Metabolic Acidosis in Patients with Chronic Kidney Disease: Diagnosis, Pathogenesis, and Treatment—A Narrative Review
Abstract
1. Introduction
2. Definition and Epidemiology of Metabolic Acidosis in Chronic Kidney Disease
3. Diagnosis of Metabolic Acidosis
4. Special Conditions to Consider in a Patient with CKD and MA
4.1. The Effect of Albumin Concentration on the Anion Gap
4.2. Chronic Obstructive Pulmonary Disease
4.3. Lactic Acidosis
4.4. Diabetic Ketoacidosis
4.5. Salicylates
4.6. Drug-Induced Acidosis
- (a)
- Metformin
- (b)
- SGLT2 Inhibitors
4.7. Renal Transplant Recipients
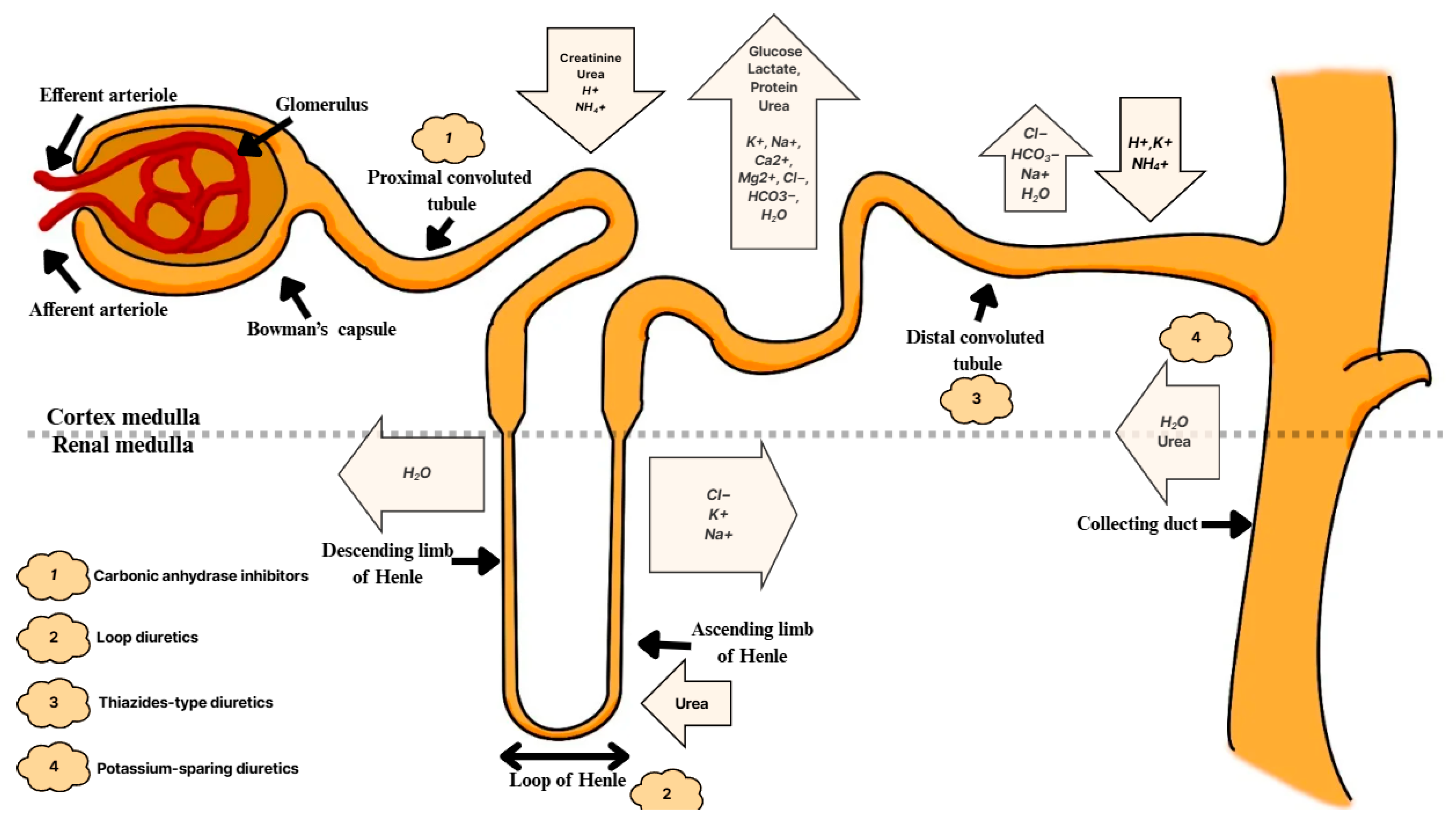
5. Acid–Base Regulation of the Kidneys
6. Metabolic Acidosis and Progression of Chronic Kidney Disease
6.1. Ammonia
6.2. Aldosterone
6.3. Endothelin
7. Consequences of Metabolic Acidosis in Chronic Kidney Disease
8. Anion Gap
9. Normal Anion Gap Metabolic Acidosis (NAGMA)
9.1. Renal Tubular Acidosis
9.2. Diagnostics of RTA
9.2.1. Urine pH
9.2.2. Urinary Excretion of Ammonium
9.2.3. Differences in the Diagnosis Between Distal Renal Tubular Acidosis and Proximal Renal Tubular Acidosis
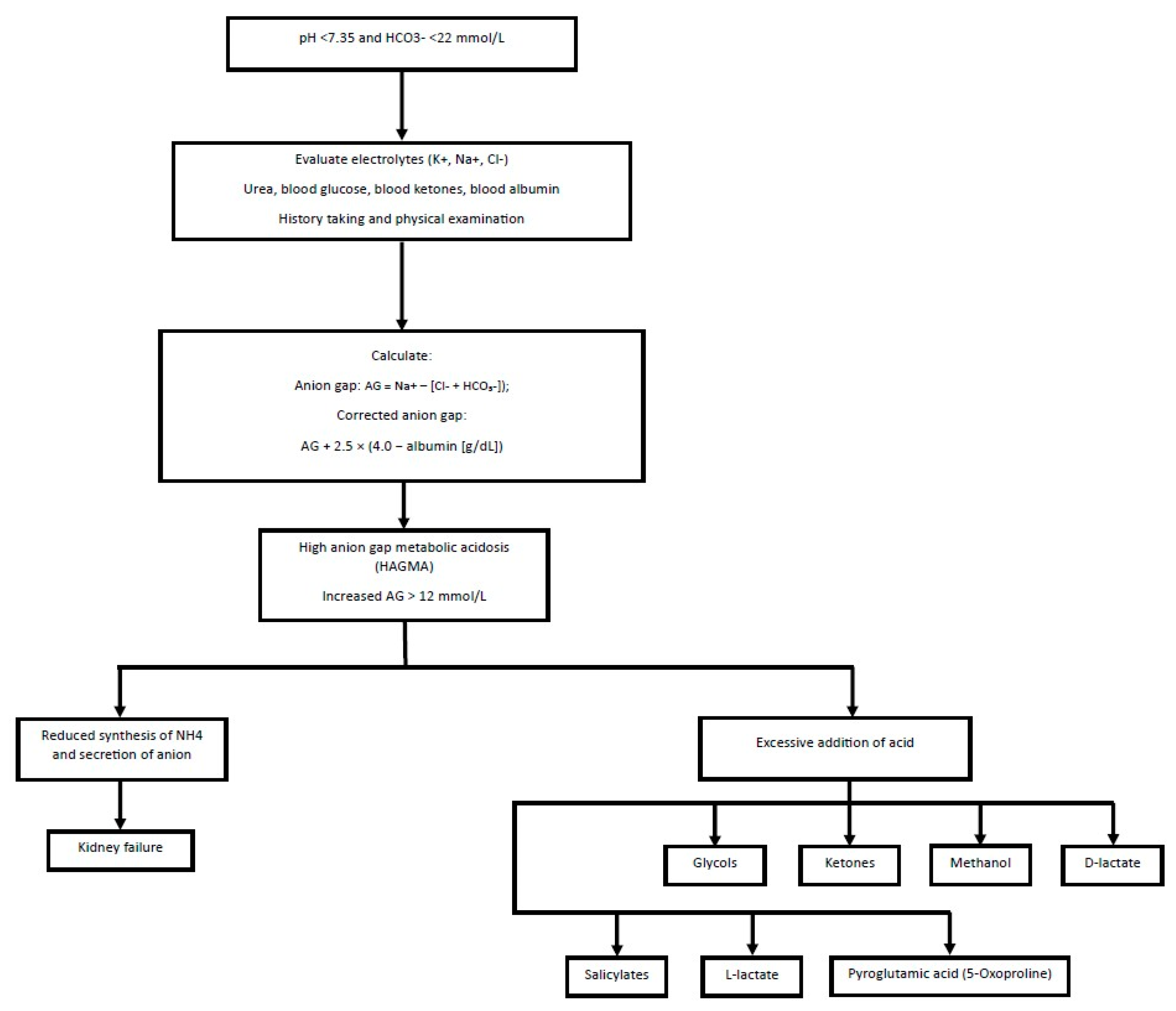
10. High Anion Gap Metabolic Acidosis (HAGMA)
11. Basic Dietary Recommendations for Patients with Metabolic Acidosis
11.1. General Information
11.2. Low-Protein Diet
11.3. Fruit and Vegetable Diet
11.4. Fiber-Rich Diet
11.5. Diet Rich in Sugars
11.6. Ketogenic Diet
12. Pharmacological Treatment of Metabolic Acidosis
12.1. Sodium Bicarbonate
12.2. Veverimer
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karim, Z.; Attmane-Elakeb, A.; Bichara, M. Renal handling of NH4+ in relation to the control of acid-base balance by the kidney. J. Nephrol. 2002, 15, S128–S134. [Google Scholar] [PubMed]
- Raphael, K.L.; Zhang, Y.; Ying, J.; Greene, T. Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology 2014, 19, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L. Metabolic Acidosis in CKD: Pathogenesis, Adverse Effects, and Treatment Effects. Int. J. Mol. Sci. 2024, 25, 5187. [Google Scholar] [CrossRef]
- Cecilia Farfan Ruiz, A.; Sriperumbuduri, S.; Shaw, J.L.; Clark, E.G. High-anion-gap metabolic acidosis during a prolonged hospitalization following perforated diverticulitis: An educational case report. Can. J. Kidney Health Dis. 2022, 9, 20543581221129753. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Chabala, J.M.; Gavrilov, K.L.; Levi-Setti, R. Effects of in vivo metabolic acidosis on midcortical bone ion composition. Am. J. Physiol.-Ren. Physiol. 1999, 277, F813–F819. [Google Scholar] [CrossRef]
- Bailey, J.L.; Wang, X.; England, B.K.; Price, S.R.; Ding, X.; Mitch, W.E. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J. Clin. Investig. 1996, 97, 1447–1453. [Google Scholar] [CrossRef]
- Bellasi, A.; Di Micco, L.; Santoro, D.; Marzocco, S.; De Simone, E.; Cozzolino, M.; Di Lullo, L.; Guastaferro, P.; Di Iorio, B.; UBI Study Investigators. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016, 17, 158. [Google Scholar] [CrossRef]
- Mandel, E.I.; Forman, J.P.; Curhan, G.C.; Taylor, E.N. Plasma bicarbonate and odds of incident hypertension. Am. J. Hypertens. 2013, 26, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Abramowitz, M.; Hostetter, T.H.; Melamed, M.L. Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am. J. Kidney Dis. 2009, 54, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Madias, N.E. Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatr. Nephrol. 2011, 26, 19–28. [Google Scholar] [CrossRef]
- Toba, K.; Hosojima, M.; Kabasawa, H.; Kuwahara, S.; Murayama, T.; Yamamoto-Kabasawa, K.; Kaseda, R.; Wada, E.; Watanabe, R.; Tanabe, N. Higher estimated net endogenous acid production with lower intake of fruits and vegetables based on a dietary survey is associated with the progression of chronic kidney disease. BMC Nephrol. 2019, 20, 421. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.; Surma, S. Metabolic acidosis in patients with CKD: Epidemiology, pathogenesis, and treatment. Kidney Dis. 2021, 7, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, M.K.; Hostetter, T.H.; Melamed, M.L. Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. Am. J. Kidney Dis. 2011, 58, 29–38. [Google Scholar] [CrossRef] [PubMed]
- May, R.; Kelly, R.; Mitch, W. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J. Clin. Investig. 1986, 77, 614–621. [Google Scholar] [CrossRef]
- Moranne, O.; Froissart, M.; Rossert, J.; Gauci, C.; Boffa, J.-J.; Haymann, J.P.; M’rad, M.B.; Jacquot, C.; Houillier, P.; Stengel, B. Timing of onset of CKD-related metabolic complications. J. Am. Soc. Nephrol. 2009, 20, 164–171. [Google Scholar] [CrossRef]
- Gołębiowski, T.; Kusztal, M.; Konieczny, A.; Kuriata-Kordek, M.; Gawryś, A.; Augustyniak-Bartosik, H.; Letachowicz, K.; Zielińska, D.; Wiśniewska, M.; Krajewska, M. Exhausted capacity of bicarbonate buffer in renal failure diagnosed using point of care analyzer. Diagnostics 2021, 11, 226. [Google Scholar] [CrossRef]
