Development and Validation of Differential Diagnosis Models and Nomograms Based on Serum D-Dimer and Other Multimodal Information for Borderline and Benign Epithelial Ovarian Tumors: A Multicenter Study
Abstract
1. Introduction
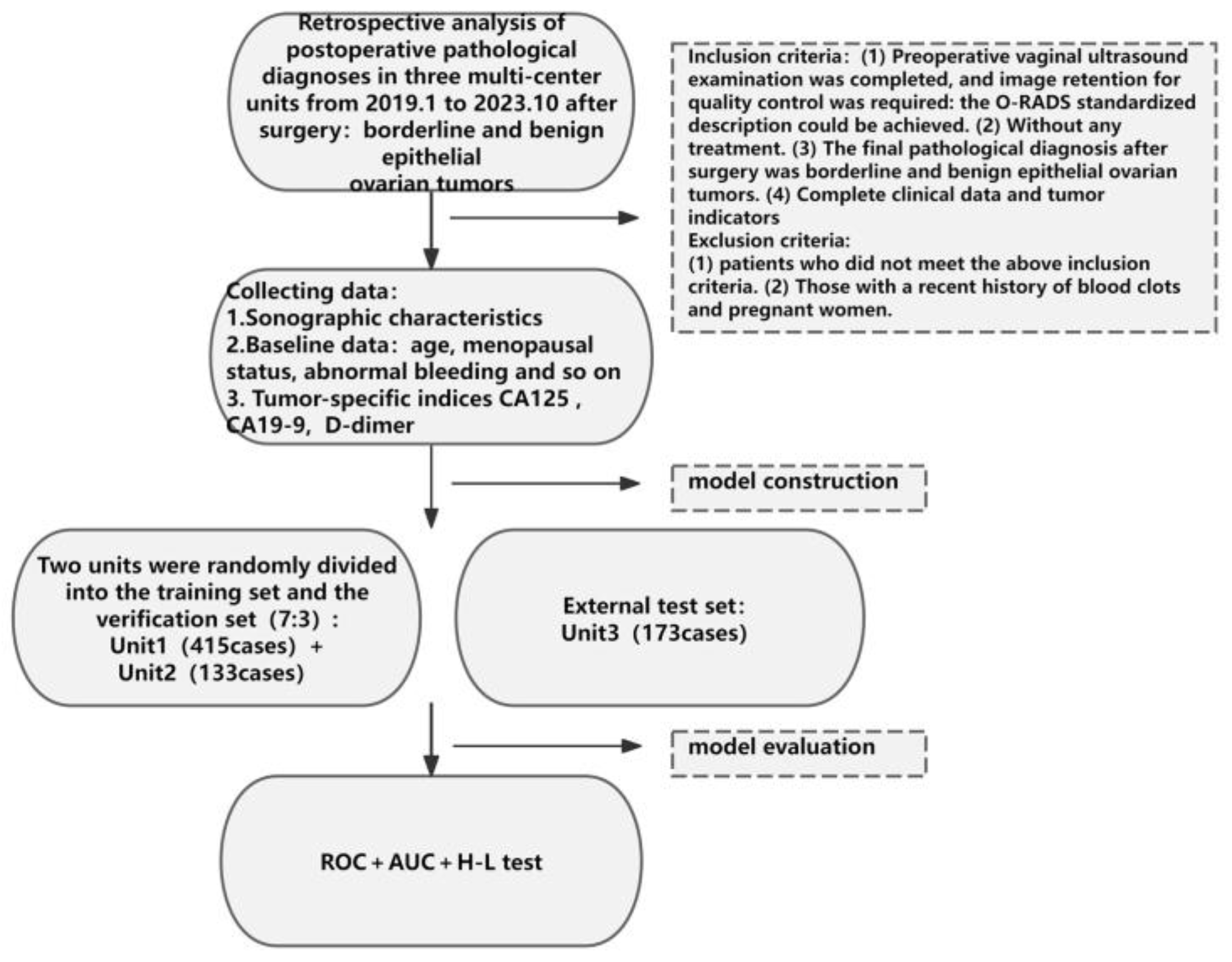
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
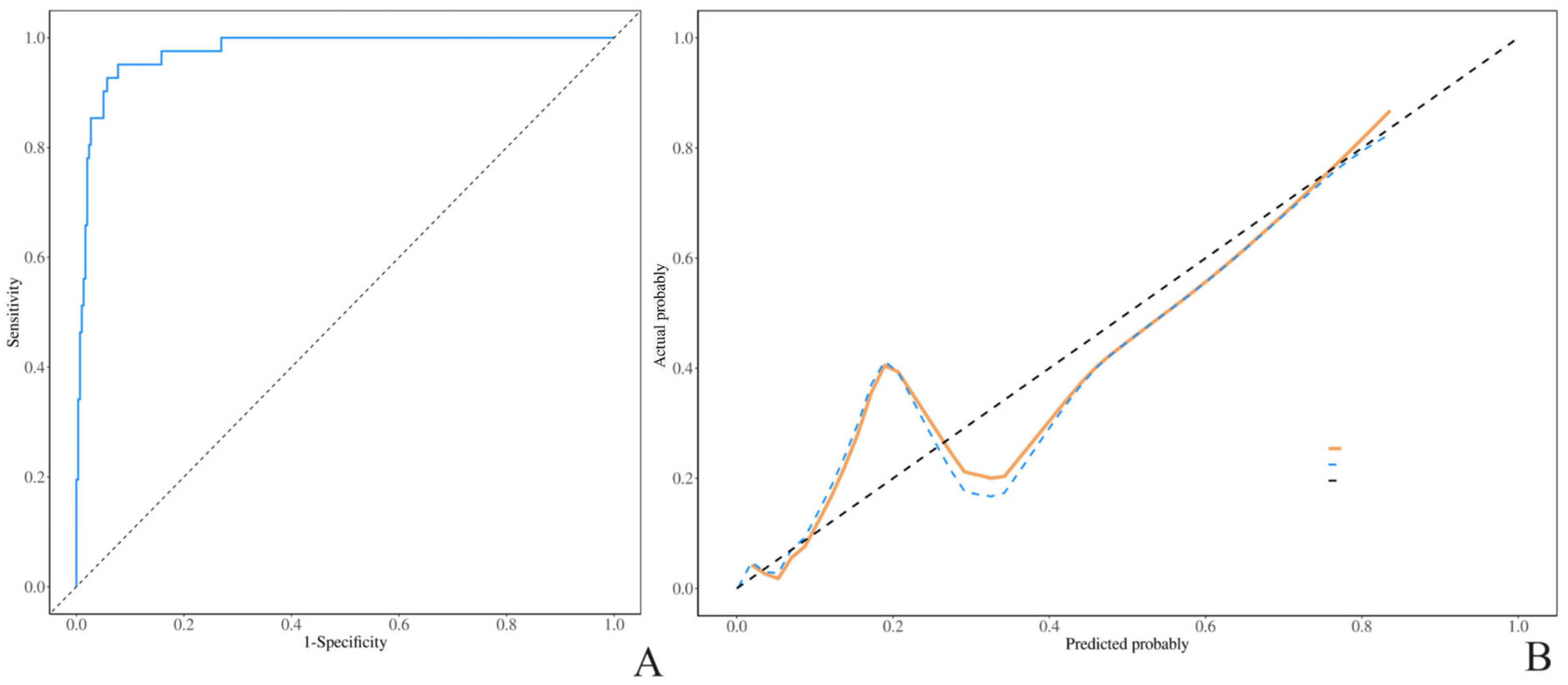
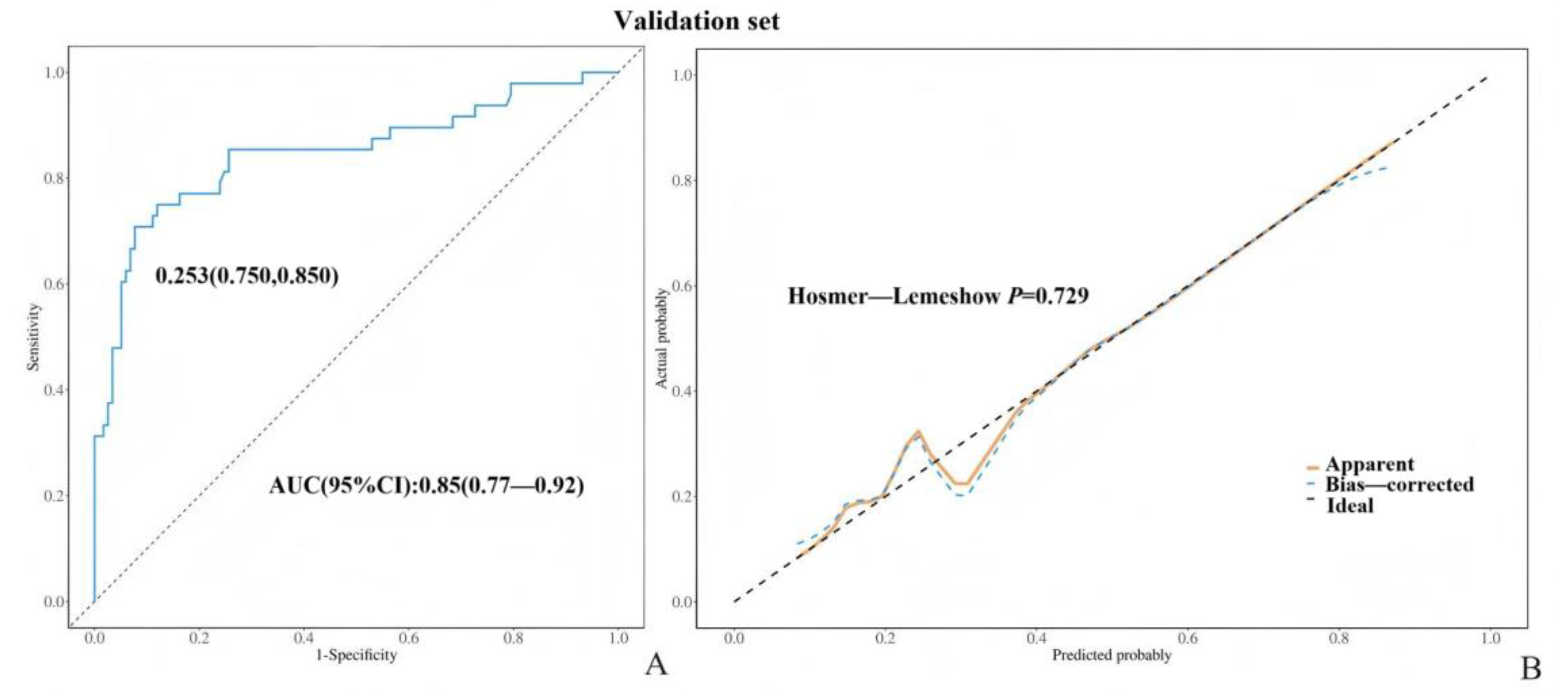
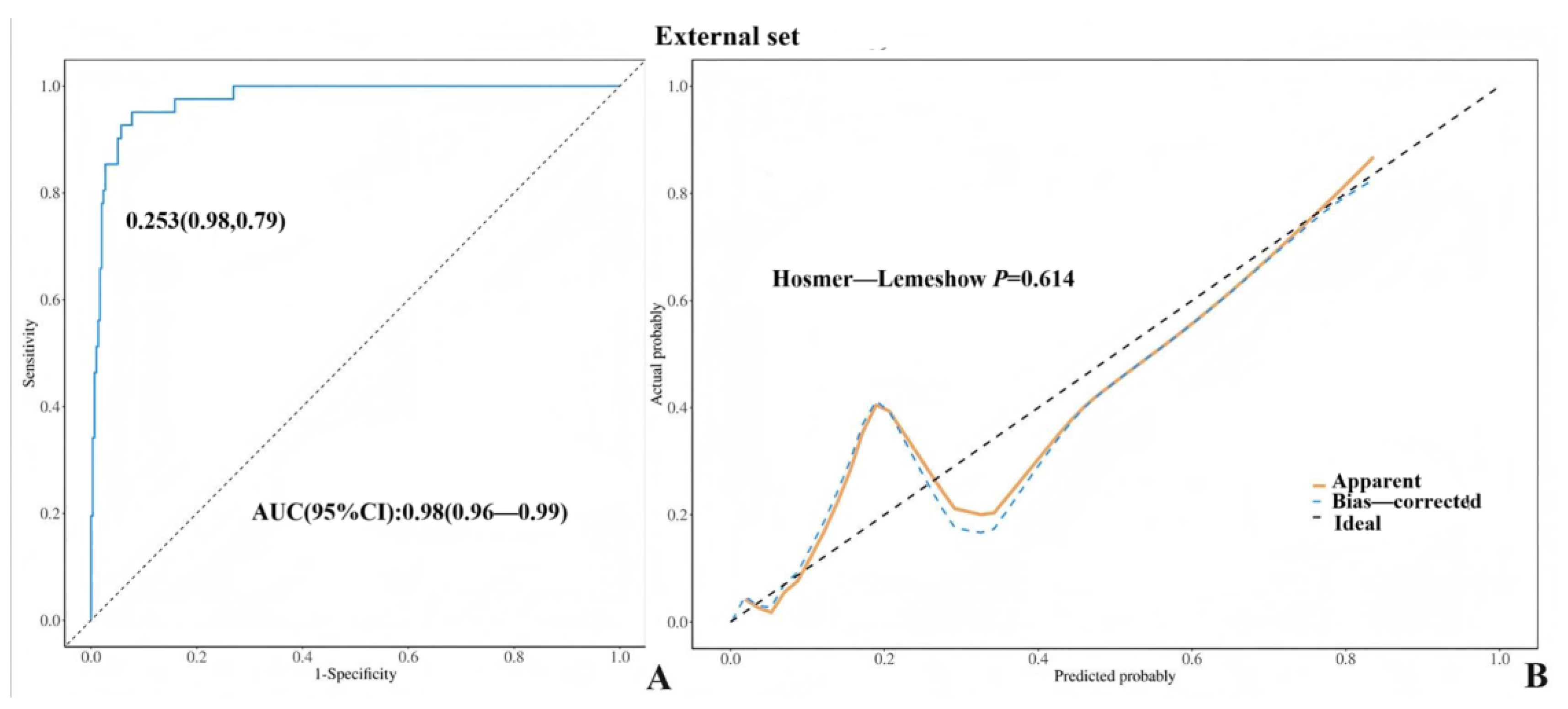
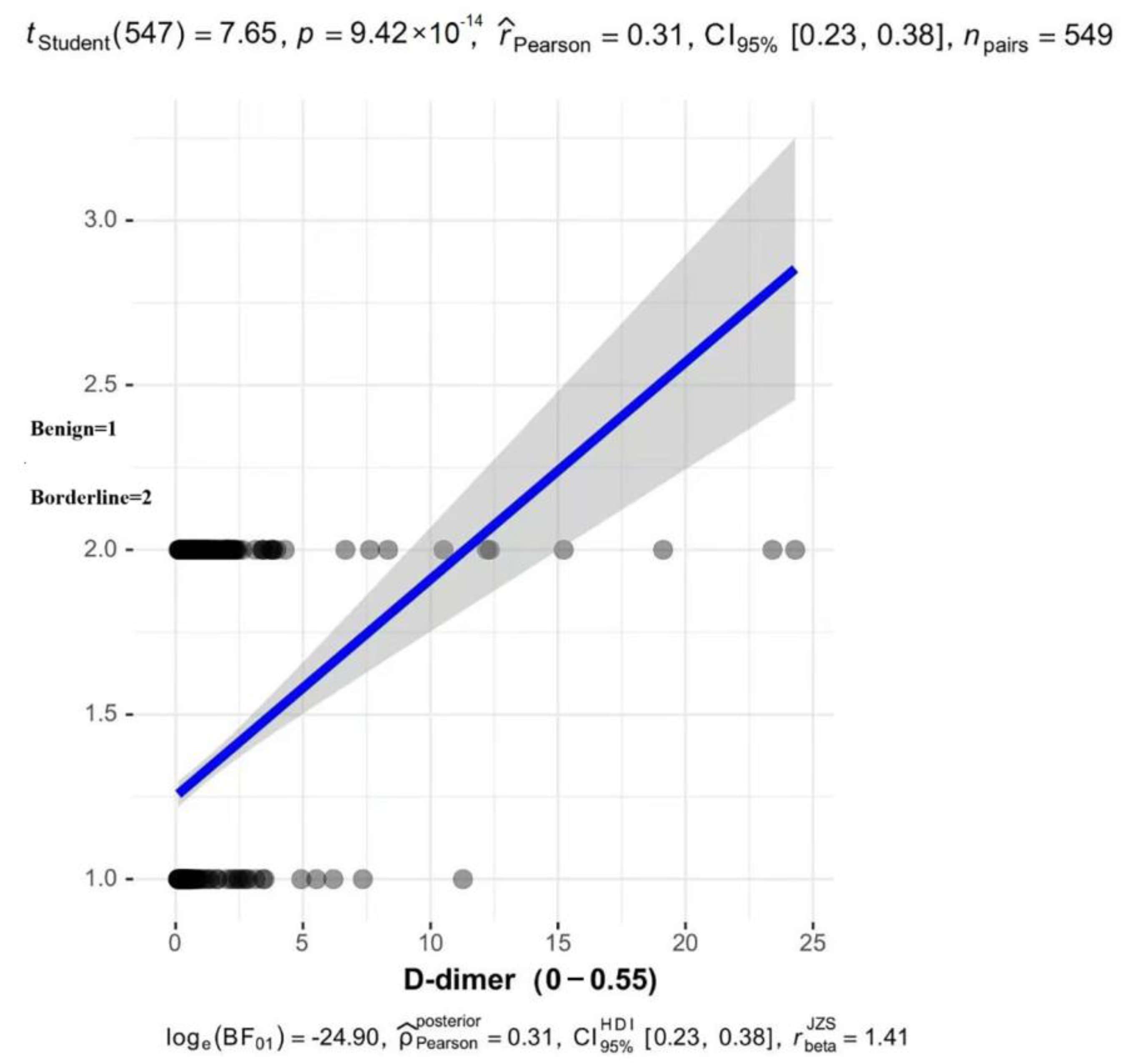
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flicek, K.T.; VanBuren, W.; Dudiak, K.; Lahkman, Y.; Chen, L.W.; Butler, K.; Menias, C.O. Borderline epithelial ovarian tumors: What the radiologist should know. Abdom. Radiol. 2021, 46, 2350–2366. [Google Scholar] [CrossRef] [PubMed]
- Margueritte, F.; Sallee, C.; Lacorre, A.; Gauroy, E.; Larouzee, E.; Chereau, E.; Rouge, T.D.L.M.; Koskas, M.; Gauthier, T. Tumeurs frontières de l’ovaire. Recommandations pour la pratique clinique du CNGOF—Épidémiologie et facteurs de risques de récidive, modalités de surveillance et intérêt d’une chirurgie de clôture [Borderline Ovarian Tumours: CNGOF Guidelines for Clinical Practice—Epidemiology and Risk Factors of Relapse, Follow-up and Interest of a Completion Surgery]. Gynecol. Obstet. Fertil. Senol. 2020, 48, 248–259. (In French) [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, Y.; Li, N.; Yuan, G.W.; Sun, Y.C.; Ma, S.K.; Zhang, X.; Wu, L.Y. The clinicopathological features and risk factors of recurrence in patients with mucinous borderline ovarian tumors. Zhonghua Zhong Liu Za Zhi 2017, 39, 589–594. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, S.; Friedrich, K.; Redline, R.; Avril, S. Ovarian borderline tumors in the 2014 WHO classification: Evolving concepts and diagnostic criteria. Virchows Arch. 2017, 470, 125–142. [Google Scholar] [CrossRef]
