The Utility of Intravoxel Incoherent Motion Metrics in Assessing Disability in Relapsing–Remitting Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Participants
2.2. EDSS Evaluation and Relapse History
2.3. MRI Acquisition Protocol
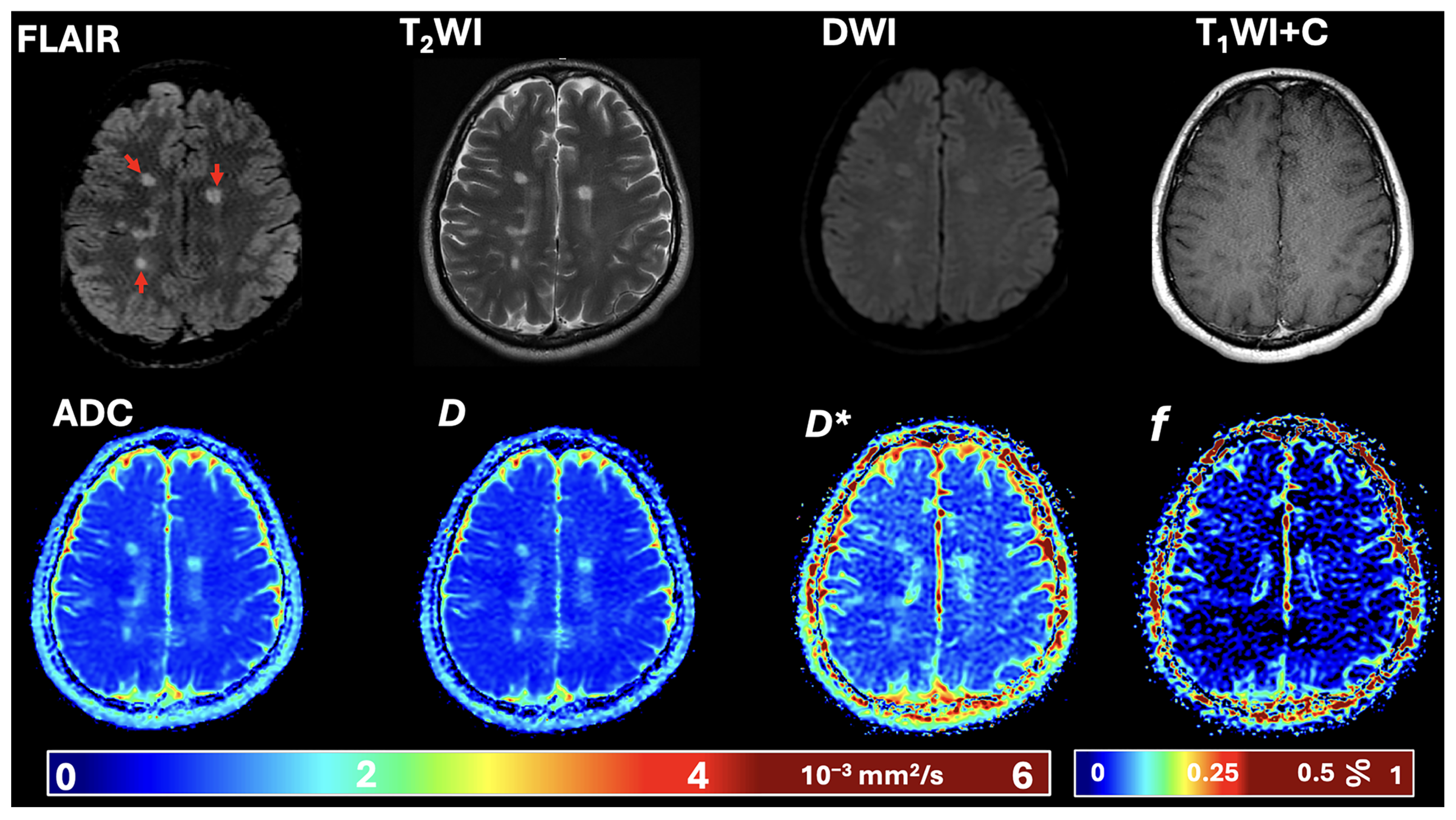
2.4. Image Post-Processing
2.5. Data Analysis
3. Results
3.1. Descriptive Statistics
3.2. Bivariate Analysis
3.2.1. Association Between IVIM Parameters and Clinical Variables
3.2.2. Disease Burden and Disability Measures
3.3. Correlation Analysis
3.4. Group Comparison According to EDSS Level
3.5. Multivariate Regression Analysis
4. Discussion
4.1. MRI Biomarkers and Clinical Associations
4.2. Disability Scores and Clinical/Demographic Correlates
4.3. EDSS Stratification Threshold
4.4. Correlation and Predictive Analyses
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| RR-MS | Relapsing–remitting multiple sclerosis |
| EDSS | Expanded Disability Status Scale |
| DMT | Disease-modifying therapy |
| IVIM | Intravoxel incoherent motion |
| DWI | Diffusion-weighted imaging |
| ADC | Apparent diffusion coefficient |
| D | Pure molecular diffusion |
| D* | Pseudo-diffusion |
| WI | Weighted imaging |
| FLAIR | Fluid-attenuated inversion recovery |
References
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lapiscina, E.H.; Fraga-Pumar, E.; Gabilondo, I.; Martínez-Heras, E.; Torres-Torres, R.; Ortiz-Pérez, S.; Llufriu, S.; Tercero, A.; Andorra, M.; Roca, M.F.; et al. The multiple sclerosis visual pathway cohort: Understanding neurodegeneration in MS. BMC Res. Notes 2014, 7, 910. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S.A. Assessment of MRI Safety Practices in Saudi Arabia. Risk Manag. Healthc. Policy 2023, 16, 199–208. [Google Scholar] [CrossRef]
- Alomair, O.I. Conventional and Advanced Magnetic Resonance Imaging Biomarkers of Multiple Sclerosis in the Brain. Cureus 2025, 17, e79914. [Google Scholar] [CrossRef]
- Rovira, À.; Auger, C.; Sceppacuercia, S.; Torres, C. Typical and Emerging Diagnostic MRI Features in Multiple Sclerosis. Can. Assoc. Radiol. J. 2025, 76, 122–144. [Google Scholar] [CrossRef]
- Isaksson, A.K.; Ahlström, G.; Gunnarsson, L.G. Quality of life and impairment in patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 64–69. [Google Scholar] [CrossRef]
- Zwibel, H.L. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv. Ther. 2009, 26, 1043–1057. [Google Scholar] [CrossRef]
- Schwenkenbecher, P.; Wurster, U.; Konen, F.F.; Gingele, S.; Sühs, K.W.; Wattjes, M.P.; Stangel, M.; Skripuletz, T. Impact of the McDonald Criteria 2017 on Early Diagnosis of Relapsing-Remitting Multiple Sclerosis. Front. Neurol. 2019, 10, 188. [Google Scholar] [CrossRef]
