Prognostic Significance of Nomogram and T-Score in Locally Advanced Cervical Cancer Patients Treated with Curative Chemoradiotherapy and Image-Guided Brachytherapy: A Single-Center Retrospective Study
Abstract
1. Introduction
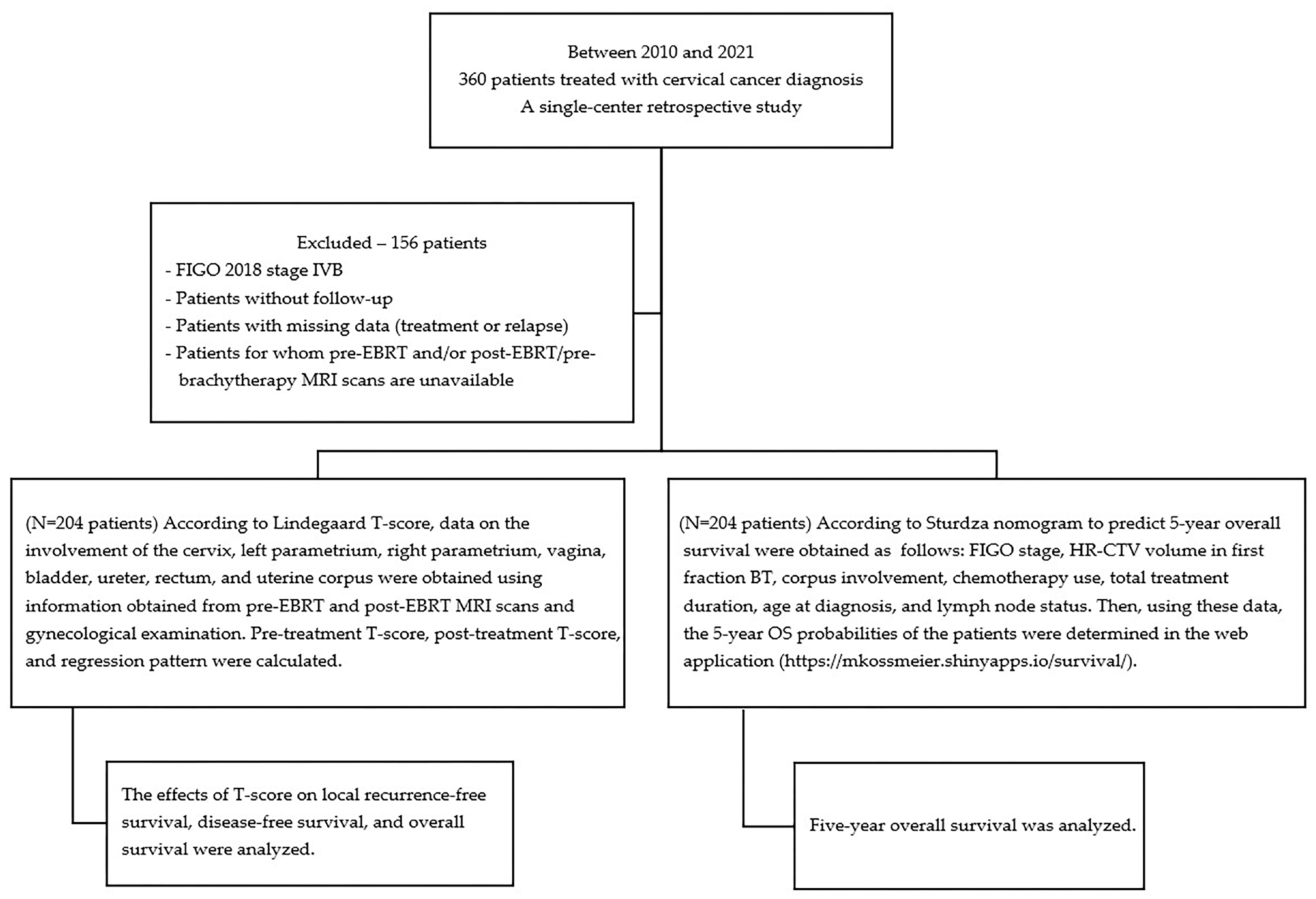
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D-CRT | Three-dimensional conformal radiotherapy |
| BT | Brachytherapy |
| CRT | Chemoradiotherapy |
| CT | Computed tomography |
| DFS | Disease-free survival |
| EBRT | External beam radiotherapy |
| EQD2 | Equivalent dose of 2Gy |
| FIGO | International Federation of Gynecology and Obstetrics |
| Gy | Gray |
| HL | High–Low |
| HR | Hazard ratio |
| HR-CTV D90 | 90% of the high risk-clinical target volume |
| IGABT | Image-guided adaptive brachytherapy |
| IMRT | Intensity-Modulated Radiation Therapy |
| IQR | Interquartile range |
| KPS | Karnofsky performance scale |
| LL | Low–Low |
| LRFS | Local recurrence-free survival |
| MRI | Magnetic resonance imaging |
| OS | Overall survival |
| SCC | Squamous cell carcinoma |
| TSBT | Pre-treatment T-score at brachytherapy |
| T-score | Tumor score |
| TSD | Pre-treatment T-score at diagnosis |
References
- Sahasrabuddhe, V.V. Cervical Cancer: Precursors and prevention. Hematol. Oncol. Clin. N. Am. 2024, 38, 771–781. [Google Scholar] [CrossRef]
- Ferrall, L.; Lin, K.Y.; Roden, R.B.S.; Hung, C.F.; Wu, T.C. Cervical Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2021, 27, 4952–4973. [Google Scholar] [CrossRef]
- Pimple, S.; Mishra, G. Cancer cervix: Epidemiology and disease burden. Cytojournal 2022, 19, 21. [Google Scholar] [CrossRef]
- Kido, A.; Nakamoto, Y. Implications of the new FIGO staging and the role of imaging in cervical cancer. Br. J. Radiol. 2021, 94, 20201342. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Milosevic, M.; Flys, A.; Pintilie, M.; Viswanathan, A.N. Trends in the utilisation of brachytherapy in cervical cancer in the United States. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Creutzberg, C.L.; Craighead, P.; McCormack, M.; Toita, T.; Narayan, K.; Reed, N.; Long, H.; Kim, H.J.; Marth, C.; et al. International brachytherapy practice patterns: A survey of the Gynecologic Cancer Intergroup (GCIG). Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 250–255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karnofsky, D.A.; Burchenal, J.H. Experimental observations on the effects of the nitrogen mustards on neoplastic tissues. Cancer Res. 1947, 7, 50. [Google Scholar] [PubMed]
- You, X.; Hou, F.Y. Clinical efficacy of image-guided radiation therapy for cervical cancer and its impact on patients’ serum tumor markers and KPS scores. J. Oncol. 2022, 2022, 8536554. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Tanderup, K.; Kirisits, C.; Mahantshetty, U.; Swamidas, J.; Jürgenliemk-Schulz, I.; Lindegaard, J.; de Leeuw, A.; Nesvacil, N.; Assenholt, M.; et al. Education and training for image-guided adaptive brachytherapy for cervix cancer-The (GEC)-ESTRO/EMBRACE perspective. Brachytherapy 2020, 19, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.E.; Pötter, R.; Kossmeier, M.; Kirchheiner, K.; Mahantshetty, U.; Haie-Meder, C.; Lindegaard, J.C.; Jurgenliemk-Schulz, I.; Tan, L.T.; Hoskin, P.; et al. Nomogram Predicting Overall Survival in Patients With Locally Advanced Cervical Cancer Treated With Radiochemotherapy Including Image-Guided Brachytherapy: A Retro-EMBRACE Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, J.C.; Petric, P.; Lindegaard, A.M.; Tanderup, K.; Fokdal, L.U. Evaluation of a new prognostic tumour score in locally advanced cervical cancer integrating clinical examination and magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, J.C.; Petric, P.; Schmid, M.P.; Nesvacil, N.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. Prognostic Implications of Uterine Cervical Cancer Regression During Chemoradiation Evaluated by the T-Score in the Multicenter EMBRACE I Study. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.A., 3rd; Liu, P.Y.; Barrett, R.J., 2nd; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.Q.; Liang, Z.G.; Ye, J.X.; Li, J.; Wang, J.L.; Chen, C.X.; Song, H.L. Significant Efficacy of Additional Concurrent Chemotherapy with Radiotherapy for Postoperative Cervical Cancer with Risk Factors: A Systematic Review and Meta-analysis. Asian Pac. J. Cancer Prev. 2016, 17, 3945–3951. [Google Scholar] [PubMed]