- Laban, M.; Chen, X.; Guo, B. Seromucinous and Mucinous Borderline Ovarian Tumors: We Need to Know More. Reprod. Sci. 2023, 30, 1684–1685. [Google Scholar] [CrossRef]
- Folkins, A.K.; Longacre, T.A. Low-grade Serous Neoplasia of the Female Genital Tract. Surg. Pathol. Clin. 2019, 12, 481–513. [Google Scholar] [CrossRef]
- Raad, J.; Rolland, L.; Grynberg, M.; Courbiere, B.; D’argent, E.M. Tumeurs frontières de l’ovaire. Recommandations pour la pratique clinique du CNGOF—Fertilité [Borderline Ovarian Tumours: CNGOF Guidelines for Clinical Practice—Fertility]. Gynecol. Obstet. Fertil. Senol. 2020, 48, 330–336. (In French) [Google Scholar] [CrossRef]
- Otify, M.; Laios, A.; Elshamy, T.; D’aNgelo, A.; Amso, N. A systematic review and meta-analysis of the use of ultrasound to diagnose borderline ovarian tumours. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 120–127. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, J.; Jia, X. The Diagnosis, Treatment, Prognosis and Molecular Pathology of Borderline Ovarian Tumors: Current Status and Perspectives. Cancer Manag. Res. 2020, 12, 3651–3659. [Google Scholar] [CrossRef]
- Gershenson, D.M. Management of borderline ovarian tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 49–59. [Google Scholar] [CrossRef]
- Luis Alcázar, J.; Ramón Pérez-Vidal, J.; Tameish, S.; Chacón, E.; Manzour, N.; Mínguez, J.Á. Ultrasound for assessing tumor spread in ovarian cancer. A systematic review of the literature and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 194–200. [Google Scholar] [CrossRef]
- Froyman, W.; Timmerman, D. Methods of Assessing Ovarian Masses: International Ovarian Tumor Analysis Approach. Obstet. Gynecol. Clin. N. Am. 2019, 46, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, B.; Zhou, S.; Wen, L.; Liu, J.; Fu, Y.; Xu, F.; Liu, M. A comparison of the diagnostic performance of the O-RADS, RMI4, IOTA LR2, and IOTA SR systems by senior and junior doctors. Ultrasonography 2022, 41, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Gotta, J.; Gruenewald, L.D.; Eichler, K.; Martin, S.S.; Mahmoudi, S.; Booz, C.; Biciusca, T.; Reschke, P.; Bernatz, S.; Dos Santos, D.P.; et al. Unveiling the diagnostic enigma of D-dimer testing in cancer patients: Current evidence and areas of application. Eur. J. Clin. Investig. 2023, 53, e14060. [Google Scholar] [CrossRef]
- Li, J.; Yan, S.; Zhang, X.; Xiang, M.; Zhang, C.; Gu, L.; Wei, X.; You, C.; Chen, S.; Zeng, D.; et al. Circulating D- dimers increase the risk of mortality and venous thromboembolism in patients with lung cancer: A systematic analysis combined with external validation. Front. Med. 2022, 9, 853941. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Huang, F.; Gao, Y.; Li, X.; Chi, J.; Xie, J.; Zhou, L.; Feng, Y.; Huang, H.; Deng, T.; et al. Artificial intelligence-based models enabling accurate diagnosis of ovarian cancer using laboratory tests in China: A multicentre, retrospective cohort study. Lancet Digit. Health 2024, 6, e176–e186. [Google Scholar] [CrossRef]
- Dai, H.; Zhou, H.; Sun, Y.; Xu, Z.; Wang, S.; Feng, T.; Zhang, P. D-Dimer as a potential clinical marker for predicting metastasis and progression in cancer. Biomed. Rep. 2018, 9, 453–457. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, Y.; Jia, Y.; Zhang, X.; Li, K. Prognostic and predictive value of plasma D-dimer levels in patients with small-cell lung cancer. Int. J. Clin. Oncol. 2018, 23, 1070–1075. [Google Scholar] [CrossRef]
- Andreotti, R.F.; Timmerman, D.; Strachowski, L.M.; Froyman, W.; Benacerraf, B.R.; Bennett, G.L.; Bourne, T.; Brown, D.L.; Coleman, B.G.; Frates, M.C.; et al. O-RADS US risk stratification and management system: A consensus guideline from the ACR ovarian-adnexal reporting and data system committee. Radiology 2020, 294, 168–185. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours: Female Genital Tumours; IARC Publications: Lyon, France, 2020; pp. 1–632. [Google Scholar]