- Kuczera, P.; Ciaston-Mogilska, D.; Oslizlo, B.; Hycki, A.; Wiecek, A.; Adamczak, M. The prevalence of metabolic acidosis in patients with different stages of chronic kidney disease: Single-centre study. Kidney Blood Press. Res. 2020, 45, 863–872. [Google Scholar] [CrossRef]
- Goutham, K.C.; Harichandrakumar, K.; Dhanin, P.; Priyamvada, P.; Haridasan, S.; Parameswaran, S. Persistent metabolic acidosis on regular hemodialysis or peritoneal dialysis. Indian J. Nephrol. 2019, 29, 84–89. [Google Scholar] [CrossRef]
- Makówka, A.; Nowicki, M. Bicarbonate-buffered dialysis solutions—A breakthrough in peritoneal dialysis therapy? Ren. Dis. Transplant. Forum 2018, 18, 9–14. [Google Scholar]
- Himmele, R.; Jensen, L.; Fenn, D.; Ho, C.-H.; Sawin, D.-A.; Diaz–Buxo, J.A. A new neutral-pH low-GDP peritoneal dialysis fluid. Perit. Dial. Int. 2012, 32, 444–452. [Google Scholar] [CrossRef]
- Vashistha, T.; Kalantar-Zadeh, K.; Molnar, M.Z.; Torlen, K.; Mehrotra, R. Dialysis modality and correction of uremic metabolic acidosis: Relationship with all-cause and cause-specific mortality. Clin. J. Am. Soc. Nephrol. 2013, 8, 254–264. [Google Scholar] [CrossRef]
- Szeto, C.-C.; Kwan, B.C.-H.; Chow, K.-M.; Chung, S.; Yu, V.; Cheng, P.M.-S.; Leung, C.-B.; Law, M.-C.; Li, P.K.-T. Predictors of residual renal function decline in patients undergoing continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 2015, 35, 180–188. [Google Scholar] [CrossRef]
- Selby, N.M.; Kazmi, I. Peritoneal dialysis has optimal intradialytic hemodynamics and preserves residual renal function: Why isn’t it better than hemodialysis? Semin. Dial. 2019, 32, 3–8. [Google Scholar] [CrossRef]
- Tam, P. Peritoneal dialysis and preservation of residual renal function. Perit. Dial. Int. 2009, 29, 108–110. [Google Scholar] [CrossRef]
- Adamczak, M.; Masajtis-Zagajewska, A.; Mazanowska, O.; Madziarska, K.; Stompór, T.; Więcek, A. Diagnosis and treatment of metabolic acidosis in patients with chronic kidney disease–position statement of the working group of the Polish Society of Nephrology. Kidney Blood Press. Res. 2018, 43, 959–969. [Google Scholar] [CrossRef]
- Farrell, D.R.; Vassalotti, J.A. Screening, identifying, and treating chronic kidney disease: Why, who, when, how, and what? BMC Nephrol. 2024, 25, 34. [Google Scholar] [CrossRef]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-base homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Haller, C. Hypoalbuminemia in renal failure: Pathogenesis and therapeutic considerations. Kidney Blood Press. Res. 2006, 28, 307–310. [Google Scholar] [CrossRef]
- Klaerner, G.; Shao, J.; Biyani, K.; Kade, M.; Kierstead, P.; Gbur, R.; Tabakman, S.; Nguyen, S.; Buysse, J. Mechanism of action of veverimer: A novel, orally administered, nonabsorbed, counterion-free, hydrochloric acid binder under development for the treatment of metabolic acidosis in chronic kidney disease. J. Pharmacol. Exp. Ther. 2020, 375, 439–450. [Google Scholar] [CrossRef]
- Feldman, M.; Soni, N.; Dickson, B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J. Lab. Clin. Med. 2005, 146, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Fenves, A.Z.; Emmett, M. Approach to patients with high anion gap metabolic acidosis: Core curriculum 2021. Am. J. Kidney Dis. 2021, 78, 590–600. [Google Scholar] [CrossRef]
- Figge, J.; Jabor, A.; Kazda, A.; Fencl, V. Anion gap and hypoalbuminemia. Crit. Care Med. 1998, 26, 1807–1810. [Google Scholar] [CrossRef]
- Sue, D.Y.; Wasserman, K.; Moricca, R.B.; Casaburi, R. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease: Use of the V-slope method for anaerobic threshold determination. Chest 1988, 94, 931–938. [Google Scholar] [CrossRef]
- Indrayanti, L.; Mulyono, H. Profil Asam Laktat Penderita Diabetes Mellitus Terkendali (Kontrol) Dan Tidak Terkendali (Kontrol). Indones. J. Clin. Pathol. Med. Lab. 2008, 14, 97–101. [Google Scholar] [CrossRef][Green Version]
- Kamel, K.S.; Oh, M.S.; Halperin, M.L. L-lactic acidosis: Pathophysiology, classification, and causes; emphasis on biochemical and metabolic basis. Kidney Int. 2020, 97, 75–88. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Lactic acidosis: Current treatments and future directions. Am. J. Kidney Dis. 2016, 68, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Calimag, A.P.P.; Chlebek, S.; Lerma, E.V.; Chaiban, J.T. Diabetic ketoacidosis. Dis.-A-Mon. 2023, 69, 101418. [Google Scholar] [CrossRef]
- Dhatariya, K.K.; Glaser, N.S.; Codner, E.; Umpierrez, G.E. Diabetic ketoacidosis. Nat. Rev. Dis. Primers 2020, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Kitabchi, A.E. Diabetic ketoacidosis: Risk factors and management strategies. Treat. Endocrinol. 2003, 2, 95–108. [Google Scholar] [CrossRef]
- Buisman, L.; Riphagen, I.J.; Kwant, M.; Themmen, M.D.; Bouma, M.; Bethlehem, C. Case report: Salicylate intoxication can present with a normal anion gap metabolic acidosis depending on method used for measuring chloride. Br. J. Clin. Pharmacol. 2022, 88, 4933–4936. [Google Scholar] [CrossRef]
- Needs, C.J.; Brooks, P.M. Clinical pharmacokinetics of the salicylates. Clin. Pharmacokinet. 1985, 10, 164–177. [Google Scholar] [CrossRef]
