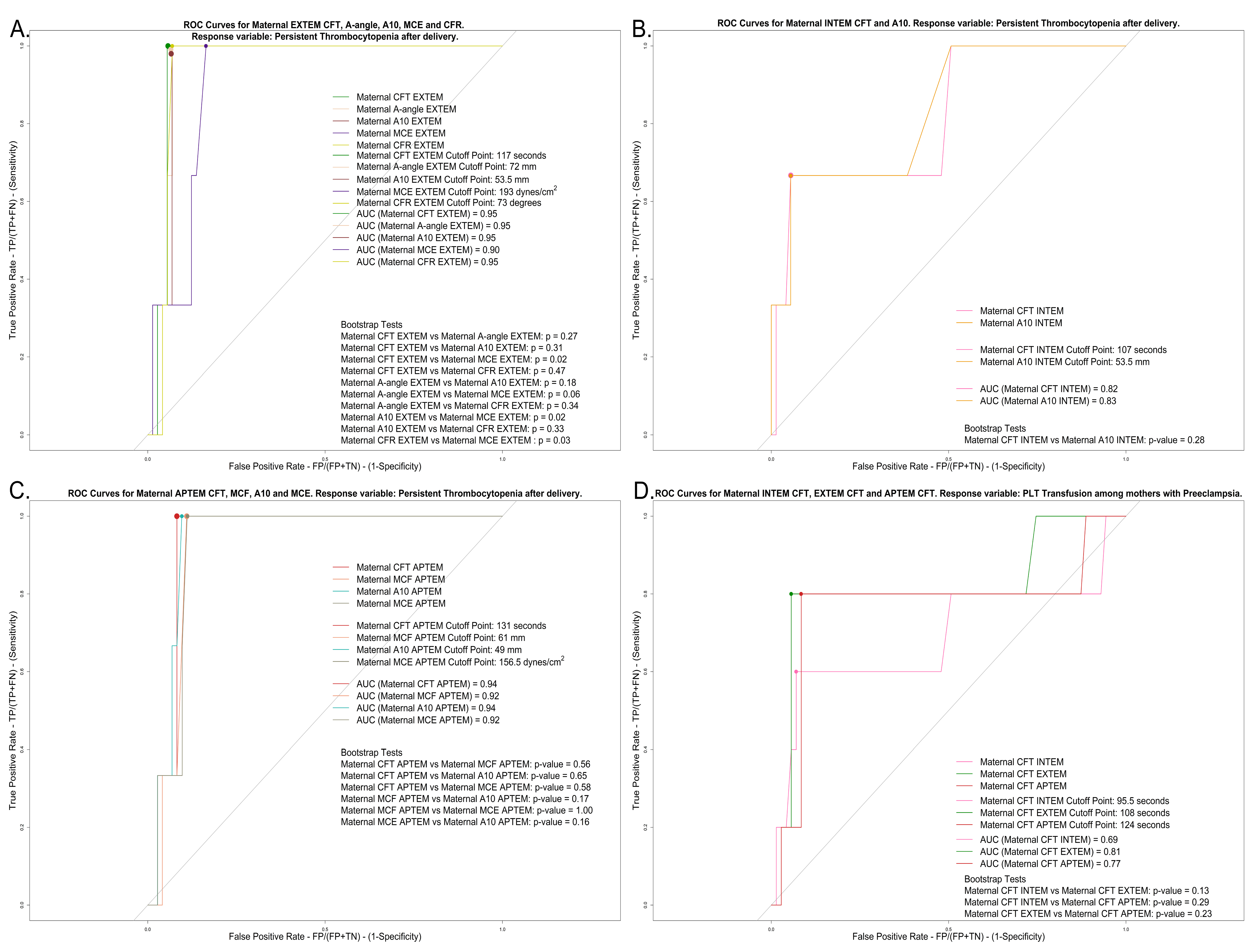
3.4. Correlations, Regressions, and ROCs
4. Discussion
4.1. Main Findings
4.2. Pregnant Women
4.3. Neonates
4.4. Limitations and Strengths
4.5. Future Implications
- Large prospective studies should develop ROTEM reference patterns for PE and specify quantitative thresholds for predicting severe outcomes (e.g., progression to HELLP). This could confirm specific ROTEM parameters as predictive factors and improve maternal risk assessment.
- Trials are required to evaluate ROTEM-guided care methods in PE. Randomized trials could compare conventional care to ROTEM to administer tailored transfusions or to adjust delivery timing. Such trials would establish whether ROTEM-guided, tailored therapy improves maternal and newborn outcomes (by averting catastrophic bleeding or thrombosis) compared with standard management.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A10, 20, 30 | Amplitude at 10, 20, 30 min after Clotting Time |
| ACF | Actual Clot Firmness |
| ALT | Alanine Aminotransferase |
| APTEM | Aprotinin Rotational Thromboelastometry |
| APTT | Activated Partial Thromboplastin Time |
| AST | Aspartate Aminotransferase |
| AUC | Area Under Curve |
| CCTs | Conventional Coagulation Tests |
| CFR | Clot Formation Rate |
| CFT | Clot Formation Time |
| CI | Coagulation Index |
| CT | Clotting Time |
| EXTEM | Extrinsic Rotational Thromboelastometry |
| FIBTEM | Fibrinogen Rotational Thromboelastometry |
| GA | Gestational Age |
| GWs | Gestational Weeks |
| HELLP | Hemolysis, Elevated Liver enzymes, Low Platelets |
| HEPTEM | Heparinase Rotational Thromboelastometry |
| INR | International Normalized Ratio |
| INTEM | Intrinsic Rotational Thromboelastometry |
| K | Clot Kinetics |
| LDH | Lactate Dehydrogenase |
| LI60 | Lysis Index at 60 min after Clotting Time |
| LY60 | Lysis Index or Clot Lysis at 60 min after Maximum Amplitude |
| MA | Maximum Amplitude |
| MCE | Maximum Clot Elasticity |
| MCF | Maximum Clot Firmness |
| ML | Maximum Lysis |
| PAI-1, 2 | Plasminogen Activator Inhibitor 1, 2 |
| PE | Preeclampsia |
| PLT(s) | Platelet(s) |
| PT | Prothrombin Time |
| R | Reaction Time |
| ROC | Receiver Operating Characteristic |
| ROTEM | Rotational Thromboelastometry |
| TEG | Thromboelastography |
| tPA | tissue Plasminogen Activator |
| uPA | urokinase-type Plasminogen Activator |
References
- Kenny, L.C.; McCrae, K.R.; Cunningham, F.G. Platelets, coagulation, and the liver. In Chesley’s Hypertensive Disorders in Pregnancy, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 379–396. [Google Scholar] [CrossRef]
- Khadijah Ismail, S.; Higgins, J. Hemostasis in Pre-Eclampsia. Semin. Thromb. Hemost. 2011, 37, 111–117. [Google Scholar] [CrossRef]
- Millar, C.M.; Laffan, M. Hemostatic Changes in Normal Pregnancy. In Disorders of Thrombosis and Hemostasis in Pregnancy; Cohen, H., O’Brien, P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–13. ISBN 978-3-319-15119-9. [Google Scholar]
- Warren, B.B.; Moyer, G.C.; Manco-Johnson, M.J. Hemostasis in the Pregnant Woman, the Placenta, the Fetus, and the Newborn Infant. Semin. Thromb. Hemost. 2023, 49, 319–329. [Google Scholar] [CrossRef]
- Kontovazainitis, C.-G.; Gialamprinou, D.; Theodoridis, T.; Mitsiakos, G. Hemostasis in Pre-Eclamptic Women and Their Offspring: Current Knowledge and Hemostasis Assessment with Viscoelastic Tests. Diagnostics 2024, 14, 347. [Google Scholar] [CrossRef]