- Babaoglu, S.; Atas, A.E.; Kerimoglu, U.; İyisoy, M.S.; Kilinc, F. Differentiation of Borderline Epithelial Ovarian Tumors from Benign and Malignant Epithelial Ovarian Tumors by MRI Scoring. Curr. Med. Imaging 2024, 20, e060623217706. [Google Scholar] [CrossRef]
- Borrelli, G.M.; de Mattos, L.A.; de P Andres, M.; Gonçalves, M.O.; Kho, R.M.; Abrão, M.S. Role of imaging tools for the diagnosis of BOTs: A systematic review and meta-analysis. J. Minim. Invasive Gynecol. 2017, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Global epidemiology of epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Miao, K.; Zhao, N.; Lv, Q.; He, X.; Xu, M.; Dong, X.; Li, D.; Shao, X. Prediction of benign and malignant ovarian tumors using Resnet34 on ultrasound images. J. Obstet. Gynaecol. Res. 2023, 49, 2910–2917. [Google Scholar] [CrossRef] [PubMed]
- Hiett, A.K.; Sonek, J.D.; Guy, M.; Reid, T.J. Performance of IOTA Simple Rules, Simple Rules risk assessment, ADNEX model and O-RADS in differentiating between benign and malignant adnexal lesions in North American women. Ultrasound Obstet. Gynecol. 2022, 59, 668–676. [Google Scholar] [CrossRef]
- Nyangoh-Timoh, K.; Bendifallah, S.; Dion, L.; Ouldamer, L.; Levêque, J. Recommandations pour la pratique clinique du CNGOF—Pertinence des marqueurs tumoraux [Borderline Ovarian Tumours: CNGOF Guidelines for Clinical Practice—Value of Tumor Markers]. Gynecol. Obstet. Fertil. Senol. 2020, 48, 277–286. (In French) [Google Scholar] [CrossRef]
- Trillsch, F.; Mahner, S.; Ruetzel, J.; Harter, P.; Ewald-Riegler, N.; Jaenicke, F.; du Bois, A. Clinical management of borderline ovarian tumors. Expert Rev. Anticancer Ther. 2010, 10, 1115–1124. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.N.; Huang, Z.; Chu, R.; Dong, T.T.; Zhang, Y.Z.; Yang, X.S.; Jiang, J.; Cui, B.X.; Song, K.; et al. Prognosis and fertility outcomes of patients with borderline ovarian tumors after fertility-sparing surgery. Zhonghua Yi Xue Za Zhi 2022, 102, 1999–2004. (In Chinese) [Google Scholar] [CrossRef]