- Wiederkehr, M.R.; Benevides, R., Jr.; Santa Ana, C.A.; Emmett, M. Pseudohyperchloremia and Negative Anion Gap–Think Salicylate! Am. J. Med. 2021, 134, 1170–1174. [Google Scholar] [CrossRef]
- Moe, O.W.; Pham, A.Q.T.; Xu, L.H.R. Drug-Induced Metabolic Acidosis. F1000Research 2015, 4, 1460. [Google Scholar] [CrossRef] [PubMed]
- Blough, B.; Moreland, A.; Mora, A., Jr. Metformin-induced lactic acidosis with emphasis on the anion gap. Bayl. Univ. Med. Cent. Proc. 2015, 28, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Regolisti, G.; Antoniotti, R.; Fani, F.; Greco, P.; Fiaccadori, E. Treatment of metformin intoxication complicated by lactic acidosis and acute kidney injury: The role of prolonged intermittent hemodialysis. Am. J. Kidney Dis. 2017, 70, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Euglycemic ketoacidosis as a complication of SGLT2 inhibitor therapy. Clin. J. Am. Soc. Nephrol. 2021, 16, 1284–1291. [Google Scholar] [CrossRef]
- Breuer, T.; Kampmann, K.; Wutzler, A.; Steinfort, C.; Uhl, W.; Schmidt, W.; Meier, J. Severe atypical ketoacidosis due to SGLT2-inhibitor therapy: Two case reports. Der Internist 2018, 59, 282–287. [Google Scholar] [CrossRef]
- Qiu, H.; Novikov, A.; Vallon, V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes/Metab. Res. Rev. 2017, 33, e2886. [Google Scholar] [CrossRef]
- Tariq, H.; Dobre, M. Metabolic acidosis post kidney transplantation. Front. Physiol. 2022, 13, 989816. [Google Scholar] [CrossRef]
- Mohebbi, N.; Mihailova, M.; Wagner, C.A. The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am. J. Physiol.-Ren. Physiol. 2009, 297, F499–F509. [Google Scholar] [CrossRef]
- Bonar, P.T.; Casey, J.R. Plasma membrane Cl-/HCO3-exchangers: Structure, mechanism and physiology. Channels 2008, 2, 337–345. [Google Scholar] [CrossRef]
- Steinmetz, P.R.; Eisinger, R.P.; Lowenstein, J. The excretion of acid in unilateral renal disease in man. J. Clin. Investig. 1965, 44, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Raphael, K.L. Metabolic acidosis and subclinical metabolic acidosis in CKD. J. Am. Soc. Nephrol. 2018, 29, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Abramowitz, M.K. Advances in management of chronic metabolic acidosis in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 409–416. [Google Scholar] [CrossRef]
- Peyton, K.J.; Liu, X.-M.; Yu, Y.; Yates, B.; Behnammanesh, G.; Durante, W. Glutaminase-1 stimulates the proliferation, migration, and survival of human endothelial cells. Biochem. Pharmacol. 2018, 156, 204–214. [Google Scholar] [CrossRef]
- Davies, G.F.; Khandelwal, R.L.; Wu, L.; Juurlink, B.H.; Roesler, W.J. Inhibition of phosphoenolpyruvate carboxykinase (PEPCK) gene expression by troglitazone: A peroxisome proliferator-activated receptor-γ (PPARγ)-independent, antioxidant-related mechanism. Biochem. Pharmacol. 2001, 62, 1071–1079. [Google Scholar] [CrossRef]
- Nwia, S.M.; Li, X.C.; Leite, A.P.d.O.; Hassan, R.; Zhuo, J.L. The Na+/H+ exchanger 3 in the intestines and the proximal tubule of the kidney: Localization, physiological function, and key roles in angiotensin II-induced hypertension. Front. Physiol. 2022, 13, 861659. [Google Scholar] [CrossRef]
- Goraya, N.; Munoz-Maldonado, Y.; Simoni, J.; Wesson, D.E. Treatment of chronic kidney disease-related metabolic acidosis with fruits and vegetables compared to NaHCO3 yields more and better overall health outcomes and at comparable five-year cost. J. Ren. Nutr. 2021, 31, 239–247. [Google Scholar] [CrossRef]
- Kretschmer, R. Chemotaxis. Mol. Cells Parasites Immunol. 1980, 9, S299–S302. [Google Scholar]
- Halperin, M.L.; Ethier, J.H.; Kamel, K.S. Ammonium excretion in chronic metabolic acidosis: Benefits and risks. Am. J. Kidney Dis. 1989, 14, 267–271. [Google Scholar] [CrossRef]
- Verma, A.; Vaidya, A.; Subudhi, S.; Waikar, S.S. Aldosterone in chronic kidney disease and renal outcomes. Eur. Heart J. 2022, 43, 3781–3791. [Google Scholar] [CrossRef]
- Ponda, M.P.; Hostetter, T.H. Aldosterone antagonism in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2006, 1, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Mc Causland, F.R.; Lefkowitz, M.P.; Claggett, B.; Anavekar, N.S.; Senni, M.; Gori, M.; Jhund, P.S.; McGrath, M.M.; Packer, M.; Shi, V. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation 2020, 142, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Eckenstaler, R.; Sandori, J.; Gekle, M.; Benndorf, R.A. Angiotensin II receptor type 1–An update on structure, expression and pathology. Biochem. Pharmacol. 2021, 192, 114673. [Google Scholar] [CrossRef] [PubMed]
- Kohan, D.E.; Barton, M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014, 86, 896–904. [Google Scholar] [CrossRef]
- Wesson, D.E. Endogenous endothelins mediate increased distal tubule acidification induced by dietary acid in rats. J. Clin. Investig. 1997, 99, 2203–2211. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Mehrotra, R.; Fouque, D.; Kopple, J.D. Poor nutritional status and inflammation: Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin. Dial. 2004, 17, 455–465. [Google Scholar] [CrossRef]