- Bergmann, C.; Becker, S.; Watts, A.; Sullivan, C.; Wilken, J.; Golan, D.; Zarif, M.; Bumstead, B.; Buhse, M.; Kaczmarek, O.; et al. Multiple sclerosis and quality of life: The role of cognitive impairment on quality of life in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 79, 104966. [Google Scholar] [CrossRef]
- Safiri, S.; Ghaffari Jolfayi, A.; Mousavi, S.E.; Nejadghaderi, S.A.; Sullman, M.J.M.; Kolahi, A.-A. Global burden of multiple sclerosis and its attributable risk factors, 1990–2019. Front. Neurol. 2024, 15, 1448377. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Anzalone, N.; Storelli, L.; Del Poggio, A.; Cacciaguerra, L.; Manfredi, A.A.; Meani, A.; Filippi, M. Deep Learning on Conventional Magnetic Resonance Imaging Improves the Diagnosis of Multiple Sclerosis Mimics. Investig. Radiol. 2021, 56, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.V.; Hagemeier, J.; Bergsland, N.; Jakimovski, D.; Dwyer, M.G.; Ramasamy, D.P.; Lizarraga, A.A.; Hojnacki, D.; Kolb, C.; Weinstock-Guttman, B.; et al. Atrophied Brain T2 Lesion Volume at MRI Is Associated with Disability Progression and Conversion to Secondary Progressive Multiple Sclerosis. Radiology 2019, 293, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Preziosa, P.; Arnold, D.L.; Barkhof, F.; Harrison, D.M.; Maggi, P.; Mainero, C.; Montalban, X.; Sechi, E.; Weinshenker, B.G.; et al. Present and future of the diagnostic work-up of multiple sclerosis: The imaging perspective. J. Neurol. 2023, 270, 1286–1299. [Google Scholar] [CrossRef]
- McFarland, H.F.; Barkhof, F.; Antel, J.; Miller, D.H. The role of MRI as a surrogate outcome measure in multiple sclerosis. Mult. Scler. 2002, 8, 40–51. [Google Scholar] [CrossRef]
- Zivadinov, R. Can imaging techniques measure neuroprotection and remyelination in multiple sclerosis? Neurology 2007, 68, S72–S82; discussion S76–S91. [Google Scholar] [CrossRef]
- Zivadinov, R.; Leist, T.P. Clinical-magnetic resonance imaging correlations in multiple sclerosis. J. Neuroimaging 2005, 15, 10s–21s. [Google Scholar] [CrossRef]
- Rocca, M.A.; Preziosa, P.; Barkhof, F.; Brownlee, W.; Calabrese, M.; De Stefano, N.; Granziera, C.; Ropele, S.; Toosy, A.T.; Vidal Jordana, À.; et al. Current and future role of MRI in the diagnosis and prognosis of multiple sclerosis. Lancet Reg. Health Eur. 2024, 44, 100978. [Google Scholar] [CrossRef]