- Committee on Practice Bulletin. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- Valensise, H.; Vasapollo, B.; Gagliardi, G.; Novelli, G.P. Early and Late Preeclampsia: Two Different Maternal Hemodynamic States in the Latent Phase of the Disease. Hypertension 2008, 52, 873–880. [Google Scholar] [CrossRef]
- Lidan, H.; Jianbo, W.; Liqin, G.; Jifen, H.; Lin, L.; Xiuyan, W. The diagnostic efficacy of thrombelastography (TEG) in patients with preeclampsia and its association with blood coagulation. Open Life Sci. 2019, 14, 335–341. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Z.; Cheng, Q.X.; Xu, J.; Liang, C. The ability of thromboelastography parameters to predict severe pre-eclampsia when measured during early pregnancy. Int. J. Gynecol. Obstet. 2019, 145, 170–175. [Google Scholar] [CrossRef]
- Murray, E.K.I.; Murphy, M.S.Q.; Smith, G.N.; Graham, C.H.; Othman, M. Thromboelastographic analysis of haemostasis in preeclamptic and normotensive pregnant women. Blood Coagul. Fibrinolysis 2018, 29, 567–572. [Google Scholar] [CrossRef]
- Spiezia, L.; Bogana, G.; Campello, E.; Maggiolo, S.; Pelizzaro, E.; Carbonare, C.D.; Gervasi, M.T.; Simioni, P. Whole blood thromboelastometry profiles in women with preeclampsia. Clin. Chem. Lab. Med. CCLM 2015, 53, 1793–1798. [Google Scholar] [CrossRef]
- De Lange, N.M.; Van Rheenen-Flach, L.E.; Lancé, M.D.; Mooyman, L.; Woiski, M.; Van Pampus, E.C.; Porath, M.; Bolte, A.C.; Smits, L.; Henskens, Y.M.; et al. Peri-partum reference ranges for ROTEM® thromboelastometry. Br. J. Anaesth. 2014, 112, 852–859. [Google Scholar] [CrossRef]
- Orlikowski, C.E.; Rocke, D.A.; Murray, W.B.; Gouws, E.; Moodley, J.; Kenoyer, D.G.; Byrne, S. Thrombelastography changes in pre-eclampsia and eclampsia. Br. J. Anaesth. 1996, 77, 157–161. [Google Scholar] [CrossRef]
- Ahmad, A.; Kohli, M.; Malik, A.; Kohli, M.; Bogra, J.; Abbas, H.; Gupta, R.; Kushwaha, B.B. Role of Thromboelastography Versus Coagulation Screen as a Safety Predictor in Pre-eclampsia/Eclampsia Patients Undergoing Lower-Segment Caesarean Section in Regional Anaesthesia. J. Obstet. Gynecol. India 2016, 66, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswami, S.; Butwick, A. Latest advances in postpartum hemorrhage management. Best Pract. Res. Clin. Anaesthesiol. 2022, 36, 123–134. [Google Scholar] [CrossRef]
- Kim, S.-M.; Sohn, C.H.; Kwon, H.; Ryoo, S.M.; Ahn, S.; Seo, D.W.; Kim, W.Y. Thromboelastography as an early prediction method for hypofibrinogenemia in emergency department patients with primary postpartum hemorrhage. Scand. J. Trauma Resusc. Emerg. Med. 2024, 32, 85. [Google Scholar] [CrossRef]
- Burkhart, J.G.; Smith, R.P.; Hill, T.M.; Winfield, R.D. What, When, and Why: Viscoelastic Hemostatic Assays and Their Uses in Trauma Resuscitation. Am. Surg. 2025, 91, 626–632. [Google Scholar] [CrossRef]
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef]
- Amgalan, A.; Allen, T.; Othman, M.; Ahmadzia, H.K. Systematic review of viscoelastic testing (TEG/ROTEM) in obstetrics and recommendations from the women’s SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 1813–1838. [Google Scholar] [CrossRef]
- NICE Guideline Hypertension in Pregnancy: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng133/resources/hypertension-in-pregnancy-diagnosis-and-management-pdf-66141717671365 (accessed on 18 August 2022).