- Deme, D.; Telekes, A. A keresztkötött fibrindegradációs termékek (D-dimer) prognosztikai jelentõsége az onkológiában [Prognostic importance of cross-linked fibrin degradation products (D-dimer) in oncology]. Magy. Onkol. 2017, 61, 319–326. [Google Scholar]
- Durand, M.K.; Bødker, J.S.; Christensen, A.; Dupont, D.M.; Hansen, M.; Jensen, J.K.; Kjelgaard, S.; Mathiasen, L.; Pedersen, K.E.; Skeldal, S.; et al. Plasminogen activator inhibitor-I and tumour growth, invasion, and metastasis. Thromb. Haemost. 2004, 91, 438–449. [Google Scholar] [CrossRef]
- Ge, L.; Liu, G.; Hu, K.; Huang, K.; Zhang, M.; Zhou, J.; Teng, F.; Cao, J.; Dai, C.; Jia, X. A New Risk Index Combining d-Dimer, Fibrinogen, HE4, and CA199 Differentiates Suspecting Endometrial Cancer from Patients with Abnormal Vaginal Bleeding or Discharge. Technol. Cancer Res. Treat. 2020, 19, 1533033819901117. [Google Scholar] [CrossRef]
| Variate | Total (n = 548) | Validation Set (n = 165) | Training Set (n = 383) | Z/χ2 | p |
|---|---|---|---|---|---|
| age | 37.00 (29.00, 49.00) | 35.00 (27.00, 49.00) | 37.00 (30.00, 49.00) | −1.67 | 0.096 |
| pathology | 0.41 | 0.521 | |||
| benign | 378 (68.98) | 117 (70.91) | 261 (68.15) | ||
| borderline | 170 (31.02) | 48 (29.09) | 122 (31.85) | ||
| menopause | 0.26 | 0.611 | |||
| no | 448 (81.75) | 137 (83.03) | 311 (81.20) | ||
| yes | 100 (18.25) | 28 (16.97) | 72 (18.80) | ||
| abnormal bleeding | 0.08 | 0.784 | |||
| no | 542 (98.91) | 164 (99.39) | 378 (98.69) | ||
| yes | 6 (1.09) | 1 (0.61) | 5 (1.31) | ||
| CA125 | 1.68 | 0.194 | |||
| normal | 326 (59.49) | 105 (63.64) | 221 (57.70) | ||
| abnormal | 222 (40.51) | 60 (36.36) | 162 (42.30) | ||
| CA19-9 | 0.01 | 0.903 | |||
| normal | 417 (76.09) | 125 (75.76) | 292 (76.24) | ||
| abnormal | 131 (23.91) | 40 (24.24) | 91 (23.76) | ||