- Alomair, O.I.; Alghamdi, S.A.; Abujamea, A.H.; AlfIfi, A.Y.; Alashban, Y.I.; Kurniawan, N.D. Investigating the Role of Intravoxel Incoherent Motion Diffusion-Weighted Imaging in Evaluating Multiple Sclerosis Lesions. Diagnostics 2025, 15, 1260. [Google Scholar] [CrossRef]
- Martinelli Boneschi, F.; Rovaris, M.; Comi, G.; Filippi, M. The use of magnetic resonance imaging in multiple sclerosis: Lessons learned from clinical trials. Mult. Scler. 2004, 10, 341–347. [Google Scholar] [CrossRef]
- Valizadeh, A.; Moassefi, M.; Barati, E.; Ali Sahraian, M.; Aghajani, F.; Fattahi, M.R. Correlation between the clinical disability and T1 hypointense lesions’ volume in cerebral magnetic resonance imaging of multiple sclerosis patients: A systematic review and meta-analysis. CNS Neurosci. Ther. 2021, 27, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Maggi, P.; Absinta, M. Emerging MRI biomarkers for the diagnosis of multiple sclerosis. Mult. Scler. 2024, 30, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Heales, C. Evaluation of IVIM in the Spinal Cord of Multiple Sclerosis Patients. Radiol. Technol. 2024, 95, 248–255. [Google Scholar]
- Lipiński, K.; Bogorodzki, P. Evaluation of Whole Brain Intravoxel Incoherent Motion (IVIM) Imaging. Diagnostics 2024, 14, 653. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef]
- Federau, C.; Sumer, S.; Becce, F.; Maeder, P.; O’Brien, K.; Meuli, R.; Wintermark, M. Intravoxel incoherent motion perfusion imaging in acute stroke: Initial clinical experience. Neuroradiology 2014, 56, 629–635. [Google Scholar] [CrossRef]
- Paschoal, A.M.; Leoni, R.F.; Dos Santos, A.C.; Paiva, F.F. Intravoxel incoherent motion MRI in neurological and cerebrovascular diseases. Neuroimage Clin. 2018, 20, 705–714. [Google Scholar] [CrossRef]
- Le Bihan, D. What can we see with IVIM MRI? Neuroimage 2019, 187, 56–67. [Google Scholar] [CrossRef]
- Iima, M. Perfusion-driven Intravoxel Incoherent Motion (IVIM) MRI in Oncology: Applications, Challenges, and Future Trends. Magn. Reson. Med. Sci. 2021, 20, 125–138. [Google Scholar] [CrossRef]
- Sener, R.N. Diffusion MRI: Apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput. Med. Imaging Graph. 2001, 25, 299–326. [Google Scholar] [CrossRef] [PubMed]
- Zacharzewska-Gondek, A.; Pokryszko-Dragan, A.; Budrewicz, S.; Sąsiadek, M.; Trybek, G.; Bladowska, J. The role of ADC values within the normal-appearing brain in the prognosis of multiple sclerosis activity during interferon-β therapy in the 3-year follow-up: A preliminary report. Sci. Rep. 2020, 10, 12828. [Google Scholar] [CrossRef] [PubMed]