- Haie-Meder, C.; Pötter, R.; Van Limbergen, E.; Briot, E.; De Brabandere, M.; Dimopoulos, J.; Dumas, I.; Hellebust, T.P.; Kirisits, C.; Lang, S.; et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother. Oncol. 2005, 74, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Haie-Meder, C.; Van Limbergen, E.; Barillot, I.; De Brabandere, M.; Dimopoulos, J.; Dumas, I.; Erickson, B.; Lang, S.; Nulens, A.; et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother. Oncol. 2006, 78, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Mahantshetty, U.; Poetter, R.; Beriwal, S.; Grover, S.; Lavanya, G.; Rai, B.; Petric, P.; Tanderup, K.; Carvalho, H.; Hegazy, N.; et al. IBS-GEC ESTRO-ABS recommendations for CT based contouring in image guided adaptive brachytherapy for cervical cancer. Radiother. Oncol. 2021, 160, 273–284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCormack, M.; Eminowicz, G.; Gallardo, D.; Diez, P.; Farrelly, L.; Kent, C.; Hudson, E.; Panades, M.; Mathew, T.; Anand, A.; et al. Induction chemotherapy followed by standard chemoradiotherapy versus standard chemoradiotherapy alone in patients with locally advanced cervical cancer (GCIG INTERLACE): An international, multicentre, randomised phase 3 trial. Lancet 2024, 404, 1525–1535, Erratum in: Lancet 2025, 405, 468. https://doi.org/10.1016/S0140-6736(25)00207-7. [Google Scholar] [CrossRef] [PubMed]
- Mileshkin, L.R.; Moore, K.N.; Barnes, E.H.; Gebski, V.; Narayan, K.; King, M.T.; Bradshaw, N.; Lee, Y.C.; Diamante, K.; Fyles, A.W.; et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 468–482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monk, B.J.; Toita, T.; Wu, X.; Vázquez Limón, J.C.; Tarnawski, R.; Mandai, M.; Shapira-Frommer, R.; Mahantshetty, U.; Del Pilar Estevez-Diz, M.; Zhou, Q.; et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Sukhin, V.; Cloven, N.; Gomes, A.J.P.d.S.; et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind, phase 3 clinical trial. Lancet 2024, 403, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Coldon-Fearon, D.; Liu, Z.A.; Viswanathan, A.N. Updated trends in the utilisation of brachytherapy in cervical cancer in the United States: A survelliance, epidemiology, and end-results study. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Ayala-Peacock, D.N.; Stephens, S.J.; Chino, J.P. Recent advances in gynecologic radiation oncology. Cancer 2025, 131, e35888. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, J.C.; Petric, P.; Tan, L.T.; Hoskin, P.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Mahantshetty, U.; Kirisits, C.; Pötter, R. Are we making progress in curing advanced cervical cancer-again? Int. J. Gynecol. Cancer. 2024, 34, 1940–1945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jian, K.; Ali, Y.; Li, Y.; Jia, L. Nomogram models for the prognosis of cervical cancer: A SEER-based study. Front. Oncol. 2022, 12, 961678. [Google Scholar] [CrossRef]
- Chen, W.; Xia, X.; Xie, X.; Wei, Y.; Wu, R.; Cai, W.; Hong, J. Nomogram for prognosis of elderly patients with cervical cancer who receive combined radiotherapy. Sci. Rep. 2023, 13, 13299. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; Zhao, Q.; Zhang, L.; Yin, M.; Zhou, J.; Zhu, J.; Qin, S. CT-based radiomics nomogram for overall survival prediction in patients with cervical cancer treated with concurrent chemotherapy. Front. Oncol. 2023, 13, 1287121. [Google Scholar] [CrossRef]
- Polterauer, S.; Grimm, C.; Hofstetter, G.; Concin, N.; Natter, C.; Sturdza, A.; Pötter, R.; Marth, C.; Reinthaller, A.; Heinze, G. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br. J. Cancer 2012, 107, 918–924. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, Y.; Weng, T. Construction and validation of an MRI-based radiomic nomogram to predict overall survival in patients with locally advanced cervical cancer: A multicenter study. Acad. Radiol. 2024, 31, 4372–4382. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, P.; Chen, Z.; Li, W.; Dong, B.; Zhang, Y. Efficacy and safety of different chemotherapy regimens concurrent with radiotherapy in the treatment of locally advanced cervical cancer. BMC Cancer 2024, 24, 589. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Coza, O.; Ordeanu, C.; Trăilă, A.; Rancea, A.; Todor, N.; Ghilezan, N. Radiotherapy versus concurrent 5-day cisplatin and radiotherapy in locally advanced cervical carcinoma. Long-term results of a phase III randomized trial. Strahlenther. Onkol. 2009, 185, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Wu, J.; Li, J.; Hou, Q.; Tang, B. Prognostic significance of clinicopathological factors influencing overall survival and event-free survival of patients with cervical cancer: A systematic review and meta-analysis. Med. Sci. Monit. 2022, 28, e934588. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.P.; Lindegaard, J.C.; Mahantshetty, U.; Tanderup, K.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.; Hoskin, P.; Segedin, B.; et al. Risk Factors for Local Failure Following Chemoradiation and Magnetic Resonance Image-Guided Brachytherapy in Locally Advanced Cervical Cancer: Results From the EMBRACE-I Study. J. Clin. Oncol. 2023, 41, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Schernberg, A.; Bockel, S.; Annede, P.; Fumagalli, I.; Escande, A.; Mignot, F.; Kissel, M.; Morice, P.; Bentivegna, E.; Gouy, S.; et al. Tumor shrinkage during chemoradiation in locally advanced cervical cancer patients: Prognostic significance, and impact for image-guided adaptive brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 362–372. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Treatment Characteristics | ||
|---|---|---|---|
| Characteristics | Median (Min.–Max.) N (%) | Characteristics | Median (Min.–Max.) N (%) |
| Age (years) | 52 (29–89) | EBRT technique 3D-CRT IMRT/VMAT | 112 (54.9%) 92 (45.1%) |
| KPS score 100 90 80 70 | 100 (70–100) 134 (65.7%) 59 (28.8%) 10 (4.9%) 1 (0.5%) | Total EBRT doses (Gy) | 50 (45–64.4) |
| Total EBRT fractions | 25 (23–30) | ||
| Pre-treatment hemoglobin (g/dL) | 11.85 (7.2–15.2) | Brachytherapy dose per fraction (Gy) | 6.35 (5–8.5) |