- Xie, Q.W.; Whisnant, R.; Nathan, C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J. Exp. Med. 1993, 177, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Bellocq, A.; Suberville, S.; Philippe, C.; Bertrand, F.; Perez, J.; Fouqueray, B.; Cherqui, G.; Baud, L. Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages: Evidence for involvement of nuclear factor-κB activation. J. Biol. Chem. 1998, 273, 5086–5092. [Google Scholar] [CrossRef]
- Celotto, A.; Ferreira, L.; Capellini, V.; Albuquerque, A.; Rodrigues, A.J.; Evora, P.R.B. Acute but not chronic metabolic acidosis potentiates the acetylcholine-induced reduction in blood pressure: An endothelium-dependent effect. Braz. J. Med. Biol. Res. 2015, 49, e5007. [Google Scholar] [CrossRef]
- Pedoto, A.; Caruso, J.E.; Nandi, J.; Oler, A.; Hoffmann, S.P.; Tassiopoulos, A.K.; McGRAW, D.J.; Camporesi, E.M.; Hakim, T.S. Acidosis stimulates nitric oxide production and lung damage in rats. Am. J. Respir. Crit. Care Med. 1999, 159, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Gaynullina, D.K.; Tarasova, O.S.; Shvetsova, A.A.; Borzykh, A.A.; Schubert, R. The effects of acidosis on eNOS in the systemic vasculature: A focus on early postnatal ontogenesis. Int. J. Mol. Sci. 2022, 23, 5987. [Google Scholar] [CrossRef]
- Orchard, C.; Kentish, J.C. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol.-Cell Physiol. 1990, 258, C967–C981. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Kurtz, I. Metabolic acidosis of CKD: Diagnosis, clinical characteristics, and treatment. Am. J. Kidney Dis. 2005, 45, 978–993. [Google Scholar] [CrossRef]
- Krieger, N.S.; Bushinsky, D.A.; Frick, K.K. RENAL RESEARCH INSTITUTE SYMPOSIUM: Cellular Mechanisms of Bone Resorption Induced by Metabolic Acidosis. Semin. Dial. 2003, 16, 463–466. [Google Scholar] [CrossRef]
- Kopple, J.D.; Kalantar-Zadeh, K.; Mehrotra, R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int. 2005, 67, S21–S27. [Google Scholar] [CrossRef]
- Dobre, M. Acidosis in renal disease: Should we be concerned? Nephrol. Dial. Transplant. 2022, 37, 1043–1045. [Google Scholar] [CrossRef]
- Yenchek, R.; Ix, J.H.; Rifkin, D.E.; Shlipak, M.G.; Sarnak, M.J.; Garcia, M.; Patel, K.V.; Satterfield, S.; Harris, T.B.; Newman, A.B. Association of serum bicarbonate with incident functional limitation in older adults. Clin. J. Am. Soc. Nephrol. 2014, 9, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Orchard, C.H.; Cingolani, H.E. Acidosis and arrhythmias in cardiac muscle. Cardiovasc. Res. 1994, 28, 1312–1319. [Google Scholar] [CrossRef]
- Kim, H.J. Metabolic acidosis in chronic kidney disease: Pathogenesis, clinical consequences, and treatment. Electrolytes Blood Press. 2021, 19, 29. [Google Scholar] [CrossRef]
- London, G.M.; Drüeke, T.B. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997, 51, 1678–1695. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Anderson, J.E.; Kalantar-Zadeh, K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol. Dial. Transplant. 2009, 24, 1232–1237. [Google Scholar] [CrossRef]
- Collister, D.; Ferguson, T.W.; Funk, S.E.; Reaven, N.L.; Mathur, V.; Tangri, N. Metabolic acidosis and cardiovascular disease in CKD. Kidney Med. 2021, 3, 753–761.e751. [Google Scholar] [CrossRef] [PubMed]
- Dobre, M.; Pajewski, N.M.; Beddhu, S.; Chonchol, M.; Hostetter, T.H.; Li, P.; Rahman, M.; Servilla, K.; Weiner, D.E.; Wright, J.T. Serum bicarbonate and cardiovascular events in hypertensive adults: Results from the Systolic Blood Pressure Intervention Trial. Nephrol. Dial. Transplant. 2020, 35, 1377–1384. [Google Scholar] [CrossRef]
- Magni, G.; Unwin, R.J.; Moochhala, S.H. Renal tubular acidosis (RTA) and kidney stones: Diagnosis and management. Arch. Esp. Urol. 2021, 74, 123–128. [Google Scholar]
- Guimerà, J.; Martínez, A.; Tubau, V.; Sabate, A.; Bauza, J.L.; Rios, A.; Lopez, M.; Piza, P.; Grases, F.; Pieras, E. Prevalence of distal renal tubular acidosis in patients with calcium phosphate stones. World J. Urol. 2020, 38, 789–794. [Google Scholar] [CrossRef]
- Peitzman, S.J. The flame photometer as engine of nephrology: A biography. Am. J. Kidney Dis. 2010, 56, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, T.; Zmonarski, S.; Rożek, W.; Powązka, M.; Jerzak, P.; Gołębiowski, M.; Kusztal, M.; Olczyk, P.; Stojanowski, J.; Letachowicz, K. Point-of-Care Testing to Differentiate Various Acid–Base Disorders in Chronic Kidney Disease. Diagnostics 2023, 13, 3367. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kang, E.; Ryu, H.; Han, M.; Lee, K.-B.; Kim, Y.-S.; Sung, S.; Ahn, C.; Oh, K.-H. Metabolic acidosis is associated with pulse wave velocity in chronic kidney disease: Results from the KNOW-CKD Study. Sci. Rep. 2019, 9, 16139. [Google Scholar] [CrossRef]
- Emmett, M.; Kelepouris, E. Overview and Pathophysiology of Renal Tubular Acidosis and the Effect on Potassium Balance. UpToDate. [cited 23 September 2016]. Last Updated: 30 June 2015. Available online: https://www.uptodate.com/contents/overview-and-pathophysiology-of-renal-tubular-acidosis-and-the-effect-on-potassium-balance (accessed on 8 August 2025).