- Robillard, P.; Dekker, G.; Scioscia, M.; Bonsante, F.; Iacobelli, S.; Boukerrou, M.; Hulsey, T.C. Validation of the 34-week gestation as definition of late onset preeclampsia: Testing different cutoffs from 30 to 37 weeks on a population-based cohort of 1700 preeclamptics. Acta Obstet. Gynecol. Scand. 2020, 99, 1181–1190. [Google Scholar] [CrossRef]
- Backes, C.H.; Markham, K.; Moorehead, P.; Cordero, L.; Nankervis, C.A.; Giannone, P.J. Maternal Preeclampsia and Neonatal Outcomes. J. Pregnancy 2011, 2011, 214365. [Google Scholar] [CrossRef]
- Committee on Practice Bulletin. ACOG Practice Bulletin No. 203: Chronic Hypertension in Pregnancy. Obstet. Gynecol. 2019, 133, e26–e50. [Google Scholar] [CrossRef]
- Mitsiakos, G.; Gialamprinou, D.; Kontovazainitis, C.-G.; Moraitis, A.; Katsaras, G.; Pouliakis, A.; Diamanti, E. Coagulation assessment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infected pregnant women and their offspring by using rotational thromboelastometry (ROTEM). J. Perinat. Med. 2024, 52, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Gialamprinou, D.; Kontovazainitis, C.-G.; Pouliakis, A.; Fleva, A.; Markopoulou, M.; Bessina, M.; Katsaras, G.N.; Chatziioannidis, I.; Giannakou, A.; Roilides, E.; et al. Sepsis-induced coagulopathy in preterm neonates with Gram-positive sepsis presents with hypercoagulation and reduced platelet activation compared with healthy preterm neonates. Res. Pract. Thromb. Haemost. 2023, 7, 100100. [Google Scholar] [CrossRef]
- Sharma, S.K.; Philip, J.; Whitten, C.W.; Padakandla, U.B.; Landers, D.F. Assessment of Changes in Coagulation in Parturients with Preeclampsia Using Thromboelastography. Anesthesiology 1999, 90, 385–390. [Google Scholar] [CrossRef]
- Xie, X.; Wang, M.; Lu, Y.; Zeng, J.; Wang, J.; Zhang, C.; Zhu, H.; Song, Y.; Han, L.; Liu, Y.; et al. Thromboelastography (TEG) in normal pregnancy and its diagnostic efficacy in patients with gestational hypertension, gestational diabetes mellitus, or preeclampsia. J. Clin. Lab. Anal. 2021, 35, e23623. [Google Scholar] [CrossRef]
- Davidge, S.T.; Signorella, A.P.; Lykins, D.L.; Gilmour, C.H.; Roberts, J.M. Evidence of endothelial activation and endothelial activators in cord blood of infants of preeclamptic women. Am. J. Obstet. Gynecol. 1996, 175, 1301–1306. [Google Scholar] [CrossRef]
- Catarino, C.; Rebelo, I.; Belo, L.; Rocha, S.; Castro, E.B.; Patrício, B.; Quintanilha, A.; Santos-Silva, A. Fetal and maternal angiogenic/anti-angiogenic factors in normal and preeclamptic pregnancy. Growth Factors 2009, 27, 345–351. [Google Scholar] [CrossRef]
- Bulbul, M.; Atalay, M.A.; Demir, B.C.; Turker, G.; Esmer, A. Detecting coagulability status by thromboelastography in women with the history of preeclampsia and inherited thrombophilia. Clin. Exp. Obstet. Gynecol. 2015, 42, 462–468. [Google Scholar] [CrossRef]
- Andersson, M.; Bengtsson, P.; Karlsson, O.; Thörn, S.-E.; Thorgeirsdottir, L.; Bergman, L.; Oras, J.; Romlin, B. Platelet aggregation and thromboelastometry monitoring in women with preeclampsia: A prospective observational study. Int. J. Obstet. Anesth. 2025, 61, 104297. [Google Scholar] [CrossRef]
- Fiol, A.G.; Yoo, J.; Yanez, D.; Fardelmann, K.L.; Salimi, N.; Alian, M.; Mancini, P.; Alian, A. Baseline rotational thromboelastometry (ROTEM) values in a healthy, diverse obstetric population and parameter changes by pregnancy-induced comorbidities. Bayl. Univ. Med. Cent. Proc. 2023, 36, 562–571. [Google Scholar] [CrossRef]
- Stallmach, T.; Karolyi, L.; Lichtlen, P.; Maurer, M.; Hebisch, G.; Joller, H.; Marti, H.H.; Gassmann, M. Fetuses from Preeclamptic Mothers Show Reduced Hepatic Erythropoiesis. Pediatr. Res. 1998, 43, 349–354. [Google Scholar] [CrossRef]
- Reverdiau-Moalic, P.; Delahousse, B.; Body, G.; Bardos, P.; Leroy, J.; Gruel, Y. Evolution of blood coagulation activators and inhibitors in the healthy human fetus. Blood 1996, 88, 900–906. [Google Scholar] [CrossRef]
- Zanardo, V.; Savio, V.; Sabrina, G.; Franzoi, M.; Zerbinati, P.; Fadin, M.; Tognin, G.; Tormene, D.; Pagnan, A.; Simioni, P. The effect of pre-eclampsia on the levels of coagulation and fibrinolysis factors in umbilical cord blood of newborns. Blood Coagul. Fibrinolysis 2005, 16, 177–181. [Google Scholar] [CrossRef]