- Eisele, P.; Szabo, K.; Griebe, M.; Rossmanith, C.; Förster, A.; Hennerici, M.; Gass, A. Reduced diffusion in a subset of acute MS lesions: A serial multiparametric MRI study. AJNR Am. J. Neuroradiol. 2012, 33, 1369–1373. [Google Scholar] [CrossRef]
- Asaadi, F.; Faeghi, F.; Ashrafi, F.; Sanei Taheri, M. Clinical Significance of Diffusion-weighted Magnetic Resonance Imaging on Treatment Efficacy in MS Patients with Acute Attacks. Basic Clin. Neurosci. 2021, 12, 729–736. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Jankowska, A.; Chwojnicki, K.; Szurowska, E. The diagnosis of multiple sclerosis: What has changed in diagnostic criteria? Pol. J. Radiol. 2023, 88, e574–e581. [Google Scholar] [CrossRef] [PubMed]
- Hartung, H.P.; Graf, J.; Aktas, O.; Mares, J.; Barnett, M.H. Diagnosis of multiple sclerosis: Revisions of the McDonald criteria 2017—Continuity and change. Curr. Opin. Neurol. 2019, 32, 327–337. [Google Scholar] [CrossRef]
- El-Nahas, A.R.; El-Assmy, A.M.; Mansour, O.; Sheir, K.Z. A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: The value of high-resolution noncontrast computed tomography. Eur. Urol. 2007, 51, 1688–1693, discussion 1693–1684. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Greve, D.N.; Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009, 48, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Preziosa, P.; Barkhof, F.; Ciccarelli, O.; Cossarizza, A.; De Stefano, N.; Gasperini, C.; Geraldes, R.; Granziera, C.; Haider, L.; et al. The ageing central nervous system in multiple sclerosis: The imaging perspective. Brain 2024, 147, 3665–3680. [Google Scholar] [CrossRef] [PubMed]
- Rzepiński, Ł.; Zawadka-Kunikowska, M.; Maciejek, Z.; Newton, J.L.; Zalewski, P. Early Clinical Features, Time to Secondary Progression, and Disability Milestones in Polish Multiple Sclerosis Patients. Medicina 2019, 55, 232. [Google Scholar] [CrossRef] [PubMed]
- Leray, E.; Yaouanq, J.; Le Page, E.; Coustans, M.; Laplaud, D.; Oger, J.; Edan, G. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010, 133, 1900–1913. [Google Scholar] [CrossRef]
- Hohol, M.J.; Orav, E.J.; Weiner, H.L. Disease steps in multiple sclerosis: A longitudinal study comparing disease steps and EDSS to evaluate disease progression. Mult. Scler. 1999, 5, 349–354. [Google Scholar] [CrossRef]
- Sellner, J.; Kraus, J.; Awad, A.; Milo, R.; Hemmer, B.; Stüve, O. The increasing incidence and prevalence of female multiple sclerosis--a critical analysis of potential environmental factors. Autoimmun. Rev. 2011, 10, 495–502. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Thygesen, L.C.; Stenager, E.; Laursen, B.; Magyari, M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 2018, 90, e1954–e1963. [Google Scholar] [CrossRef]