| Menopause status Postmenopause Premenopause Perimenopause | 117 (57.4%) 76 (37.3%) 11 (5.4%) | Brachytherapy fraction number | 4 (2–6) |
| Pathology Squamous cell carcinoma Adenocarcinoma Adenosquamous carcinoma Serous papillary carcinoma Mucinous papillary carcinoma | 188 (92.2%) 12 (5.9%) 2 (1%) 1 (0.5%) 1 (0.5%) | HR-CTV volume cc | 30.8 (4.33–111.78) |
| HR-CTV volume group ≤30 cc >30 cc | 98 (48.04%) 106 (51.96%) | ||
| Involved lymph node Yes No | 92 (45.1%) 112 (54.9%) | HR-CTV D90 EQD2 (Gy) | 81.65 (70.1–97.2) |
| Involved lymph node region Pelvic region Pelvic + para-aortic region Para-aortic region No | 79 (38.7%) 11 (5.4%) 2 (1%) 112 (54.9%) | Total treatment time group ≤80 days >80 days | 105 (51.47%) 99 (48.53%) |
| FIGO 2018 Staging IB2 IB3 IIA2 IIB IIIA IIIB IIIC1 IIIC2 IVA | 6 (2.9%) 4 (2%) 2 (1%) 84 (41.2%) 3 (1.5%) 11 (5.4%) 76 (37.3%) 13 (6.4%) 5 (2.5%) | Concurrent chemotherapy drugs Cisplatin Carboplatin Low dose paclitaxel–carboplatin No | 198 (97.1%) 1 (0.5%) 2 (1%) 3 (1.5%) |
| Concurrent chemotherapy cycles | 5 (0–7) | ||
| FIGO 2018 Staging Group Stage I-II Stage III-IV | 96 (47.1%) 108 (52.9%) | 60-month estimates by Sturdza et al. | 70.35 (20.9–87.1) |
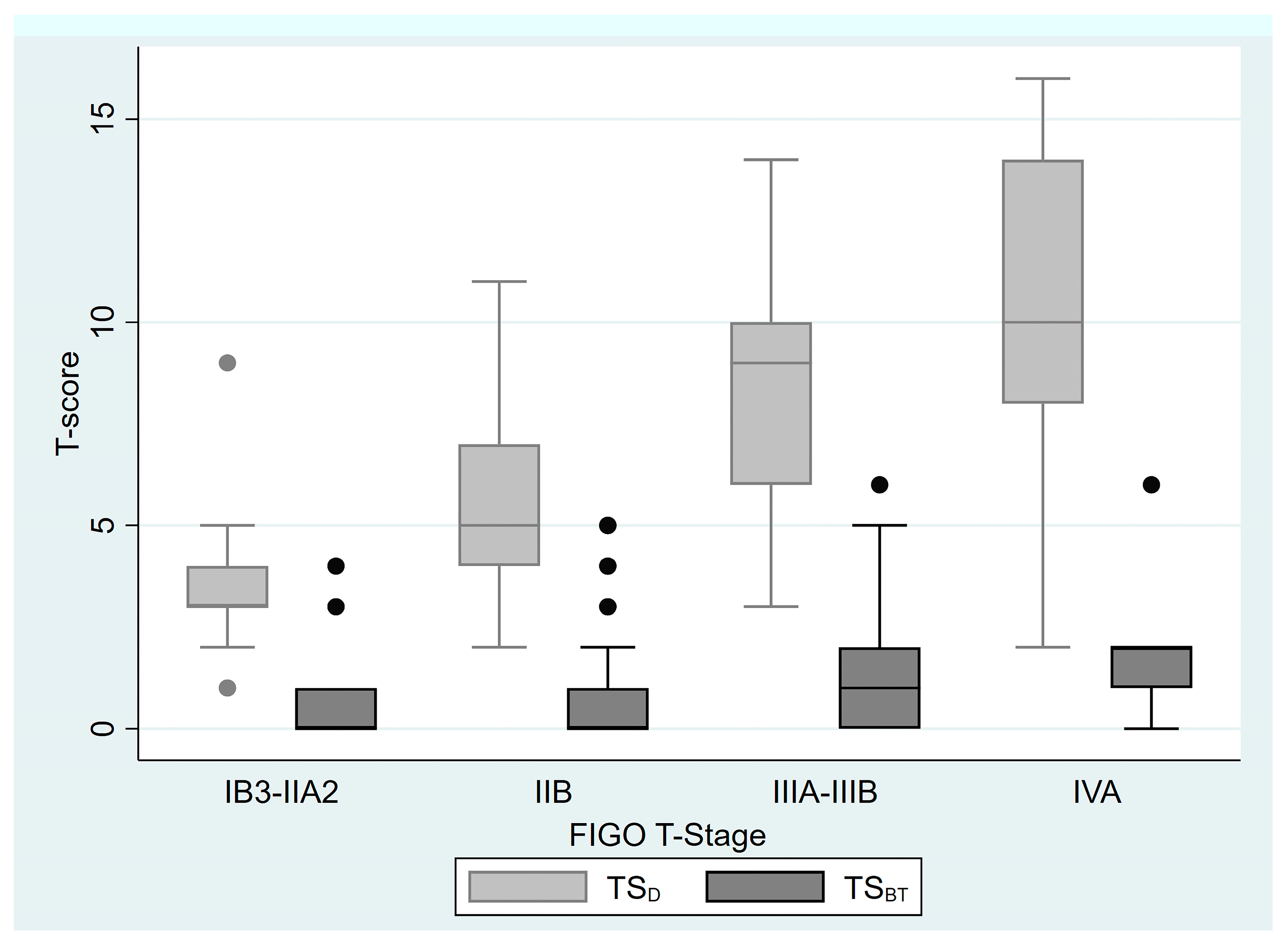
| FIGO 2018 T Staging IB2 IB3 IIA1 IIA2 IIB IIIA IIIB IVA | 11 (5.4%) 8 (3.9%) 4 (2%) 2 (2%) 143 (70.1%) 4 (2%) 27 (13.2%) 5 (2.5%) | TSD | 5.5 (1–16) |
| TSBT | 1 (0–6) | ||
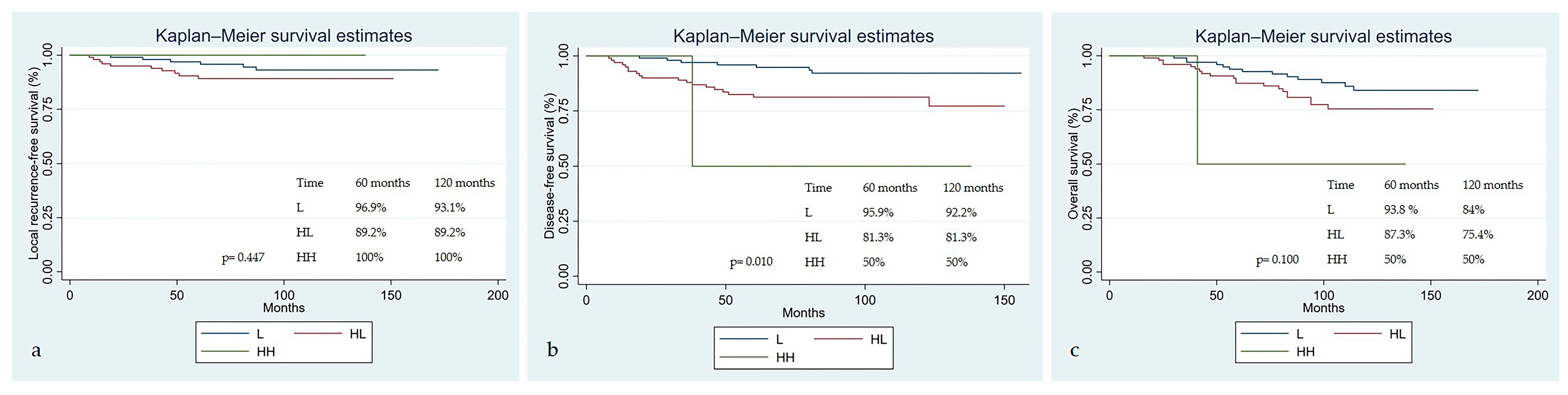
| FIGO 2018 T Staging Group Stage I-II Stage III-IV | 168 (82.4%) 36 (17.6%) | Regression pattern L HL HH | 102 (50%) 100 (49.02%) 2 (0.98%) |
| Variable | Local Recurrence-Free Survival p-Value | Disease-Free Survival p-Value | Overall Survival p-Value |
|---|---|---|---|
| Age ≥ 65 years | 0.341 | 0.591 | 0.367 |
| Pathology type | 0.604 | 0.247 | 0.209 |
| Regression pattern | 0.448 | 0.010 | 0.101 |
| Pelvic and/or para-aortic lymphadenopathy | 0.031 | 0.988 | 0.665 |
| FIGO 2018 stage group | 0.024 | 0.965 | 0.755 |
| FIGO 2018 T stage group | 0.623 | 0.528 | 0.046 |
| Pelvic radiotherapy technique | 0.377 | 0.080 | 0.281 |
| HR-CTV volume ≥ 30 cc | 0.561 | 0.474 | 0.347 |
| Treatment duration (>80 days) | 0.478 | 0.435 | 0.879 |