- Haugen, G.; Hanson, M.; Kiserud, T.; Crozier, S.; Inskip, H.; Godfrey, K.M. Fetal Liver-Sparing Cardiovascular Adaptations Linked to Mother’s Slimness and Diet. Circ. Res. 2005, 96, 12–14. [Google Scholar] [CrossRef]
- Karapati, E.; Valsami, S.; Sokou, R.; Pouliakis, A.; Tsaousi, M.; Sulaj, A.; Iliodromiti, Z.; Iacovidou, N.; Boutsikou, T. Hemostatic Profile of Intrauterine Growth-Restricted Neonates: Assessment with the Use of NATEM Assay in Cord Blood Samples. Diagnostics 2024, 14, 178. [Google Scholar] [CrossRef]
- Mitsiakos, G.; Papaioannou, G.; Papadakis, E.; Chatziioannidis, E.; Giougi, E.; Karagianni, P.; Evdoridou, J.; Malindretos, P.; Athanasiou, M.; Athanassiadou, F.; et al. Haemostatic profile of full-term, healthy, small for gestational age neonates. Thromb. Res. 2009, 124, 288–291. [Google Scholar] [CrossRef]
- Katsaras, G.; Gialamprinou, D.; Kontovazainitis, C.-G.; Psaroulaki, E.; Mitsiakos, G. Neonatal hemostasis and the use of thromboelastography/rotational thromboelastometry in the neonatal period. Minerva Pediatr. 2024, 76, 425–438. [Google Scholar] [CrossRef]
- Katsaras, G.Ν.; Sokou, R.; Tsantes, A.G.; Piovani, D.; Bonovas, S.; Konstantinidi, A.; Ioakeimidis, G.; Parastatidou, S.; Gialamprinou, D.; Makrogianni, A.; et al. The use of thromboelastography (TEG) and rotational thromboelastometry (ROTEM) in neonates: A systematic review. Eur. J. Pediatr. 2021, 180, 3455–3470. [Google Scholar] [CrossRef]
- Delaney, J.; Nunes, G.D.C.; Simoneau, J.; Beltempo, M.; Malhamé, I.; Goudie, C.; Altit, G. Thrombocytopenia and neonatal outcomes among extremely premature infants exposed to maternal hypertension. Pediatr. Blood Cancer 2023, 70, e30131. [Google Scholar] [CrossRef]
- Lox, C.; Word, R.; Corrigan, J. Effects of Preeclampsia on Maternal and Cord Blood Clotting Activity. Am. J. Perinatol. 1985, 2, 279–282. [Google Scholar] [CrossRef]
- Kalagiri, R.; Choudhury, S.; Carder, T.; Govande, V.; Beeram, M.; Uddin, M. Neonatal Thrombocytopenia as a Consequence of Maternal Preeclampsia. Am. J. Perinatol. Rep. 2015, 06, e42–e47. [Google Scholar] [CrossRef]
- Bayoumi, M.A.A.; Ali, A.A.H.; Hamad, S.G.; Ali, A.A.M.; Elmalik, E.E.; Elkalaf, M.M.I.R.; Moustafa, B.A.A.; Shaltout, D.A.D.A.; Chandra, P.; Langtree, L.J.; et al. Effect of Maternal Preeclampsia on Hematological Profile of Newborns in Qatar. BioMed Res. Int. 2020, 2020, 7953289. [Google Scholar] [CrossRef]
- Baschat, A. Absent umbilical artery end-diastolic velocity in growth-restricted fetuses: A risk factor for neonatal thrombocytopenia. Obstet. Gynecol. 2000, 96, 162–166. [Google Scholar] [CrossRef]
- Marins, L.R.; Anizelli, L.B.; Romanowski, M.D.; Sarquis, A.L. How does preeclampsia affect neonates? Highlights in the disease’s immunity. J. Matern. Fetal Neonatal Med. 2019, 32, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Tanjung, M.T.; Siddik, H.D.; Hariman, H.; Koh, S.C.L. Coagulation and Fibrinolysis in Preeclampsia and Neonates. Clin. Appl. Thromb. 2005, 11, 467–473. [Google Scholar] [CrossRef]
- Higgins, J.R.; Bonnar, J.; Norris, L.A.; Darling, M.R.N.; Walshe, J.J. The Effect of Pre-eclampsia on Coagulation and Fibrinolytic Activation in the Neonate. Thromb. Res. 2000, 99, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Roes, E.M.; Sweep, C.G.F.; Thomas, C.M.; Zusterzeel, P.L.; Geurts-Moespot, A.; Peters, W.H.; Steegers, E.A. Levels of plasminogen activators and their inhibitors in maternal and umbilical cord plasma in severe preeclampsia. Am. J. Obstet. Gynecol. 2002, 187, 1019–1025. [Google Scholar] [CrossRef]

| Pregnant Women | PE 1 Group (N = 31) | Pregnant Controls (N = 45) | p-Value | Neonates | Neonates Born to Mothers with PE (N = 34) | Neonates Born to Pregnant Controls (N = 47) | p-Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 34 ± 5.9 | 33 ± 5.7 | 0.45 | GA 6 (weeks) | 33 (30–36) | 36 (31.5–38) | 0.07 |
| Age above 40 | 4/31 (12.9%) | 5/44 (11.4%) | 1.00 | Gender (male) | 17/34 (50%) | 26/47 (55.3%) | 0.64 |