- Kappy, M.S.; Morrow, G., III. A diagnostic approach to metabolic acidosis in children. Pediatrics 1980, 65, 351–356. [Google Scholar] [CrossRef]
- Thomlinson, P.; Carpenter, M.; D’Alessandri-Silva, C. The evaluation and treatment of metabolic acidosis. Curr. Treat. Options Pediatr. 2020, 6, 29–37. [Google Scholar] [CrossRef]
- Gilbert-Barness, E.; Farrell, P.M. Approach to diagnosis of metabolic diseases. Transl. Sci. Rare Dis. 2016, 1, 3–22. [Google Scholar] [CrossRef]
- Nagami, G.T. Hyperchloremia–Why and how. Nefrologia 2016, 36, 347–353. [Google Scholar] [CrossRef]
- Emmett, M.; Palmer, B.F. Treatment of Distal (Type 1) and Proximal (Type 2) Renal Tubular Acidosis. 2016. Available online: https://www.uptodate.com/contents/treatment-of-distal-type-1-and-proximal-type-2-renal-tubular-acidosis (accessed on 8 August 2025).
- Bhandarkar, A.; Varmudy, A.; Boro, H.; Bhat, S. Renal tubular acidosis: Varied aetiologies and clinical presentations: Three case reports. World J. Nephrol. 2025, 14, 104760. [Google Scholar] [CrossRef]
- Dutta, D.; Sein, S.; Gupta, M.; Mehrotra, A.; Ahmad, D. Hypokalemic Periodic Paralysis with Renal Tubular Acidosis in a Patient with Autoimmune Disorder. J. Assoc. Physicians India. 2025, 73, e41–e42. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, M.; Obialo, C.; Hruska, K.A. Renai Tubular Acidosis. Endocrinol. Metab. Clin. 1990, 19, 869–887. [Google Scholar] [CrossRef]
- Richardson, R.M.; Halperin, M.L. The urine pH: A potentially misleading diagnostic test in patients with hyperchloremic metabolic acidosis. Am. J. Kidney Dis. 1987, 10, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vaswani, M.; Srivastava, R.; Bagga, A. Urinary net charge in hyperchloremic metabolic acidosis. Indian Pediatr. 1998, 35, 13–18. [Google Scholar] [PubMed]
- Batlle, D.C.; Hizon, M.; Cohen, E.; Gutterman, C.; Gupta, R. The use of the urinary anion gap in the diagnosis of hyperchloremic metabolic acidosis. N. Engl. J. Med. 1988, 318, 594–599. [Google Scholar] [CrossRef]
- Rastogi, S.P.; Crawford, C.; Wheeler, R.; Flanigan, W.; Arruda, J. Effect of furosemide on urinary acidification in distal renal tubular acidosis. J. Lab. Clin. Med. 1984, 104, 271–282. [Google Scholar]
- Rehman, M.Z.; Melamed, M.; Harris, A.; Shankar, M.; Rosa, R.M.; Batlle, D. Urinary ammonium in clinical medicine: Direct measurement and the urine anion gap as a surrogate marker during metabolic acidosis. Adv. Kidney Dis. Health 2023, 30, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Brenes, L.G.; Sanchez, M.I. Impaired urinary ammonium excretion in patients with isolated proximal renal tubular acidosis. J. Am. Soc. Nephrol. 1993, 4, 1073–1078. [Google Scholar] [CrossRef]
- Kamel, K.S.; Ethier, J.H.; Richardson, R.M.; Bear, R.A.; Halperin, M.L. Urine electrolytes and osmolality: When and how to use them. Am. J. Nephrol. 1990, 10, 89–102. [Google Scholar] [CrossRef]
- Emmett, M.; Palmer, B. Etiology and Diagnosis of Distal (Type 1) and Proximal (Type 2) Renal Tubular Acidosis-UpToDate. Topic 2328 Version 24.0. Available online: https://www.uptodate.com/contents/etiology-and-diagnosis-of-distal-type-1-and-proximal-type-2-renal-tubular-acidosis (accessed on 8 August 2025).