| Local Recurrence-Free Survival | Disease-Free Survival | Overall Survival | |
|---|---|---|---|
| Variable | HR (95% CI), p-Value | HR (95% CI), p-Value | HR (95% CI), p-Value |
| Age | 0.977 (0.933–1.022), 0.312 | 1.001 (0.968–1.034), 0.970 | 1.018 (0.988–1.048), 0.241 |
| KPS score (0–100) | 1.026 (0.937–1.123), 0.584 | 0.995 (0.935–1.058), 0.862 | 0.992 (0.938–1.049), 0.771 |
| Hemoglobin level (g/dL) | 1.015 (0.730–1.412), 0.930 | 1.006 (0.786–1.288), 0.962 | 0.962 (0.764–1.211), 0.741 |
| Menopause status (compared to peri) | |||
| Post | 0.959 (0.123–7.499), 0.969 | 0.924 (0.215–3.976), 0.915 | 2.909 (0.394–21.498), 0.295 |
| Pre | 0.702 (0.082–6.014), 0.747 | 0.421 (0.085–2.090), 0.290 | 1.420 (0.177–11.380), 0.741 |
| Pathology (compared to SCC) | 1.477 (0.336–6.497), 0.606 | 1.855 (0.641–5.370), 0.255 | 1.820 (0.704–4.703), 0.217 |
| Presence of lymphadenopathy | 3.633 (1.035–12.748) 0.044 | 1.006 (0.471–2.149), 0.988 | 0.860 (0.434–1.703), 0.665 |
| FIGO 2018 T stage group (compared to I-II) | 0.691 (0.157–3.043), 0.625 | 1.338 (0.540–3.317), 0.529 | 2.081 (0.995–4.354), 0.052 |
| FIGO 2018 group (compared to I-II) | 3.413 (1.101–10.583), 0.033 | 0.983 (0.462–2.092), 0.965 | 0.899 (0.459–1.761), 0.756 |
| 3D-CRT (compared to IMRT/VMAT) | 1.606 (0.556–4.634), 0.381 | 2.126 (0.896–5.043), 0.087 | 1.500 (0.713–3.153), 0.285 |
| HR-CTV volume | 1.022 (0.995–1.050), 0.114 | 1.017 (0.995–1.039), 0.140 | 1.010 (0.986–1.034), 0.423 |
| HR-CTV of EQD2 D90 (Gy) | 1.030 (0.936–1.133), 0.543 | 1.000 (0.928–1.077), 0.998 | 1.002 (0.938–1.071), 0.947 |
| Treatment duration (in months) | 1.007 (0.982–1.033), 0.577 | 1.006 (0.986–1.026), 0.585 | 1.007 (0.990–1.025), 0.438 |
| 60-month estimates by Sturdza et al. | 0.993 (0.959–1.027), 0.670 | 0.973 (0.950–0.997), 0.028 | 0.977 (0.957–0.997), 0.025 |
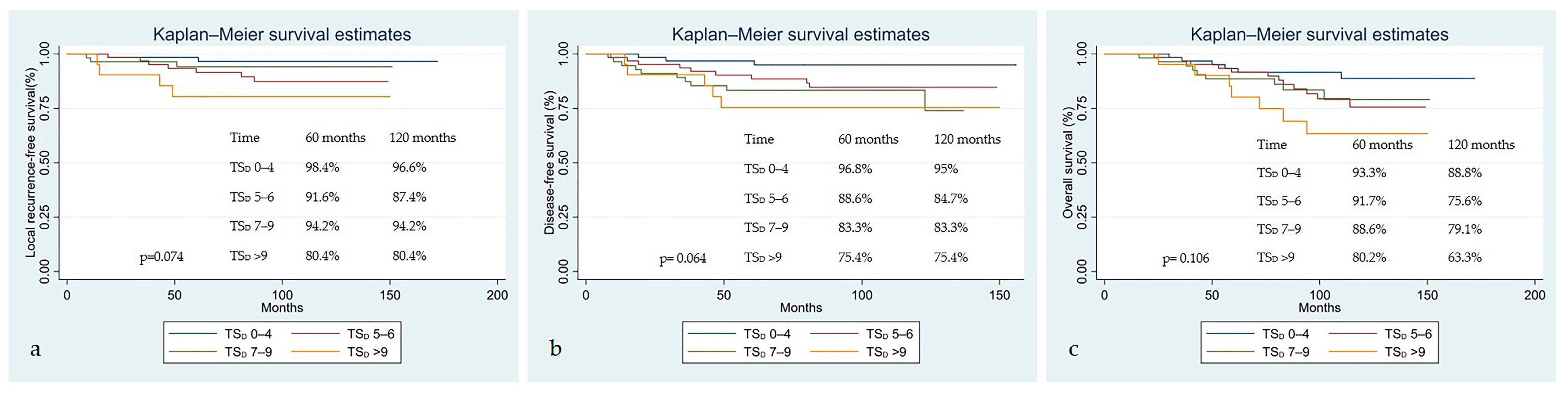
| TSD | 1.184 (1.002–1.400), 0.047 | 1.193 (1.052–1.354), 0.006 | 1.126 (1.001–1.265), 0.048 |
| TSBT | 0.997 (0.670–1.484), 0.988 | 1.297 (1.037–1.622), 0.023 | 1.339 (1.096–1.637), 0.004 |
| Regression group (compared to L) | |||