| Race | All Caucasian | All Caucasian | - | Preterm | 28/34 (82.3%) | 32/47 (68.1%) | 0.15 |
| BMI 2 before (kg/m2) | 26.3 (23.5–28.4) | 23.3 (21.2–27.7) | 0.06 | Extremely preterm | 4/28 (14.2%) | 4/32 (12.5%) | 1.00 |
| BMI at labor (kg/m2) | 29.6 (28.8–31.2) | 28.3 (25.3–31.6) | 0.12 | Very preterm | 8/28 (28.6%) | 8/32 (25%) | 0.76 |
| BMI difference (kg/m2) | 3.6 (1.7–5) | 3.4 (2.5–5.5) | 0.57 | Moderate preterm | 8/28 (28.6%) | 8/32 (25%) | 0.76 |
| Smoking before pregnancy | 7/31 (22.6%) | 15/45 (33.3%) | 0.31 | Late preterm | 8/28 (28.6%) | 12/32 (37.5%) | 0.46 |
| Smoking during pregnancy | 2/31 (6.5%) | 9/45 (20%) | 0.18 | Birthweight (grams) | 2035 (1475–2752.5) | 2450 (1435–3140) | 0.27 |
| Obstetric History | Below 1000 g | 7/34 (20.6%) | 5/47 (10.6%) | 0.21 | |||
| Gravidity | 2 (1–3) | 2 (2–3) | 0.06 | Below 2500 g | 22/34 (64.7%) | 25/47 (53.2%) | 0.30 |
| Parity | 1 (1–2) | 2 (1–2) | 0.049 | Birthweight percentile | 46.8 ± 30.7 | 45.3 ± 28 | 0.82 |
| PE in previous pregnancies | 3/31 (9.7%) | 3/45 (6.7%) | 0.68 | Length (cm) | 44.4 (36–49) | 47 (40.5–50) | 0.20 |
| Pregnancy losses | 0 (0–0.5) | 1 (0–1) | 0.03 | Length percentile | 57 (26.8–86.3) | 56 (24.8–86.4) | 0.79 |
| Drugs during Pregnancy | Head circumference (cm) | 31 (28.3–33.4) | 32.5 (29.2–34.5) | 0.39 | |||
| Aspirin | 21/31 (67,7%) | 24/45 (53.3%) | 0.21 | Circumference percentile | 54.5 (42.8–87.3) | 68.9 (42.5–83.5) | 0.86 |
| LMWH 3 | 12/31 (38.7%) | 16/45 (35.6%) | 0.78 | SGA 7 | 4/34 (11.8%) | 5/47 (10.6%) | 1.00 |
| Progesterone | 6/31 (19.4%) | 11/45 (24.4%) | 0.60 | IUGR 8 | 8/34 (23.5%) | 10/47 (21.3%) | 0.81 |
| Thyroxine | 14/31 (45.2%) | 17/45 (37.8%) | 0.52 | Pregnancy Characteristics | |||
| Steroids | 21/31 (67.7%) | 28/45 (62.2%) | 0.62 | Twins | 6/34 (17.7%) | 4/47 (8.5%) | 0.31 |
| Comorbidities and Major Complications | ART 9 | 12/34 (35.3%) | 13/47 (27.7%) | 0.46 | |||
| Hypothyroidism | 14/31 (45.2%) | 17/45 (37.8%) | 0.52 | Mean uterine artery’s PI 10 | 0.9 (0.8–1.5) | 0.7 (0.6–0.9) | 0.01 |
| Hypercholesterolemia | 1/31 (3.2%) | 4/45 (8.9%) | 0.64 | Umbilical artery’s PI | 1 ± 0.2 | 1 ± 0.1 | 0.77 |
| Persistent thrombocytopenia after delivery | 3/31 (9.7%) | 0/45 (0%) | 0.06 | Delivery Characteristics | |||
| Platelet transfusion | 5/31 (16.1%) | 0/45 (0%) | 0.009 | Delivery Type (CS 11) | 33/34 (97.1%) | 42/47 (89.4%) | 0.39 |
| ICU 4 admission | 1/31 (3.2%) | 0/45 (0%) | 0.41 | Placenta’s weight (grams) | 385 (302–487.5) | 450 (390–500) | 0.10 |
| Death | 0/31 (0%) | 0/45 (0%) | - | Umbilical cord’s clamp (seconds) | 60 (42.5–60) | 60 (40–60) | 0.85 |
| PE Type | Apgar score at 1 min | 8 (7–8) | 8 (7–8) | 0.50 | |||
| Early-onset PE | 19/31 (61.3%) | NA | - | Apgar score at 5 min | 8 (8–9) | 9 (8–9) | 0.03 |
| Late-onset PE | 12/31 (38.7%) | NA | - | Neonatal Major Complications | |||
| Severe PE | 11/31 (35.5%) | NA | - | NICU 12 admission | 24/34 (70.6%) | 24/47 (51.1%) | 0.08 |
| HELLP 5 syndrome | 2/31 (6.5%) | NA | - | Respiratory assistance | 21/34 (61.8%) | 26/47 (55.3%) | 0.56 |
| Infection | 11/34 (32.4%) | 9/47 (19.2%) | 0.17 | ||||
| Sepsis | 4/34 (11.8%) | 2/47 (4.3%) | 0.23 | ||||
| RDS 13 | 13/34 (38.2%) | 14/47 (29.8%) | 0.43 | ||||
| BPD 14 | 5/34 (14.7%) | 2/47 (4.3%) | 0.12 | ||||
| IVH 15 | 2/34 (5.9%) | 2/47 (4.3%) | 1.00 | ||||
| NEC 16 | 2/34 (5.9%) | 1/47 (2.1%) | 0.57 | ||||
| Thrombocytopenia | 3/34 (8.8%) | 1/47 (2.1%) | 0.30 | ||||
| Transfusion (blood/platelet/plasma) | 7/34 (20.6%) | 11/47 (23.4%) | 0.76 | ||||
| Death | 1/34 (2.9%) | 0/47 (0%) | 0.42 | ||||
| Pregnant Women | PE 1 Group (N = 31) | Pregnant Controls (N = 45) | p-Value | Neonates | Neonates Born to Mothers with PE (N = 34) | Neonates Born to Pregnant Controls (N = 47) | Adjusted p-Value (Adjusted for GA 20 and Apgar Score at 5 min) | ||
|---|---|---|---|---|---|---|---|---|---|
| Platelet count × 109/L | 167 (138.5–201.5) | 234 (197–271) | <0.001 | Platelet count × 10/L | 230 (168–257) | 282 (226.5–322.5) | 0.002 | ||
| MPV 2 (fL) | 9.8 (8.4–10.8) | 10.2 (9.4–10.7) | 0.61 | MPV (fL) | 7.4 (6.9–8) | 9.1 (7.4–9.8) | 0.01 | ||
