Characteristics and Outcomes of Diffuse Interstitial Pneumonias Discovered in the ICU: A Retrospective Monocentric Study—The “IPIC” (Interstitial Pneumonia in Intensive Care) Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aMDA-5 | anti-MDA-5 antibodies |
| ATS | American Thoracic Society |
| ARDS | Acute respiratory distress syndrome |
| ARF | Acute respiratory failure |
| BAL | Bronchoalveolar lavage |
| CYC | Cyclophosphamide |
| CTD | Connective tissue disease |
| COP | Cryptogenic organising pneumonia |
| ECMO | Extracorporeal membrane oxygenation |
| ERS | European Thoracic Society |
| HFNO | High-flow nasal oxygen |
| HRCT | High-resolution computed tomography |
| ICU | Intensive care unit |
| ILD | Interstitial lung disease |
| IMV | Invasive mechanical ventilation |
| MMF | Mycophenolate mofetil |
| MDD | Multidisciplinary discussion |
| NSIP | Non-specific interstitial pneumonia |
| PDS | Protected distal sampling |
| PaO2:FiO2 | Ratio of the oxygen pressure to the fraction of inspired oxygen |
| PEP | Positive end-expiratory pressure |
| SOFA | Sepsis-related organ failure assessment |
| VATS | Video-assisted thoracoscopic surgery |
References
- American Thoracic Society; European Respiratory Society American Thoracic Society/European Respiratory Society Inter-national Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This Joint Statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) Was Adopted by the ATS Board of Directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 2002, 165, 277–304. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Coultas, D.B.; Zumwalt, R.E.; Black, W.C.; Sobonya, R.E. The Epidemiology of Interstitial Lung Diseases. Am. J. Respir. Crit. Care Med. 1994, 150, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Huapaya, J.A.; Wilfong, E.M.; Harden, C.T.; Brower, R.G.; Danoff, S.K. Risk Factors for Mortality and Mortality Rates in Interstitial Lung Disease Patients in the Intensive Care Unit. Eur. Respir. Rev. 2018, 27, 180061. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.; Suzuki, A.; Maher, T.M. Interstitial Lung Diseases. Lancet 2022, 400, 769–786. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute Respiratory Distress Syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Gibelin, A.; Parrot, A.; Maitre, B.; Brun-Buisson, C.; Mekontso Dessap, A.; Fartoukh, M.; de Prost, N. Acute Respiratory Distress Syndrome Mimickers Lacking Common Risk Factors of the Berlin Definition. Intensive Care Med. 2016, 42, 164–172. [Google Scholar] [CrossRef]
- Papazian, L.; Calfee, C.S.; Chiumello, D.; Luyt, C.-E.; Meyer, N.J.; Sekiguchi, H.; Matthay, M.A.; Meduri, G.U. Diagnostic Workup for ARDS Patients. Intensive Care Med. 2016, 42, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Mikolasch, T.A.; Garthwaite, H.S.; Porter, J.C. Update in Diagnosis and Management of Interstitial Lung Disease. Clin. Med. 2017, 17, 146–153. [Google Scholar] [CrossRef]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA Score to Assess the Incidence of Organ Dysfunction/Failure in Intensive Care Units: Results of a Multicenter, Prospective Study. Working Group on “Sepsis-Related Problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An Official European Respiratory Society/American Thoracic Society Research Statement: Interstitial Pneumonia with Autoimmune Features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Gertler, R. Respiratory Mechanics. Anesthesiol. Clin. 2021, 39, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Duron, L.; Cohen-Aubart, F.; Diot, E.; Borie, R.; Abad, S.; Richez, C.; Banse, C.; Vittecoq, O.; Saadoun, D.; Haroche, J.; et al. Shrinking Lung Syndrome Associated with Systemic Lupus Erythematosus: A Multicenter Collaborative Study of 15 New Cases and a Review of the 155 Cases in the Literature Focusing on Treatment Response and Long-Term Outcomes. Autoimmun. Rev. 2016, 15, 994–1000. [Google Scholar] [CrossRef]
- Gannon, W.D.; Lederer, D.J.; Biscotti, M.; Javaid, A.; Patel, N.M.; Brodie, D.; Bacchetta, M.; Baldwin, M.R. Outcomes and Mortality Prediction Model of Critically Ill Adults With Acute Respiratory Failure and Interstitial Lung Disease. Chest 2018, 153, 1387–1395. [Google Scholar] [CrossRef]