- Yaxley, J.; Pirrone, C. Review of the diagnostic evaluation of renal tubular acidosis. Ochsner J. 2016, 16, 525–530. [Google Scholar]
- Gowda, Y.H.; Jagtap, N.; Karyampudi, A.; Rao, N.P.; Deepika, G.; Sharma, M.; Gupta, R.; Tandan, M.; Ramchandani, M.; John, P. Fractional excretion of sodium and urea in differentiating acute kidney injury phenotypes in decompensated cirrhosis. J. Clin. Exp. Hepatol. 2022, 12, 899–907. [Google Scholar] [CrossRef]
- Funes, S.; de Morais, H.A. A Quick Reference on High Anion Gap Metabolic Acidosis. Vet. Clin. North Am. Small Anim. Pract. 2016, 47, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Asahina, Y.; Sakaguchi, Y.; Kajimoto, S.; Hattori, K.; Doi, Y.; Oka, T.; Kaimori, J.-Y.; Isaka, Y. Association of time-updated anion gap with risk of kidney failure in advanced CKD: A cohort study. Am. J. Kidney Dis. 2022, 79, 374–382. [Google Scholar] [CrossRef]
- Kaimori, J.-Y.; Sakaguchi, Y.; Kajimoto, S.; Asahina, Y.; Oka, T.; Hattori, K.; Doi, Y.; Isaka, Y. Diagnosing metabolic acidosis in chronic kidney disease: Importance of blood pH and serum anion gap. Kidney Res. Clin. Pract. 2022, 41, 288. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Kelly, J.T.; Gonzalez-Ortiz, A.; St-Jules, D.E.; Carrero, J.J. Animal Protein Intake and Possible Cardiovascular Risk in People With Chronic Kidney Disease: Mechanisms and Evidence. Adv. Kidney Dis. Health 2023, 30, 480–486. [Google Scholar] [CrossRef]
- Siener, R. Dietary treatment of metabolic acidosis in chronic kidney disease. Nutrients 2018, 10, 512. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional approaches for the management of metabolic acidosis in chronic kidney disease. Nutrients 2021, 13, 2534. [Google Scholar] [CrossRef]
- Czekalski, S.; Rutkowski, B.; Małgorzewicz, S.; w imieniu Zespołu, A.D.-Ś. Stanowisko Zespołu Krajowego Konsultanta Medycznego w dziedzinie Nefrologii dotyczące stosowania ketoanalogów aminokwasów i roztworu aminokwasów w leczeniu niedożywienia i zapobieganiu mu u dorosłych chorych z przewlekłą chorobą nerek. Ren. Dis. Transplant. Forum 2011, 4, 183–188. [Google Scholar]
- Yen, C.-L.; Fan, P.-C.; Chen, J.-J.; Kuo, G.; Hsiao, C.-C.; Chen, C.-Y.; Tu, Y.-R.; Hsu, H.-H.; Chen, Y.-C.; Chang, C.-H. Ketoanalogues supplemental low protein diet safely decreases short-term risk of dialysis among CKD stage 4 patients. Nutrients 2022, 14, 4020. [Google Scholar] [CrossRef]
- Ko, G.-J.; Kalantar-Zadeh, K. How important is dietary management in chronic kidney disease progression? A role for low protein diets. Korean J. Intern. Med. 2021, 36, 795. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, C.-A.; Simionescu, T.P.; Mocanu, A.E.; Garneata, L. Plant-based versus animal-based low protein diets in the management of chronic kidney disease. Nutrients 2021, 13, 3721. [Google Scholar] [CrossRef] [PubMed]
- Stremke, E.R.; Biruete, A.; Hill Gallant, K.M. Dietary protein intake and bone across stages of chronic kidney disease. Curr. Osteoporos. Rep. 2020, 18, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Bushinsky, D.A.; Krieger, N.S. Effects of acid on bone. Kidney Int. 2022, 101, 1160–1170. [Google Scholar] [CrossRef]
- Martin, T.J. PTH1R actions on bone using the cAMP/protein kinase a pathway. Front. Endocrinol. 2022, 12, 833221. [Google Scholar] [CrossRef]
- Kramer, H. Diet and chronic kidney disease. Adv. Nutr. 2019, 10, S367–S379. [Google Scholar] [CrossRef]
- Kuhn, C.; Mohebbi, N.; Ritter, A. Metabolic acidosis in chronic kidney disease: Mere consequence or also culprit? Pflüg. Arch.-Eur. J. Physiol. 2024, 476, 579–592. [Google Scholar] [CrossRef]
- Naude, M.; Francis, D. Chronic Sub-Clinical Systemic Metabolic Acidosis–A Review with Implications for Clinical Practice. J. Evid.-Based Integr. Med. 2022, 27, 2515690X221142352. [Google Scholar] [CrossRef]
- Cases, A.; Cigarrán-Guldrís, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-based diets for chronic kidney disease? It is time to reconsider. Nutrients 2019, 11, 1263. [Google Scholar] [CrossRef]
- Joshi, S.; Shah, S.; Kalantar-Zadeh, K. Adequacy of plant-based proteins in chronic kidney disease. J. Ren. Nutr. 2019, 29, 112–117. [Google Scholar] [CrossRef]
- Qiu, R.; Cao, W.-T.; Tian, H.-Y.; He, J.; Chen, G.-D.; Chen, Y.-M. Greater intake of fruit and vegetables is associated with greater bone mineral density and lower osteoporosis risk in middle-aged and elderly adults. PLoS ONE 2017, 12, e0168906. [Google Scholar] [CrossRef]
- Frassetto, L.; Remer, T.; Banerjee, T. Dietary contributions to metabolic acidosis. Adv. Chronic Kidney Dis. 2022, 29, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B. Acid–base balance of the diet—Implications for bone and muscle. Eur. J. Clin. Nutr. 2020, 74, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.A.; Ronco, A.L. Reduced dietary acid load in U.S. vegetarian adults: Results from the National Health and Nutrition Examination Survey. Food Sci. Nutr. 2022, 10, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Zimmermann-Klemd, A.M.; Lederer, A.-K.; Hannibal, L.; Kowarschik, S.; Huber, R.; Storz, M.A. A vegan diet is associated with a significant reduction in dietary acid load: Post hoc analysis of a randomized controlled trial in healthy individuals. Int. J. Environ. Res. Public Health 2021, 18, 9998. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Kaimori, J.-Y.; Isaka, Y. Plant-dominant low protein diet: A potential alternative dietary practice for patients with chronic kidney disease. Nutrients 2023, 15, 1002. [Google Scholar] [CrossRef] [PubMed]
- Cigarrán Guldris, S.; Latorre Catalá, J.A.; Sanjurjo Amado, A.; Menéndez Granados, N.; Piñeiro Varela, E. Fibre intake in chronic kidney disease: What fibre should we recommend? Nutrients 2022, 14, 4419. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gu, X.; Qiu, L.; Wang, X.; Li, Y. The effect of dietary fiber on hyperkalemia in maintenance hemodialysis patients: A cross-sectional study. J. Ren. Nutr. 2025, 35, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.M.; Berg, P.; Rix, M.; Pareek, M.; Leipziger, J.; Kamper, A.-L.; Astrup, A.; Sorensen, M.V.; Salomo, L. The New Nordic Renal Diet induces a pronounced reduction of urine acid excretion and uremic toxins in chronic kidney disease patients (stage 3 and 4). J. Ren. Nutr. 2023, 33, 412–419. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Hong, Y.; Zhong, J.; Li, Y.; Zhu, W.; Ma, J.; Huang, W.; Li, Y.; Li, Y. High fat diet and high sucrose intake divergently induce dysregulation of glucose homeostasis through distinct gut microbiota-derived bile acid metabolism in mice. J. Agric. Food Chem. 2023, 72, 230–244. [Google Scholar] [CrossRef]
- Álvarez-Herms, J. Summatory effects of anaerobic exercise and a ‘westernized athletic diet’on gut dysbiosis and chronic Low-grade metabolic acidosis. Microorganisms 2024, 12, 1138. [Google Scholar] [CrossRef]
- Ward, K.E.; Ramsay, J.; Vu, B.J.; Ward, K. A Case of Severe Metabolic Acidosis in the Setting of a Strict Ketogenic Diet. Cureus 2023, 15, e38741. [Google Scholar] [CrossRef]
- Vincent-Johnson, A.; Scialla, J.J. Importance of metabolic acidosis as a health risk in chronic kidney disease. Adv. Chronic Kidney Dis. 2022, 29, 329–336. [Google Scholar] [CrossRef]