| Platelet count < 100 × 109/L | 3/31 (9.7%) | 0/45 (0%) | 0.06 | Platelet count < 100 × 109/L | 0/33 (0%) | 0/47 (0%) | - | ||
| Platelet count < 150 × 109/L | 12/31 (38.7%) | 1/45 (2.2%) | <0.001 | Platelet count < 150 × 109/L | 5/33 (15.2%) | 1/47 (2.1%) | 0.08 | ||
| Antithrombin III (%) | 92.8 ± 14.8 | 97.6 ± 9.9 | 0.13 | - | - | - | - | ||
| Protein C activity (%) | 101 (86.5–115.5) | 103 (97–111) | 0.29 | - | - | - | - | ||
| Free Protein S Ag (%) | 28.7 ± 4.9 | 39.5 ± 6.3 | <0.001 | - | - | - | - | ||
| CCTs 3 | PT 8 (seconds) | 12.5 (12.1–13.1) | 12.8 (12.3–13.4) | 0.28 | CCTs | PT (seconds) | 14.3 (13.4–16.5) | 14.6 (13.4–15.6) | 0.87 |
| APTT 9 (seconds) | 29.1 (26.7–30.3) | 28.5 (27.4–29.5) | 0.44 | APTT (seconds) | 42 (37.3–52.9) | 42.5 (38.2–48.6) | 0.70 | ||
| INR 10 | 0.92 (0.91–0.96) | 0.95 (0.92–0.99) | 0.11 | INR | 1.06 (0.99–1.19) | 1.08 (0.98–1.14) | 0.76 | ||
| Fibrinogen (mg/dL) | 514.6 ± 110.8 | 456.5 ± 108.8 | 0.03 | Fibrinogen (mg/dL) | 208.5 (161.5–276) | 214 (178.3–280.5) | 0.68 | ||
| D-Dimers (ng/mL) | 1980 (1610–2600) | 1122.5 (880–1565) | <0.001 | D-Dimers (ng/mL) | 2003.5 (907.5–2702.5) | 870 (571–1200) | <0.001 | ||
| INTEM 4 | CT 11 (seconds) | 163.4 ± 26.3 | 169.9 ± 29 | 0.32 | INTEM | CT (seconds) | 217 (197.3–250.8) | 214 (189.5–247) | 0.66 |
| CFT 12 (seconds) | 60 (48.5–65.5) | 64 (54–73) | 0.19 | CFT (seconds) | 98 (88–128.8) | 77 (64–119.5) | 0.005 | ||
| MCF 13 (mm) | 73 (69–74.5) | 70 (66–72) | 0.053 | MCF (mm) | 50.4 ± 6.2 | 55.7 ± 8.4 | 0.003 | ||
| A-angle (° degrees) | 79 (77–80) | 77 (76–79) | 0.08 | A-angle (° degrees) | 71.5 (67.3–74) | 75 (69–77) | 0.01 | ||
| A10 14 (mm) | 65 (60.5–68) | 62 (57–65) | 0.14 | A10 (mm) | 44.9 ± 6.7 | 50.7 ± 9.5 | 0.003 | ||
| A30 14 (mm) | 71 (68.5–74.5) | 69 (66–72) | 0.10 | A30 (mm) | 49.9 ± 6.1 | 55 ± 8.2 | 0.003 | ||
| MCE 15 (dynes/cm2) | 255 (224–294) | 236 (194–258) | 0.08 | MCE (dynes/cm2) | 100 (90.5–114.5) | 131 (95.5–159) | 0.003 | ||
| LI60 16 (%) | 97 (97–100) | 97 (95–98.25) | 0.06 | LI60 (%) | 91.9 ± 3.3 | 92.2 ± 3.5 | 0.65 | ||
| ML 17 (%) | 4.3 ± 3.2 | 6.2 ± 3.9 | 0.03 | ML (%) | 12 (9.3–16) | 11 (8–15.5) | 0.44 | ||
| CFR 18 (° degrees) | 80 (79–81.5) | 79 (77–80) | 0.03 | CFR (° degrees) | 74 (70–76) | 76 (70.5–78.5) | 0.06 | ||
| ACF 19 (mm) | 69 (66.3–71) | 65 (61–69) | 0.03 | ACF (mm) | 44.3 ± 5.9 | 49.2 ± 8.9 | 0.003 | ||
| EXTEM 5 | CT (seconds) | 58 (53.5–62) | 58 (52–62) | 0.79 | EXTEM | CT (seconds) | 58 (52.3–65) | 56 (51–67) | 0.53 |
| CFT (seconds) | 61 (49–78.5) | 68 (56–79) | 0.39 | CFT (seconds) | 121 (97–155) | 94 (75.5–146.5) | 0.02 | ||
| MCF (mm) | 69 (66.5–75) | 71 (67–73) | 0.82 | MCF (mm) | 51 (44–54.8) | 56 (47.5–61) | 0.006 | ||
| A-angle (° degrees) | 78.5 (75–80) | 77 (75–79) | 0.19 | A-angle (° degrees) | 67 (64.3–71) | 71 (65–74.5) | 0.04 | ||
| A10 (mm) | 63 (59.5–68.5) | 62 (58–66) | 0.55 | A10 (mm) | 44.5 (37.3–48) | 51 (40–55.5) | 0.009 | ||
| A30 (mm) | 69 (66.5–74) | 70 (66–72) | 0.93 | A30 (mm) | 50.5 (44–54) | 55 (47–60) | 0.01 | ||
| MCE (dynes/cm2) | 230.1 ± 76.1 | 237.9 ± 53.3 | 0.60 | MCE (dynes/cm2) | 103 (77.8–120.5) | 126 (90.5–154) | 0.007 | ||
| LI60 (%) | 95 (89–98) | 96 (92.8–98) | 0.32 | LI60 (%) | 90 (87–92) | 92 (89–94) | 0.004 | ||
| ML (%) | 9 (4.5–14.5) | 8 (5–13) | 0.83 | ML (%) | 17.5 (12.3–26) | 13 (9.5–19.5) | 0.06 | ||
| CFR (° degrees) | 79.5 (76–81) | 78 (76–80) | 0.26 | CFR (° degrees) | 71 (68–74) | 74 (67–76.5) | 0.07 | ||
| ACF (mm) | 62 (57.5–66) | 64 (59–67) | 0.34 | ACF (mm) | 40 (34.3–45) | 45 (41–53) | 0.001 | ||
| FIBTEM 6 | CT (seconds) | 52 (49–57.5) | 57 (53–67) | 0.02 | FIBTEM | CT (seconds) | 58.5 (51.3–73.8) | 60 (50.5–71) | 0.96 |
| CFT (seconds) | - | - | - | CFT (seconds) | - | - | - | ||
| MCF (mm) | 27.7 ± 7.5 | 24.5 ± 6.3 | 0.051 | MCF (mm) | 12 (9–14.8) | 16 (11.5–18.5) | 0.009 | ||
| A-angle (° degrees) | 77 (74–79) | 75 (71.8–77.3) | 0.04 | A-angle (° degrees) | 65.8 ± 6.2 | 69.2 ± 6.9 | 0.19 | ||
| A10 (mm) | 25.3 ± 7.7 | 22.3 ± 6.2 | 0.07 | A10 (mm) | 11 (9–13) | 14 (9.5–16.5) | 0.009 | ||
| A30 (mm) | 27.5 ± 7.6 | 24.4 ± 6.3 | 0.06 | A30 (mm) | 12 (9.3–15) | 16 (11.5–19) | 0.009 | ||