| D-dimer | 0.83 | 0.361 | |||
| normal | 370 (67.52) | 116 (70.30) | 254 (66.32) | ||
| abnormal | 178 (32.48) | 49 (29.70) | 129 (33.68) | ||
| maximum length diameter of the mass (cm) | 0.08 | 0.777 | |||
| ≤10 | 403 (73.54) | 120 (72.73) | 283 (73.89) | ||
| >10 | 145 (26.46) | 45 (27.27) | 100 (26.11) | ||
| solid parts and forms | 0.63 | 0.731 | |||
| no | 321 (58.58) | 95 (57.58) | 226 (59.01) | ||
| regular | 185 (33.76) | 59 (35.75) | 126 (32.90) | ||
| irregular | 42 (7.66) | 11 (6.67) | 31 (8.09) | ||
| single or multilocular | 0.35 | 0.553 | |||
| single | 253 (46.17) | 73 (44.24) | 180 (47.00) | ||
| multiple | 295 (53.83) | 92 (55.76) | 203 (53.00) | ||
| internal blood supply of the mass | 0.67 | 0.414 | |||
| no | 475 (86.68) | 146 (88.48) | 329 (85.90) | ||
| yes | 73 (13.32) | 19 (11.52) | 54 (14.10) | ||
| ascites | 2.95 | 0.086 | |||
| no | 524 (95.62) | 154 (93.33) | 370 (96.61) | ||
| yes | 24 (4.38) | 11 (6.67) | 13 (3.39) |
| Variate | Total (n = 383) | Benign (n = 261) | Borderline (n = 122) | Z/χ2 | p |
|---|---|---|---|---|---|
| age | 37.00 (30.00, 49.00) | 36.00 (29.00, 47.00) | 44.00 (33.00, 53.75) | −3.58 | <0.001 |
| menopause | 5.13 | 0.024 | |||
| no | 311 (81.20) | 220 (84.29) | 91 (74.59) | ||
| yes | 72 (18.80) | 41 (15.71) | 31 (25.41) | ||
| abnormal bleeding | 3.40 | 0.065 | |||
| no | 378 (98.69) | 260 (99.62) | 118 (96.72) | ||
| yes | 5 (1.31) | 1 (0.38) | 4 (3.28) | ||
| CA125 | 36.99 | <0.001 | |||
| normal | 221 (57.70) | 178 (68.20) | 43 (35.25) | ||
| abnormal | 162 (42.30) | 83 (31.80) | 79 (64.75) | ||
| CA19-9 | 14.97 | <0.001 | |||
| normal | 292 (76.24) | 214 (81.99) | 78 (63.93) | ||
| abnormal | 91 (23.76) | 47 (18.01) | 44 (36.07) | ||
| D-dimer | 168.32 | <0.001 | |||
| normal | 254 (66.32) | 229 (87.74) | 25 (20.49) | ||
| abnormal | 129 (33.68) | 32 (12.26) | 97 (79.51) | ||
| maximum length diameter of the mass (cm) | 33.40 | <0.001 | |||