- Raphael, K.L. Approach to the treatment of chronic metabolic acidosis in CKD. Am. J. Kidney Dis. 2016, 67, 696–702. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Hostetter, T.; Klaerner, G.; Stasiv, Y.; Lockey, C.; McNulty, S.; Lee, A.; Parsell, D.; Mathur, V.; Li, E. Randomized, controlled trial of TRC101 to increase serum bicarbonate in patients with CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 26–35. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Madias, N.E. Veverimer: An emerging potential treatment option for managing the metabolic acidosis of CKD. Am. J. Kidney Dis. 2020, 76, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Eiam-Ong, S.; Kurtzman, N.A.; Sabatini, S. Effect of furosemide-induced hypokalemic metabolic alkalosis on renal transport enzymes. Kidney Int. 1993, 43, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Photia, A.; Traivaree, C.; Monsereenusorn, C.; Simthamnimit, P.; Rujkijyanont, P. Clinical Usefulness of Furosemide to Prevent Volume Overload Among Children and Young Adults with Transfusion-Dependent Thalassemia: A Randomized, Open-Label, Crossover Study. J. Blood Med. 2020, 11, 503–513. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Fernández-Fernández, C.; Mouriño-Bayolo, D.; Castro-Quintela, E.; Domínguez-Montero, A. Sodium bicarbonate therapy in patients with metabolic acidosis. Sci. World J. 2014, 2014, 627673. [Google Scholar] [CrossRef] [PubMed]
- Bovée, D.M.; Roksnoer, L.C.; van Kooten, C.; Rotmans, J.I.; Vogt, L.; de Borst, M.H.; Zietse, R.; Danser, A.J.; Hoorn, E.J. Effect of sodium bicarbonate supplementation on the renin-angiotensin system in patients with chronic kidney disease and acidosis: A randomized clinical trial. J. Nephrol. 2020, 34, 1737–1745. [Google Scholar] [CrossRef]
- Melamed, M.L.; Raphael, K.L. Metabolic acidosis in CKD: A review of recent findings. Kidney Med. 2021, 3, 267–277. [Google Scholar] [CrossRef]
- Yang, T.-Y.; Lin, H.-M.; Wang, H.-Y.; Chuang, M.-H.; Hsieh, C.-C.; Tsai, K.-T.; Chen, J.-Y. Sodium bicarbonate treatment and clinical outcomes in chronic kidney disease with metabolic acidosis: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2024, 19, 959–969. [Google Scholar] [CrossRef]
- Hultin, S.; Hood, C.; Campbell, K.L.; Toussaint, N.D.; Johnson, D.W.; Badve, S.V. A systematic review and meta-analysis on effects of bicarbonate therapy on kidney outcomes. Kidney Int. Rep. 2021, 6, 695–705. [Google Scholar] [CrossRef]
- Cheng, F.; Li, Q.; Wang, J.; Wang, Z.; Zeng, F.; Zhang, Y. The effects of oral sodium bicarbonate on renal function and cardiovascular risk in patients with chronic kidney disease: A systematic review and meta-analysis. Ther. Clin. Risk Manag. 2021, 17, 1321–1331. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Bellasi, A.; Raphael, K.L.; Santoro, D.; Aucella, F.; Garofano, L.; Ceccarelli, M.; Di Lullo, L.; Capolongo, G.; Di Iorio, M. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: The UBI Study. J. Nephrol. 2019, 32, 989–1001. [Google Scholar] [CrossRef]
- BiCARB Study Group. Clinical and cost-effectiveness of oral sodium bicarbonate therapy for older patients with chronic kidney disease and low-grade acidosis (BiCARB): A pragmatic randomised, double-blind, placebo-controlled trial. BMC Med. 2020, 18, 91. [Google Scholar]
- Bushinsky, D.A. Tolerance to sodium in patients with CKD-induced metabolic acidosis: Does the accompanying anion matter? Am. J. Kidney Dis. 2019, 73, 858–865. [Google Scholar] [CrossRef]
- Beynon-Cobb, B.; Louca, P.; Hoorn, E.J.; Menni, C.; Padmanabhan, S. Effect of sodium bicarbonate on systolic blood pressure in CKD: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2023, 18, 435–445. [Google Scholar] [CrossRef]
- Tostivint, I.N.; Castiglione, V.; Alkouri, R.; Bertocchio, J.P.; Inaoui, R.; Daudon, M.; Dousseaux, M.-P.; Cavalier, E.; Pieroni, L.; Izzedine, H. How useful is an oral calcium load test for diagnosing recurrent calcium stone formers? Urolithiasis 2022, 50, 577–587. [Google Scholar] [CrossRef]
- Nicar, M.; Hsu, M.; Fetner, C. Urinary response to oral potassium citrate therapy for urolithiasis in a private practice setting. Clin. Ther. 1986, 8, 219–225. [Google Scholar]
- Tyson, C.C.; Luciano, A.; Modliszewski, J.L.; Corcoran, D.L.; Bain, J.R.; Muehlbauer, M.; Ilkayeva, O.; Pourafshar, S.; Allen, J.; Bowman, C. Effect of bicarbonate on net acid excretion, blood pressure, and metabolism in patients with and without CKD: The acid base compensation in CKD study. Am. J. Kidney Dis. 2021, 78, 38–47. [Google Scholar] [CrossRef]
- Repitz, A.; Aigner, C.; Sunder-Plassmann, G.; Gaggl, M. P0007 effect of sodium bicarbonate load on uric acid levels in patients with chronic kidney disease and metabolic acidosis: Preliminary results of the sobic-study. Nephrol. Dial. Transplant. 2020, 35, gfaa142.P0007. [Google Scholar] [CrossRef]
- Melamed, M.L.; Horwitz, E.J.; Dobre, M.A.; Abramowitz, M.K.; Zhang, L.; Lo, Y.; Mitch, W.E.; Hostetter, T.H. Effects of sodium bicarbonate in CKD stages 3 and 4: A randomized, placebo-controlled, multicenter clinical trial. Am. J. Kidney Dis. 2020, 75, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.S.; Buckley, N.A. Common pitfalls in the use of hypertonic sodium bicarbonate for cardiac toxic drug poisonings. Clin. Toxicol. 2024, 62, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Dosani, K.; Music Aplenc, L.; Pfeiffer, S. Therapeutic Plasma Exchange to Alleviate Ventricular Tachycardia After Diphenhydramine Ingestion. 2023. Available online: https://scholarlyexchange.childrensmercy.org/researchdays/GME_Research_Days_2023/ResearchDay4/1/ (accessed on 8 August 2025).
- Bohling, R.; Grafals, M.; Moreau, K.; You, Z.; Tommerdahl, K.L.; Bjornstad, P.; Stenson, E.K.; Andrews, E.; Ramirez-Renteria, L.; Kendrick, J. A pilot study of the safety and efficacy of alkali therapy on vascular function in kidney transplant recipients. Kidney Int. Rep. 2021, 6, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Mathur, V.; Tangri, N.; Stasiv, Y.; Parsell, D.; Li, E.; Klaerner, G.; Bushinsky, D.A. Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: A multicentre, randomised, blinded, placebo-controlled, 40-week extension. Lancet 2019, 394, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Mathur, V.; Tangri, N.; Stasiv, Y.; Parsell, D.; Li, E.; Klaerner, G.; Bushinsky, D. P0001 effects of veverimer on serum bicarbonate and physical function in patients with chronic kidney disease and metabolic acidosis are independent of albuminuria: Subgroup analyses from a randomized trial. Nephrol. Dial. Transplant. 2020, 35, gfaa142.P0001. [Google Scholar] [CrossRef]
- Mathur, V.S.; Li, E.; Wesson, D.E. Effects of veverimer on serum bicarbonate and physical function in diabetic patients with chronic kidney disease and metabolic acidosis: Subgroup analysis from a randomized, controlled trial. Nephrol. Dial. Transplant. 2022, 37, 1302–1309. [Google Scholar] [CrossRef]
- Parsell, D.; Shao, J.; Guttendorf, R.; Mathur, V.; Li, E.; Wu, Y.S.; Tsao, L.; Tabakman, S.; Stasiv, Y.; Lee, A. Assessment of the potential for veverimer drug-drug interactions. Drug Metab. Dispos. 2021, 49, 490–500. [Google Scholar] [CrossRef]