| HL | 1.850 (0.672–5.091), 0.234 | 3.090 (1.298–7.356), 0.011 | 1.795 (0.892–3.612), 0.101 |
| HH | N/A; N/A | 8.072 (0.990–65.784), 0.051 | 5.004 (0.653–38.315), 0.121 |
| Variable | Stratification | HR (95% CI) | p-Value |
|---|---|---|---|
| TSD | Continuous | 1.203 (1.021–1.417) | 0.027 |
| Lymphadenopathy | Yes vs. no | 3.968 (1.128–13.957) | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibis, K.; Ilgin, C.; Suncak, L.; Akbas, C.K.; Bolukbas, D.; Denizli, M.; Azizy, A.; Yilmaz, B.; Ozben, S.G.; Celik, A.I.; et al. Prognostic Significance of Nomogram and T-Score in Locally Advanced Cervical Cancer Patients Treated with Curative Chemoradiotherapy and Image-Guided Brachytherapy: A Single-Center Retrospective Study. Diagnostics 2025, 15, 2142. https://doi.org/10.3390/diagnostics15172142
Ibis K, Ilgin C, Suncak L, Akbas CK, Bolukbas D, Denizli M, Azizy A, Yilmaz B, Ozben SG, Celik AI, et al. Prognostic Significance of Nomogram and T-Score in Locally Advanced Cervical Cancer Patients Treated with Curative Chemoradiotherapy and Image-Guided Brachytherapy: A Single-Center Retrospective Study. Diagnostics. 2025; 15(17):2142. https://doi.org/10.3390/diagnostics15172142
Chicago/Turabian StyleIbis, Kamuran, Can Ilgin, Leyla Suncak, Canan Koksal Akbas, Deniz Bolukbas, Mustafa Denizli, Abdulmunir Azizy, Begum Yilmaz, Seda Guler Ozben, Ayca Iribas Celik, and et al. 2025. "Prognostic Significance of Nomogram and T-Score in Locally Advanced Cervical Cancer Patients Treated with Curative Chemoradiotherapy and Image-Guided Brachytherapy: A Single-Center Retrospective Study" Diagnostics 15, no. 17: 2142. https://doi.org/10.3390/diagnostics15172142
APA StyleIbis, K., Ilgin, C., Suncak, L., Akbas, C. K., Bolukbas, D., Denizli, M., Azizy, A., Yilmaz, B., Ozben, S. G., Celik, A. I., Kucucuk, N. S., & Yirgin, I. K. (2025). Prognostic Significance of Nomogram and T-Score in Locally Advanced Cervical Cancer Patients Treated with Curative Chemoradiotherapy and Image-Guided Brachytherapy: A Single-Center Retrospective Study. Diagnostics, 15(17), 2142. https://doi.org/10.3390/diagnostics15172142