- Vivó, F.; Solana, E.; Calvi, A.; Lopez-Soley, E.; Reid, L.B.; Pascual-Diaz, S.; Garrido, C.; Planas-Tardido, L.; Cabrera-Maqueda, J.M.; Alba-Arbalat, S.; et al. Microscopic fractional anisotropy outperforms multiple sclerosis lesion assessment and clinical outcome associations over standard fractional anisotropy tensor. Hum. Brain Mapp. 2024, 45, e26706. [Google Scholar] [CrossRef]
- Coll, L.; Pareto, D.; Carbonell-Mirabent, P.; Cobo-Calvo, Á.; Arrambide, G.; Vidal-Jordana, Á.; Comabella, M.; Castilló, J.; Rodríguez-Acevedo, B.; Zabalza, A.; et al. Deciphering multiple sclerosis disability with deep learning attention maps on clinical MRI. Neuroimage Clin. 2023, 38, 103376. [Google Scholar] [CrossRef]
- Dworkin, J.D.; Linn, K.A.; Oguz, I.; Fleishman, G.M.; Bakshi, R.; Nair, G.; Calabresi, P.A.; Henry, R.G.; Oh, J.; Papinutto, N.; et al. An Automated Statistical Technique for Counting Distinct Multiple Sclerosis Lesions. AJNR Am. J. Neuroradiol. 2018, 39, 626–633. [Google Scholar] [CrossRef]
- Tomassini, V.; Fanelli, F.; Prosperini, L.; Cerqua, R.; Cavalla, P.; Pozzilli, C. Predicting the profile of increasing disability in multiple sclerosis. Mult. Scler. 2019, 25, 1306–1315. [Google Scholar] [CrossRef]
- Popescu, V.; Agosta, F.; Hulst, H.E.; Sluimer, I.C.; Knol, D.L.; Sormani, M.P.; Enzinger, C.; Ropele, S.; Alonso, J.; Sastre-Garriga, J.; et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1082–1091. [Google Scholar] [CrossRef]
- Lopez-Soley, E.; Martinez-Heras, E.; Solana, E.; Solanes, A.; Radua, J.; Vivo, F.; Prados, F.; Sepulveda, M.; Cabrera-Maqueda, J.M.; Fonseca, E.; et al. Diffusion tensor imaging metrics associated with future disability in multiple sclerosis. Sci. Rep. 2023, 13, 3565. [Google Scholar] [CrossRef]
- Fisniku, L.K.; Brex, P.A.; Altmann, D.R.; Miszkiel, K.A.; Benton, C.E.; Lanyon, R.; Thompson, A.J.; Miller, D.H. Disability and T2 MRI lesions: A 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 2008, 131, 808–817. [Google Scholar] [CrossRef]
| Demographics and Outcome Variables | N (%) | Mean ± SD |
|---|---|---|
| Age groups (in years) | ||
| ≤30 | 66 (33.5%) | |
| 31 to 50 | 113 (57.4%) | |
| >50 | 18 (9.1%) | |
| Gender | ||
| Male | 59 (29.9%) | |
| Female | 138 (70.1%) | |
| Disease duration (in years) | ||
| <2 | 38 (19.3%) | |
| 2 to 5 | 72 (36.5%) | |
| >5 | 87 (44.2%) | |
| DMT | ||
| Treated | 144 (73.1%) | |
| Not treated | 53 (26.9%) | |
| Mobility | ||
| Supported | 20 (10.2%) | |
| Not supported | 177 (89.8%) | |