- Shao, J.; Parsell, D.; Guttendorf, R.; Wu, Y.S.; Tsao, L.; Tabakman, S.; Stasiv, Y.; Lee, A.; Biyani, K.; Klaerner, G. P0009 Evaluation of the potential for drug interactions with veverimer. Nephrol. Dial. Transplant. 2020, 35, gfaa142.P0009. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Dong, H.; Lu, M. Efficacy and safety of veverimer in the treatment of metabolic acidosis caused by chronic kidney disease: A meta-analysis. Front. Pharmacol. 2021, 12, 643128. [Google Scholar] [CrossRef]
- Kraut, J.A.; Raphael, K.L. Drug removal of gastric acid: A novel treatment of metabolic acidosis. Lancet 2019, 394, 363–364. [Google Scholar] [CrossRef]
- Mathur, V.S.; Bushinsky, D.A.; Inker, L.; Klaerner, G.; Li, E.; Parsell, D.; Perkovic, V.; Stasiv, Y.; Walker, M.; Wesson, D.E. Design and population of the VALOR-CKD study: A multicenter, randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of veverimer in slowing progression of chronic kidney disease in patients with metabolic acidosis. Nephrol. Dial. Transplant. 2023, 38, 1448–1458. [Google Scholar] [CrossRef]

| Parameter | Normal Value | Value in Metabolic Acidosis | Notes |
|---|---|---|---|
| pH | 7.35–7.45 | <7.35 | Diagnosis and monitoring of acid–base disorders |
| pCO2 | 35–40 mmHg | <35 mmHg | The decline in pCO2 is due to compensatory hyperventilation |
| HCO3− | 21–27 mmHg | <22 mmol/L | A low level indicates a loss of base or an excess of acid |
| BE | −2.3 do +2.3 mEg/L | <−2 mEg/L | |
| AG | 4 to 12 mmol/L | >12 mmol/L for HAGMA (N) AGMA | It is used to differentiate types of acidosis: elevated AG (e.g., lactic acidosis), normal AG (diarrhea) |
| Cl− | 96–108 mEq/L | ↑/N | An increase in chlorides indicates hyperchloremic acidosis (AG within normal range) |
| AGcorr | 8–12 mmol/L | AGcorr. is an AG corrected to albuminemia. Hypoalbuminemia masks a high anion gap |
| pH | pCO2 | HCO3− | |
|---|---|---|---|
| Uneven | |||
| ↓ | N | ↓ | |
| Partially balanced | |||
| ↓ | ↓ | ↓ | |
| Fully balanced | |||
| N | ↓ | ↓ | |
| Drug | Mechanism |
|---|---|
| Proximal RTA | |
| Carbonic anhydrase inhibitors (acetazolamide, dorzolamide, and methazolamide) | Causes isolated impairment of bicarbonate reabsorption in the proximal tubule, leading to proximal RTA. This effect is mainly due to the inhibition of the CA IV isoenzyme, without affecting the reabsorption of other dissolved substances. As a result, there is a loss of HCO3− in the urine, and the development of hyperchloremic metabolic acidosis with a normal anion gap. |
| Cisplatin | A direct toxic effect on the proximal convoluted tubule’s amino acid transporter results in toxicity, which causes cell death and renal Fanconi syndrome, impaired resorption of HCO3−. |
| Tenofovir | Builds up in the renal proximal tubule cells, where the mechanism is mitochondrial toxicity. Fanconi syndrome results from this, which impairs the reabsorption of bicarbonate, phosphate, glucose, and amino acids. |
| Ifosfamide | An alkylating agent is used to treat a variety of tumors, including testicular cancer, soft tissue sarcomas, and bone sarcomas. The exact process by which ifosfamide damages renal tubules is unknown. |
| Distal RTA | |
| Amphotericin B | Causes back-diffusion of released H+ ions and K+ squandering, which in turn causes RTA type 1 by increasing membrane permeability in the collecting duct |
| Foscarnet | It is believed that the mechanism involves mitochondrial malfunction that damages the cells of the renal tubules |
| Lithium | Lithium medication is thought to cause distal renal tubular acidosis by permitting excessive acid back-diffusion. |
| List | Parameter | Development Mechanism HAGMA |
|---|---|---|
| G | Glycols | Ethylene and propylene, propylene glycol used as a solvent, for example, in lorazepam or phenobarbital, are metabolized to D-lactate and L-lactate. |
| O | Pyroglutamic acid (5-Oxoproline) | The mechanism is based on the disruption of the gamma-glutamyl cycle, leading to the production of pyroglutamic acid. The cause is a deficiency of glutathione. The accumulation of 5-oxoprolin occurs especially in malnourished individuals with CKD, liver failure, and those chronically using paracetamol. |
| L | L-lactate | It occurs with excessive lactate production and impaired hepatic clearance. In conditions of hypoxia, tissue ischemia, and sepsis, glucose is converted to lactic acid by the enzyme lactate dehydrogenase. |
| D | D-lactate | It can develop in individuals with short bowel syndrome or after bowel resections and bacterial overgrowth in the colon. Among this group of people, undigested starch and glucose are fermented by bacteria in the colon into organic acids, including D-lactate, which is poorly metabolized by humans. |
| M | Methanol | It is converted in the liver by alcohol dehydrogenase to formic aldehyde, and then by aldehyde dehydrogenase to formic acid, which accumulates in the blood and inhibits mitochondrial cytochrome oxidase, leading to cellular hypoxia. |
| A | Aspirin | It undergoes hydrolysis to salicylic acid. Salicylates inhibit oxidative phosphorylation in the mitochondria, resulting in increased production of lactic acid and exacerbation of acidosis. They also stimulate lipolysis and ketogenesis, increasing the concentration of ketones. |
| R | Kidney failure | Acidosis develops due to the retention of organic and inorganic acids. In kidney failure, the accumulation of anions, such as sulfonic acid derivatives (indoxyl, p-cresol), is observed, leading to the accumulation of phosphoric and sulfuric acids. |
| K | Ketones | Ketone bodies (mainly acetone, acetoacetate, β-hydroxybutyrate) are produced in the liver as a result of ketogenesis—a process that mainly occurs in situations of glucose or insulin availability deficiency. These situations include fasting, untreated type 1 diabetes, or alcoholism. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korus, J.; Szymczak, M.; Gołębiowski, M.; Rydzek, J.; Majcherczyk, K.; Wilk, J.; Bułdyś, K.; Zmonarski, S.; Gołębiowski, T. Metabolic Acidosis in Patients with Chronic Kidney Disease: Diagnosis, Pathogenesis, and Treatment—A Narrative Review. Diagnostics 2025, 15, 2052. https://doi.org/10.3390/diagnostics15162052
Korus J, Szymczak M, Gołębiowski M, Rydzek J, Majcherczyk K, Wilk J, Bułdyś K, Zmonarski S, Gołębiowski T. Metabolic Acidosis in Patients with Chronic Kidney Disease: Diagnosis, Pathogenesis, and Treatment—A Narrative Review. Diagnostics. 2025; 15(16):2052. https://doi.org/10.3390/diagnostics15162052
Chicago/Turabian StyleKorus, Justyna, Maciej Szymczak, Maciej Gołębiowski, Julia Rydzek, Krzysztof Majcherczyk, Jakub Wilk, Kacper Bułdyś, Sławomir Zmonarski, and Tomasz Gołębiowski. 2025. "Metabolic Acidosis in Patients with Chronic Kidney Disease: Diagnosis, Pathogenesis, and Treatment—A Narrative Review" Diagnostics 15, no. 16: 2052. https://doi.org/10.3390/diagnostics15162052
APA StyleKorus, J., Szymczak, M., Gołębiowski, M., Rydzek, J., Majcherczyk, K., Wilk, J., Bułdyś, K., Zmonarski, S., & Gołębiowski, T. (2025). Metabolic Acidosis in Patients with Chronic Kidney Disease: Diagnosis, Pathogenesis, and Treatment—A Narrative Review. Diagnostics, 15(16), 2052. https://doi.org/10.3390/diagnostics15162052