| ≤10 | 283 (73.89) | 216 (82.76) | 67 (54.92) | ||
| >10 | 100 (26.11) | 45 (17.24) | 55 (45.08) | ||
| solid parts and forms | 110.35 | <0.001 | |||
| no | 226 (59.01) | 195 (74.71) | 31 (25.41) | ||
| regular | 126 (32.90) | 65 (24.90) | 61 (50.00) | ||
| irregular | 31 (8.09) | 1 (0.37) | 30 (24.59) | ||
| single or multilocular | 0.09 | 0.769 | |||
| single | 180 (47.00) | 124 (47.51) | 56 (45.90) | ||
| multilocular | 203 (53.00) | 137 (52.49) | 66 (54.10) | ||
| internal blood supply of the mass | 76.75 | <0.001 | |||
| no | 329 (85.90) | 252 (96.55) | 77 (63.11) | ||
| yes | 54 (14.10) | 9 (3.45) | 45 (36.89) | ||
| ascites | 2.04 | 0.153 | |||
| no | 370 (96.61) | 255 (97.70) | 115 (94.26) | ||
| yes | 13 (3.39) | 6 (2.30) | 7 (5.74) |
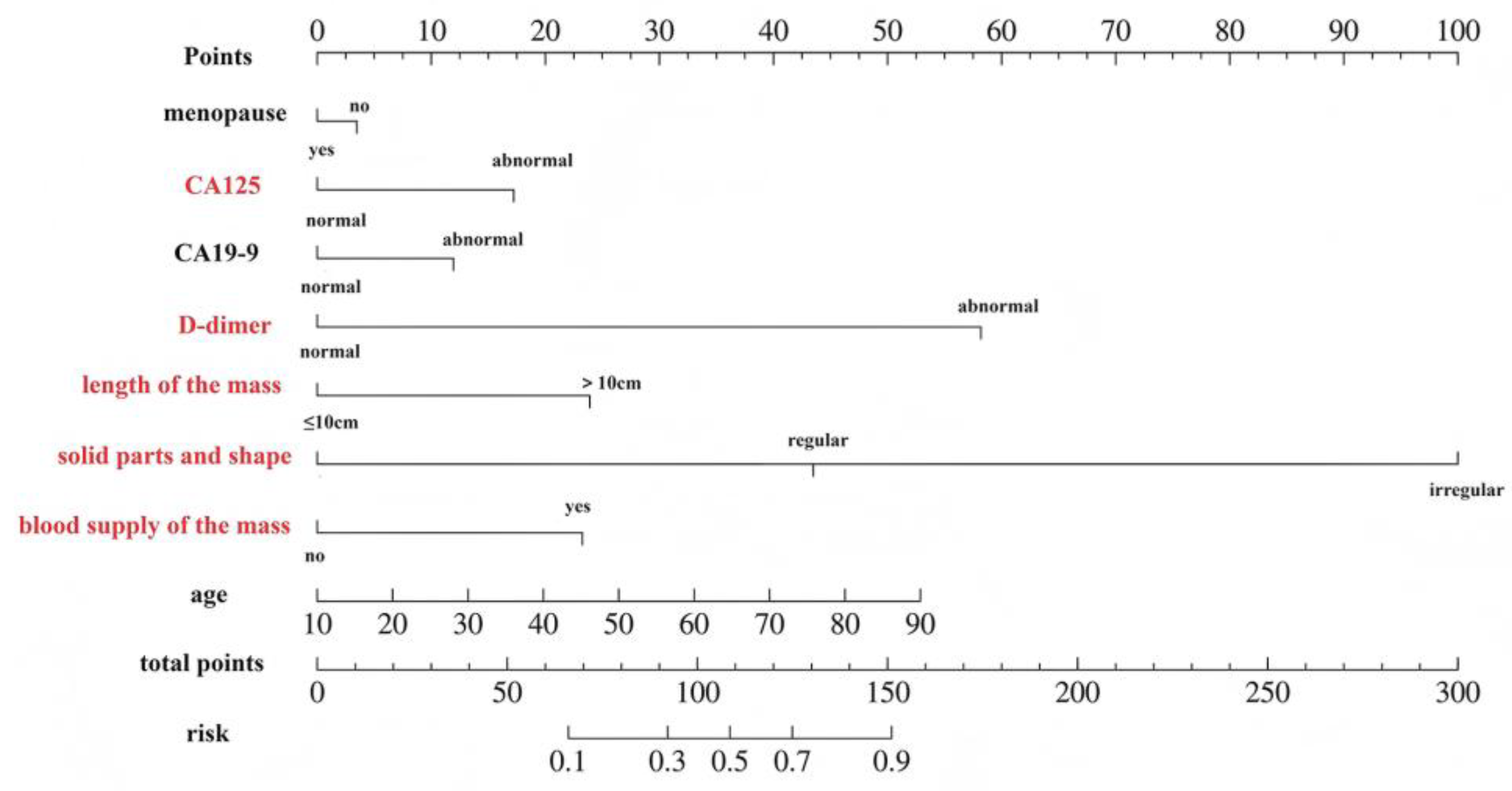
| Variate | Univariate Logistic Regression | Multivariate Logistic Regression | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| age | 1.03 (1.01~1.04) | <0.001 | 1.03 (0.99~1.08) | 0.090 |
| menopause | ||||
| no | 1.00 (Reference) | 1.00 (Reference) | ||
| yes | 1.83 (1.08~3.09) | 0.025 | 0.84 (0.21~3.37) | 0.801 |
| abnormal bleeding | ||||
| no | 1.00 (Reference) | |||
| yes | 8.81 (0.97~79.71) | 0.053 | ||
| CA125 | ||||
| normal | 1.00 (Reference) | 1.00 (Reference) | ||
| abnormal | 3.94 (2.50~6.20) | <0.001 | 2.44 (1.07~5.56) | 0.035 |
| CA19-9 | ||||
| normal | 1.00 (Reference) | 1.00 (Reference) | ||
| abnormal | 2.57 (1.58~4.18) | <0.001 | 1.85 (0.82~4.20) | 0.139 |
| D-dimer | ||||
| normal | 1.00 (Reference) | 1.00 (Reference) | ||
| abnormal | 27.77 (15.63~49.32) | <0.001 | 20.26 (9.19~44.68) | <.001 |
| maximum length diameter of the mass (cm) | ||||
| ≤10 | 1.00 (Reference) | 1.00 (Reference) | ||
| >10 | 3.94 (2.44~6.37) | <0.001 | 3.44 (1.49~7.92) | 0.004 |
| solid parts and forms | ||||
| no | 1.00 (Reference) | 1.00 (Reference) | ||
| regular | 5.90 (3.53~9.88) | <0.001 | 9.47 (4.10~21.89) | <.001 |