| ADC | 1.10 ± 0.14 | |
| D | 1.04 ± 0.14 | |
| D* | 1.30 ± 0.16 | |
| f | 0.06 ± 0.02 | |
| EDSS | 2.25 ± 1.91 | |
| No. of MS lesions | 2310 for all RR-MS patients | 11.7 ± 8.3 |
| Number of relapses | 463 for all RR-MS patients | 2.65 ± 2.03 |
| Study Variables | ADC | D | D* | f | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | p-Value and Effect Size | Mean ± SD | p-Value and Effect Size | Mean ± SD | p-Value and Effect Size | Mean ± SD | p-Value and Effect Size | |
| Age groups (in years) | NS | |||||||
| ≤30 | 1.06 ± 0.15 | <0.001 and 0.080 | 1.01 ± 0.15 | <0.001 and 0.080 | 1.23 ± 0.17 | 0.002 and 0.060 | 0.06 ± 0.01 | |
| 31–50 | 1.10 ± 0.13 | 1.04 ± 0.13 | 1.27 ± 0.15 | 0.06 ± 0.02 | ||||
| >50 | 1.22 ± 0.11 | 1.16 ± 0.10 | 1.37 ± 0.13 | 0.06 ± 0.01 | ||||
| Gender | NS | NS | NS | |||||
| Male | 1.09 ± 0.14 | 1.04 ± 0.13 | 1.24 ± 0.15 | 0.054 ± 0.01 | 0.008 and −0.417 | |||
| Female | 1.10 ± 0.15 | 1.04 ± 0.14 | 1.28 ± 0.16 | 0.061 ± 0.02 | ||||
| Disease duration (in years) | NS | |||||||
| <2 | 0.99 ± 0.11 | <0.001 and 0.185 | 0.94 ± 0.11 | <0.001 and 0.182 | 1.18 ± 0.15 | <0.001 and 0.117 | 0.06 ± 0.02 | |
| 2 to 5 | 1.08 ± 0.12 | 1.03 ± 0.12 | 1.25 ± 0.15 | 0.05 ± 0.02 | ||||
| >5 | 1.16 ± 0.14 | 1.10 ± 0.14 | 1.32 ± 0.15 | 0.06 ± 0.01 | ||||
| DMT | NS | NS | ||||||
| Treated | 1.11 ± 0.15 | 1.05 ± 0.14 | 0.036 and 0.339 | 1.27 ± 0.16 | 0.057 ± 0.01 | 0.015 and −0.394 | ||
| Not treated | 1.06 ± 0.13 | 1.01 ± 0.12 | 1.25 ± 0.16 | 0.063 ± 0.02 | ||||
| Mobility | NS | |||||||
| Supported | 1.20 ± 0.17 | 0.001 and 0.781 | 1.14 ± 0.16 | 0.002 and 0.784 | 1.37 ± 0.19 | 0.004 and 0.676 | 0.057 ± 0.01 | |
| Not supported | 1.08 ± 0.14 | 1.03 ± 0.13 | 1.25 ± 0.15 | 0.059 ± 0.02 | ||||
| Study Variables | Outcome Variables | |||||
|---|---|---|---|---|---|---|
| No. of MS Lesions | No. of Relapses | EDSS | ||||
| Mean ± SD | p-Value and Effect Size | Mean ± SD | p-Value and Effect Size | Mean ± SD | p-Value and Effect Size | |
| Age groups (in years) | NS | NS | ||||
| ≤30 | 10.68 ± 8.05 | 2.65 ± 2.45 | 1.97 ± 1.70 | |||
| 31–50 | 12.31 ± 8.57 | 2.60 ± 1.52 | 2.20 ± 1.87 | 0.007 and 0.050 | ||
| >50 | 11.89 ± 7.30 | 3.0 ± 3.36 | 3.59 ± 2.48 | |||
| Gender | NS | NS | NS | |||
| Male | 12.07 ± 8.26 | 2.59 ± 2.33 | 2.27 ± 2.0 | |||
| Female | 11.58 ± 8.32 | 2.67 ± 1.88 | 2.24 ± 1.8 | |||
| Disease duration (in years) | ||||||
| <2 | 8.53 ± 7.95 | <0.001 and 0.078 | 1.50 ± 0.80 | <0.001 and 0.150 | 1.55 ± 1.4 | <0.001 and 0.126 |
| 2 to 5 | 10.46 ± 8.28 | 2.41 ± 2.10 | 1.70 ± 1.4 | |||
| >5 | 14.17 ± 7.80 | 3.54 ± 2.05 | 3.01 ± 2.2 | |||