| MCE (dynes/cm2) | 39.8 ± 15.6 | 33.4 ± 11.8 | 0.04 | MCE (dynes/cm2) | 13.5 (10–16) | 19 (12.5–23) | 0.005 | ||
| LI60 (%) | 100 (99–100) | 100 (99–100) | 0.45 | LI60 (%) | 97 (91.3–100) | 96.5 (92.3–100) | 0.87 | ||
| ML (%) | 1 (0–2) | 0 (0–2) | 0.17 | ML (%) | 8 (2–13.8) | 7 (1–12.5) | 0.62 | ||
| CFR (° degrees) | 78.5 (75–80.8) | 76 (73–78) | 0.02 | CFR (° degrees) | 67.9 ± 5.6 | 70.7 ± 5.7 | 0.17 | ||
| ACF (mm) | 27.5 ± 7.1 | 24.6 ± 5.8 | 0.04 | ACF (mm) | 11 (9–12) | 14 (10–17.5) | 0.01 | ||
| APTEM 7 | CT (seconds) | 55 (51–59.5) | 59 (54–67) | 0.03 | APTEM | CT (seconds) | 58 (50–66.8) | 57 (47.5–70) | 0.92 |
| CFT (seconds) | 69 (52.5–117) | 75 (65–86) | 0.26 | CFT (seconds) | 133 (105–164) | 97 (79.5–143) | 0.007 | ||
| MCF (mm) | 72 (66.5–74) | 68 (65–71) | 0.06 | MCF (mm) | 48 (41.3–53) | 54 (46–58) | 0.002 | ||
| A-angle (° degrees) | 78 (74.5–80) | 75 (73–77) | 0.003 | A-angle (° degrees) | 67 (62.5–71) | 71 (65–74) | 0.08 | ||
| A10 (mm) | 62 (56–67) | 58 (55–62) | 0.08 | A10 (mm) | 39.8 ± 9.3 | 47.1 ± 9.5 | 0.001 | ||
| A30 (mm) | 71 (65–74) | 67 (64–70) | 0.06 | A30 (mm) | 46.2 ± 9.5 | 52.8 ± 8 | 0.001 | ||
| MCE (dynes/cm2) | 236.6 ± 74.5 | 214.5 ± 54.3 | 0.14 | MCE (dynes/cm2) | 92 (69.3–113.5) | 119 (85.5–138.5) | 0.002 | ||
| LI60 (%) | 98 (97–99.3) | 98 (96.8–99) | 0.35 | LI60 (%) | 90 (82.3–93) | 93 (89.3–94.8) | 0.008 | ||
| ML (%) | 4 (3–8) | 6 (3–9) | 0.31 | ML (%) | 19.5 (13–27) | 14 (9–19) | 0.005 | ||
| CFR (° degrees) | 79 (76.5–81) | 76 (74–78) | 0.001 | CFR (° degrees) | 71 (67–74) | 73 (68–76) | 0.22 | ||
| ACF (mm) | 67 (61–71) | 65 (59–68) | 0.13 | ACF (mm) | 37 (32–43.8) | 45 (39.5–51.5) | <0.001 | ||
| Neonates | Neonates Born to Mothers with Non-Severe PE 1 (N = 13) | Neonates Born to Mothers with Severe PE (N = 13) | Neonates Born to Pregnant Controls (N = 21) | Adjusted p-Value (Adjusted for Gestational Age) | ||||
|---|---|---|---|---|---|---|---|---|
| Across Subgroups | Non-Severe PE vs. Severe PE | Non-Severe PE vs. Controls | Severe PE vs. Controls | |||||
| Platelet count × 109/L | 229 ± 49.3 | 198.8 ± 60.9 | 264 ± 53.3 | 0.004 | 0.65 | 0.14 | 0.004 | |
| MPV 2 (fL) | 7.7 (7.1–8) | 7.8 (6.7–9.2) | 9 (7.4–9.8) | 0.15 | - | - | - | |
| Platelet count < 100 × 109/L | 0/13 (0%) | 0/13 (0%) | 0/21 (0%) | - | - | - | - | |
| Platelet count < 150 × 109/L | 1/13 (7.7%) | 3/13 (23.1%) | 1/21 (4.8%) | 0.36 | - | - | - | |
| CCTs 3 | PT 8 (seconds) | 15.6 (13.7–16.4) | 14.1 (13.4–14.8) | 15.8 (15–17.2) | 0.16 | - | - | - |
| APTT 9 (seconds) | 44.3 (38.8–47.4) | 42 (37.1–53.2) | 46.4 (41.8–55.2) | 0.50 | - | - | - | |
| INR 10 | 1.12 (1–1.18) | 1.04 (0.99–1.1) | 1.15 (1.09–1.26) | 0.17 | - | - | - | |
| Fibrinogen (mg/dL) | 193 (159–261) | 185 (160–253) | 210 (161.5–293.8) | 0.87 | - | - | - | |
| D-Dimers (ng/mL) | 1550 (1020–2210) | 2340 (1900–2560) | 950 (680–1650) | 0.003 | 0.68 | 0.03 | 0.003 | |
| INTEM 4 | CT 11 (seconds) | 218 (189–247) | 206 (196–255) | 203 (188–247) | 0.73 | - | - | - |
| CFT 12 (seconds) | 88 (77–106) | 110 (96–131) | 70 (62–87) | 0.002 | 0.22 | 0.12 | 0.001 | |
| MCF 13 (mm) | 51.9 ± 5.5 | 48.5 ± 4.9 | 56.7 ± 8.2 | 0.005 | 0.72 | 0.14 | 0.004 | |
| A-angle (° degrees) | 73 (70–74) | 69 (65–72) | 76 (72–77) | 0.008 | 0.38 | 0.16 | 0.005 | |
| A10 14 (mm) | 47 (44–51) | 43 (41–45) | 54 (48–59) | <0.001 | 0.15 | 0.09 | <0.001 | |
| A30 14 (mm) | 51.5 ± 5.3 | 47.9 ± 4.9 | 56.1 ± 8.3 | 0.005 | 0.57 | 0.18 | 0.004 | |
| MCE 15 (dynes/cm2) | 110.9 ± 26.4 | 94.5 ± 17.2 | 137.6 ± 39.7 | 0.001 | 0.62 | 0.06 | 0.001 | |
| LI60 16 (%) | 92 (90–93) | 93 (92–94) | 93 (92–96) | 0.40 | - | - | - | |
| ML 17 (%) | 11.5 ± 4.1 | 11.9 ± 4.4 | 10 ± 4 | 0.38 | - | - | - | |
| CFR 18 (° degrees) | 75 (72–77) | 71 (67–74) | 77 (74–79) | 0.02 | 0.45 | 0.22 | 0.01 | |
| ACF 19 (mm) | 45.8 ± 4.9 | 42.9 ± 4.5 | 51.2 ± 8.4 | 0.003 | 0.76 | 0.10 | 0.003 | |
| EXTEM 5 | CT (seconds) | 57 (50–65) | 58 (56–71) | 52 (49–64) | 0.50 | - | - | - |
| CFT (seconds) | 119 (97–127) | 127 (101.8–175.5) | 91 (77–114) | 0.009 | 0.47 | 0.13 | 0.006 | |