| irregular | 188.71 (24.83~1434.10) | <0.001 | 175.96 (16.00~1934.89) | <.001 |
| single room or multiple rooms | ||||
| single room | 1.00 (Reference) | |||
| multiple room | 1.07 (0.69~1.64) | 0.769 | ||
| internal blood supply of the mass | ||||
| no | 1.00 (Reference) | 1.00 (Reference) | ||
| yes | 16.36 (7.65~34.98) | <0.001 | 3.33 (1.05~10.54) | 0.041 |
| ascites | ||||
| no | 1.00 (Reference) | |||
| yes | 2.59 (0.85~7.87) | 0.094 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Duan, Y.; He, F.; Duan, C.; Wang, J.; Zhang, C.; Zhou, Y. Development and Validation of Differential Diagnosis Models and Nomograms Based on Serum D-Dimer and Other Multimodal Information for Borderline and Benign Epithelial Ovarian Tumors: A Multicenter Study. Diagnostics 2025, 15, 2035. https://doi.org/10.3390/diagnostics15162035
Zhang Y, Duan Y, He F, Duan C, Wang J, Zhang C, Zhou Y. Development and Validation of Differential Diagnosis Models and Nomograms Based on Serum D-Dimer and Other Multimodal Information for Borderline and Benign Epithelial Ovarian Tumors: A Multicenter Study. Diagnostics. 2025; 15(16):2035. https://doi.org/10.3390/diagnostics15162035
Chicago/Turabian StyleZhang, Yiqing, Yayang Duan, Fang He, Chunhua Duan, Junli Wang, Chaoxue Zhang, and Yi Zhou. 2025. "Development and Validation of Differential Diagnosis Models and Nomograms Based on Serum D-Dimer and Other Multimodal Information for Borderline and Benign Epithelial Ovarian Tumors: A Multicenter Study" Diagnostics 15, no. 16: 2035. https://doi.org/10.3390/diagnostics15162035
APA StyleZhang, Y., Duan, Y., He, F., Duan, C., Wang, J., Zhang, C., & Zhou, Y. (2025). Development and Validation of Differential Diagnosis Models and Nomograms Based on Serum D-Dimer and Other Multimodal Information for Borderline and Benign Epithelial Ovarian Tumors: A Multicenter Study. Diagnostics, 15(16), 2035. https://doi.org/10.3390/diagnostics15162035