| DMT | NS | <0.001 and 0.651 | NS | |||
| Treated | 11.44 ± 7.46 | 3.01 ± 2.23 | 2.35 ± 2.0 | |||
| Not treated | 12.49 ± 10.23 | 1.74 ± 0.85 | 1.95 ± 1.7 | |||
| Mobility | NS | |||||
| Supported | 18.45 ± 10.15 | <0.001 and 0.918 | 3.57 ± 2.95 | 6.58 ± 0.70 | <0.001 and 4.158 | |
| Not supported | 10.97 ± 7.72 | 2.57 ± 1.92 | 1.80 ± 1.40 | |||
| IVIM and Clinical Variables | EDSS | |
|---|---|---|
| Pearson’s Correlation | p-Value | |
| ADC | 0.360 | <0.001 |
| D | 0.368 | <0.001 |
| D* | 0.283 | <0.001 |
| f | −0.106 | NS |
| No. of MS lesions | 0.372 | <0.001 |
| Number of relapses | 0.259 | 0.001 |
| EDSS | MRI and Clinical Outcome Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADC | D | D* | f | No. of Lesions | ||||||
| Mean ± SD | p-Value and Effect Size | Mean ± SD | p-Value and Effect Size | Mean ± SD | p-Value and Effect Size | Mean ± SD | p-Value and Effect Size | Mean ± SD | p-Value and Effect Size | |
| <3.0 ≥3.0 | 1.08 ± 0.13 1.15 ± 0.17 | 0.001 and −0.504 | 1.02 ± 0.13 1.10 ± 0.16 | 0.001 and −0.539 | 1.25 ± 0.15 1.30 ± 0.18 | 0.067 and −0.288 | 0.06 ± 0.02 0.05 ± 0.01 | 0.046 and 0.314 | 9.9 ± 6.80 16.1 ± 9.80 | <0.001 and −0.785 |
| Variables | β Coefficients | Standard Error | t-Value | p-Value | 95% CI for β Coefficient |
|---|---|---|---|---|---|
| Constant | −0.488 | 1.079 | −0.452 | NS | −2.62, 1.64 |
| Age | 0.025 | 0.015 | 1.651 | NS | −0.005, 0.054 |
| ADC | 0.334 | 1.052 | 0.317 | NS | −1.74, 2.41 |
| Number of MS lesions | 0.074 | 0.016 | 4.769 | <0.001 | 0.04, 0.10 |
| Number of relapses | 0.146 | 0.63 | 2.326 | 0.021 | 0.02, 0.27 |
| Disease duration | 0.010 | 0.028 | 0.374 | NS | −0.4, 0.06 |
| Variables | β Coefficients | Standard Error | t-Value | p-Value | 95% CI for β Coefficient |
|---|---|---|---|---|---|
| Constant | −0.614 | 1.058 | −580 | NS | −2.70, 1.47 |
| Age | 0.024 | 0.015 | 1.633 | NS | −005, 0.05 |
| D | 0.499 | 1.078 | 0.463 | NS | −1.63, 2.63 |
| Number of MS lesions | 0.073 | 0.015 | 4.755 | <0.001 | 0.04, 0.10 |
| Number of relapses | 0.145 | 0.063 | 2.319 | 0.022 | 0.02, 0.27 |
| Disease duration | 0.010 | 0.028 | 0.346 | NS | −0.045, 0.06 |
| Variables | β Coefficients | Standard Error | t-Value | p-Value | 95% CI for β Coefficient |
|---|---|---|---|---|---|
| Constant | 0.099 | 1.059 | 0.093 | NS | −1.99, 2.19 |
| Age | 0.027 | 0.015 | 1.772 | NS | −0.003, 0.06 |
| D* | −0.279 | 0.888 | −0.314 | NS | −2.03, 1.47 |
| Number of MS lesions | 0.077 | 0.015 | 5.063 | <0.001 | 0.05, 0.11 |
| Number of relapses | 0.144 | 0.063 | 2.287 | 0.023 | 0.02, 0.27 |