| MCF (mm) | 52 (49–55) | 49 (38–51) | 57 (48–61) | 0.005 | 0.24 | 0.20 | 0.003 | |
| A-angle (° degrees) | 68 (66–71) | 67 (64–71) | 72 (69–74) | 0.07 | - | - | - | |
| A10 (mm) | 45 (44–50) | 42 (34–45) | 52 (42–55) | 0.01 | 0.42 | 0.17 | 0.006 | |
| A30 (mm) | 52 (48–54) | 48 (38–51) | 57 (47–60) | 0.006 | 0.22 | 0.25 | 0.003 | |
| MCE (dynes/cm2) | 109.9 ± 26 | 85.7 ± 29.8 | 129.4 ± 39.3 | 0.002 | 0.31 | 0.18 | 0.001 | |
| LI60 (%) | 91 (90–92) | 89 (82–90) | 94 (91–95) | 0.008 | 0.21 | 0.32 | 0.004 | |
| ML (%) | 17 (11–23) | 16 (14–26) | 11 (6–15) | 0.06 | - | - | - | |
| CFR (° degrees) | 71 (69–75) | 71 (68–73) | 74 (72–76) | 0.16 | - | - | - | |
| ACF (mm) | 43 (37–47) | 39 (30–43) | 50 (44–53) | <0.001 | 0.22 | 0.07 | <0.001 | |
| FIBTEM 6 | CT (seconds) | 51 (49–59) | 59 (53–63) | 56 (45–72) | 0.53 | - | - | - |
| CFT (seconds) | - | - | - | - | - | - | - | |
| MCF (mm) | 13 (11–14) | 10 (9–14) | 18 (14–19) | 0.003 | 0.48 | 0.06 | 0.002 | |
| A-angle (° degrees) | 65 (62–68.5) | 70 (70–70) | 70.5 (65.3–74) | 0.49 | - | - | - | |
| A10 (mm) | 12 (11–14) | 9 (8–11) | 15 (13–18) | <0.001 | 0.20 | 0.06 | <0.001 | |
| A30 (mm) | 13 (12–15) | 10 (9–14) | 18 (14–19) | 0.002 | 0.47 | 0.048 | 0.002 | |
| MCE (dynes/cm2) | 15 (13–16) | 11 (10–14) | 21 (16–24) | <0.001 | 0.22 | 0.06 | <0.001 | |
| LI60 (%) | 96 (91–100) | 96 (90–100) | 100 (95–100) | 0.18 | - | - | - | |
| ML (%) | 9 (3–16) | 12 (2–14) | 3 (0–8) | 0.15 | - | - | - | |
| CFR (° degrees) | 68 (65–70) | 73 (73–73) | 71.5 (66.5–75) | 0.39 | - | - | - | |
| ACF (mm) | 12 ± 3.2 | 10 ± 2.4 | 16.3 ± 7.4 | 0.05 | 1.00 | 0.10 | 0.006 | |
| APTEM 7 | CT (seconds) | 52 (49–66) | 59 (55–67) | 54 (48–70) | 0.65 | - | - | - |
| CFT (seconds) | 135 (105–157) | 139 (121.5–164.3) | 95 (79–143) | 0.02 | 0.88 | 0.08 | 0.02 | |
| MCF (mm) | 49 (45–53) | 46 (41–50) | 56 (48–61) | 0.006 | 0.88 | 0.03 | 0.008 | |
| A-angle (° degrees) | 68 (65–72) | 65 (62–67) | 71 (66–74) | 0.11 | - | - | - | |
| A10 (mm) | 40.3 ± 7.4 | 38.4 ± 10.7 | 48.2 ± 8.7 | 0.005 | 1.00 | 0.04 | 0.01 | |
| A30 (mm) | 49 (44–53) | 46 (40–50) | 56 (47–61) | 0.005 | 0.91 | 0.02 | 0.007 | |
| MCE (dynes/cm2) | 92.9 ± 23.7 | 85.9 ± 29.3 | 126.6 ± 37.3 | 0.001 | 1.00 | 0.01 | 0.003 | |
| LI60 (%) | 90.7 ± 5.7 | 86.3 ± 8.1 | 93.6 ± 4.7 | 0.004 | 0.12 | 0.72 | 0.003 | |
| ML (%) | 18 (8–22) | 21 (14–29) | 11 (6–16) | 0.008 | 0.20 | 0.34 | 0.004 | |
| CFR (° degrees) | 72.5 (69–74.5) | 68 (66–73) | 73 (69–76) | 0.30 | - | - | - | |
| ACF (mm) | 40 (36–46) | 36 (32–42) | 50 (41–56) | <0.001 | 0.26 | 0.009 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontovazainitis, C.-G.; Gialamprinou, D.; Fleva, A.; Theodoridis, T.; Chatziioannidis, I.; Mitsiakou, C.; Banti, A.; Diamanti, E.; Mitsiakos, G. Rotational Thromboelastometry (ROTEM) Hemostasis Profile in Pregnant Women with Preeclampsia and Their Offspring: An Observational Study. Diagnostics 2025, 15, 2156. https://doi.org/10.3390/diagnostics15172156
Kontovazainitis C-G, Gialamprinou D, Fleva A, Theodoridis T, Chatziioannidis I, Mitsiakou C, Banti A, Diamanti E, Mitsiakos G. Rotational Thromboelastometry (ROTEM) Hemostasis Profile in Pregnant Women with Preeclampsia and Their Offspring: An Observational Study. Diagnostics. 2025; 15(17):2156. https://doi.org/10.3390/diagnostics15172156
Chicago/Turabian StyleKontovazainitis, Christos-Georgios, Dimitra Gialamprinou, Alexandra Fleva, Theodoros Theodoridis, Ilias Chatziioannidis, Christina Mitsiakou, Anastasia Banti, Elissavet Diamanti, and Georgios Mitsiakos. 2025. "Rotational Thromboelastometry (ROTEM) Hemostasis Profile in Pregnant Women with Preeclampsia and Their Offspring: An Observational Study" Diagnostics 15, no. 17: 2156. https://doi.org/10.3390/diagnostics15172156
APA StyleKontovazainitis, C.-G., Gialamprinou, D., Fleva, A., Theodoridis, T., Chatziioannidis, I., Mitsiakou, C., Banti, A., Diamanti, E., & Mitsiakos, G. (2025). Rotational Thromboelastometry (ROTEM) Hemostasis Profile in Pregnant Women with Preeclampsia and Their Offspring: An Observational Study. Diagnostics, 15(17), 2156. https://doi.org/10.3390/diagnostics15172156