| Disease duration | 0.013 | 0.027 | 0.483 | NS | −0.04, 0.07 |
| Variables | β Coefficients | Standard Error | t-Value | p-Value | 95% CI for β Coefficient |
|---|---|---|---|---|---|
| Constant | 0.219 | 0.655 | 0.335 | NS | −1.07, 1.51 |
| Age | 0.028 | 0.015 | 1.885 | NS | −0.001, 0.06 |
| f | −7.546 | 7.205 | −1.047 | NS | −21.77, 6.68 |
| Number of MS lesions | 0.074 | 0.015 | 5.079 | <0.001 | 0.04, 0.10 |
| Number of relapses | 0.138 | 0.063 | 2.198 | 0.029 | 0.014, 0.26 |
| Disease duration | 0.011 | 0.027 | 0.407 | NS | −0.04, 0.06 |
| Variables | β Coefficients | Standard Error | t-Value | p-Value | 95% CI for β Coefficient |
|---|---|---|---|---|---|
| Constant | −0.184 | 1.136 | −0.162 | NS | −2.43, 2.06 |
| Age | 0.027 | 0.015 | 1.780 | NS | −0.003, 0.06 |
| ADC | 1.262 | 5.674 | 0.222 | NS | −9.94, 12.46 |
| D | 0.007 | 5.472 | 0.001 | NS | −10.80, 10.81 |
| D* | −0.831 | 2.502 | −0.332 | NS | −5.77, 4.11 |
| f | −5.211 | 12.851 | −0.406 | NS | −30.59, 20.16 |
| Number of MS lesions | 0.072 | 0.016 | 4.506 | <0.001 | 0.041, 0.10 |
| Number of relapses | 0.138 | 0.063 | 2.179 | 0.031 | 0.013, 0.26 |
| Disease duration | 0.008 | 0.028 | 0.284 | NS | −0.05, 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomair, O.I.; Alghamdi, S.A.; Abujamea, A.H.; Aljarallah, S.; Alkhawajah, N.M.; Alshuhri, M.S.; Alashban, Y.I.; Kurniawan, N.D. The Utility of Intravoxel Incoherent Motion Metrics in Assessing Disability in Relapsing–Remitting Multiple Sclerosis. Diagnostics 2025, 15, 2113. https://doi.org/10.3390/diagnostics15162113
Alomair OI, Alghamdi SA, Abujamea AH, Aljarallah S, Alkhawajah NM, Alshuhri MS, Alashban YI, Kurniawan ND. The Utility of Intravoxel Incoherent Motion Metrics in Assessing Disability in Relapsing–Remitting Multiple Sclerosis. Diagnostics. 2025; 15(16):2113. https://doi.org/10.3390/diagnostics15162113
Chicago/Turabian StyleAlomair, Othman I., Sami A. Alghamdi, Abdullah H. Abujamea, Salman Aljarallah, Nuha M. Alkhawajah, Mohammed S. Alshuhri, Yazeed I. Alashban, and Nyoman D. Kurniawan. 2025. "The Utility of Intravoxel Incoherent Motion Metrics in Assessing Disability in Relapsing–Remitting Multiple Sclerosis" Diagnostics 15, no. 16: 2113. https://doi.org/10.3390/diagnostics15162113
APA StyleAlomair, O. I., Alghamdi, S. A., Abujamea, A. H., Aljarallah, S., Alkhawajah, N. M., Alshuhri, M. S., Alashban, Y. I., & Kurniawan, N. D. (2025). The Utility of Intravoxel Incoherent Motion Metrics in Assessing Disability in Relapsing–Remitting Multiple Sclerosis. Diagnostics, 15(16), 2113. https://doi.org/10.3390/diagnostics15162113