- Chang, S.-L.; Tsai, H.-C.; Lin, F.-C.; Chao, H.-S.; Chou, C.-W.; Chang, S.-C. Clinical Usefulness of Bronchoalveolar Lavage in Patients with Interstitial Lung Diseases: A Pilot Study. J. Thorac. Dis. 2020, 12, 3125–3134. [Google Scholar] [CrossRef]
- Schnabel, R.M.; van der Velden, K.; Osinski, A.; Rohde, G.; Roekaerts, P.M.H.J.; Bergmans, D.C.J.J. Clinical Course and Complications Following Diagnostic Bronchoalveolar Lavage in Critically Ill Mechanically Ventilated Patients. BMC Pulm. Med. 2015, 15, 107. [Google Scholar] [CrossRef]
- Gerard, L.; Bidoul, T.; Castanares-Zapatero, D.; Wittebole, X.; Lacroix, V.; Froidure, A.; Hoton, D.; Laterre, P.-F. Open Lung Biopsy in Nonresolving Acute Respiratory Distress Syndrome Commonly Identifies Corticosteroid-Sensitive Pathologies, Associated With Better Outcome. Crit. Care Med. 2018, 46, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M. Interstitial Lung Disease: A Review. JAMA 2024, 331, 1655–1665. [Google Scholar] [CrossRef]
- Petitpierre, N.; Beigelman, C.; Letovanec, I.; Lazor, R. Cryptogenic organizing pneumonia. Rev. Mal. Respir. 2016, 33, 703–717. [Google Scholar] [CrossRef]
- Matsushita, T.; Mizumaki, K.; Kano, M.; Yagi, N.; Tennichi, M.; Takeuchi, A.; Okamoto, Y.; Hamaguchi, Y.; Murakami, A.; Hasegawa, M.; et al. Antimelanoma Differentiation-Associated Protein 5 Antibody Level Is a Novel Tool for Monitoring Disease Activity in Rapidly Progressive Interstitial Lung Disease with Dermatomyositis. Br. J. Dermatol. 2017, 176, 395–402. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Ye, S. Tofacitinib in Amyopathic Dermatomyositis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 381, 291–293. [Google Scholar] [CrossRef]
- Naccache, J.-M.; Jouneau, S.; Didier, M.; Borie, R.; Cachanado, M.; Bourdin, A.; Reynaud-Gaubert, M.; Bonniaud, P.; Israël-Biet, D.; Prévot, G.; et al. Cyclophosphamide Added to Glucocorticoids in Acute Exacerbation of Idiopathic Pulmonary Fibrosis (EXAFIP): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2022, 10, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Mankikian, J.; Caille, A.; Reynaud-Gaubert, M.; Agier, M.-S.; Bermudez, J.; Bonniaud, P.; Borie, R.; Brillet, P.-Y.; Cadranel, J.; Court-Fortune, I.; et al. Rituximab and Mycophenolate Mofetil Combination in Patients with Interstitial Lung Disease (EVER-ILD): A Double-Blind, Randomised, Placebo-Controlled Trial. Eur. Respir. J. 2023, 61, 2202071. [Google Scholar] [CrossRef] [PubMed]
- Urushiyama, H.; Jo, T.; Hasegawa, W.; Yokoyama, A.; Ando, T.; Sakamoto, Y.; Kumazawa, R.; Uda, K.; Michihata, N.; Awano, N.; et al. Effect of Nintedanib on Acute Exacerbations of Fibrosing Interstitial Lung Diseases: A National Database Study in Japan. ERJ Open Res. 2022, 8, 00209–02022. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S. Outcome of Patients with Idiopathic Pulmonary Fibrosis (IPF) Ventilated in Intensive Care Unit. Respir. Med. 2008, 102, 1355–1359. [Google Scholar] [CrossRef]
- Vuillard, C.; Pineton de Chambrun, M.; de Prost, N.; Guérin, C.; Schmidt, M.; Dargent, A.; Quenot, J.-P.; Préau, S.; Ledoux, G.; Neuville, M.; et al. Clinical Features and Outcome of Patients with Acute Respiratory Failure Revealing Anti-Synthetase or Anti-MDA-5 Dermato-Pulmonary Syndrome: A French Multicenter Retrospective Study. Ann. Intensive Care 2018, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, E.; Fijolek, J. Update on Cryptogenic Organizing Pneumonia. Front. Med. 2023, 10, 1146782. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, Z.; Gupta, R. The Management of Interstitial Lung Disease in the ICU: A Comprehensive Review. J. Clin. Med. 2024, 13, 6657. [Google Scholar] [CrossRef] [PubMed]
| Demographics | Median (IQR) or Mean (%) |
|---|---|
| Age | 70 (62–72) |
| Women | 5 (25) |
| Current smoker | 4 (20) |
| Allergic rhinitis | 1 (5) |
| Cardiovascular disease | 6 (30) |
| Obese | 2 (10) |
| Known cured or evolutive cancer disease | 5 (25) |
| Connective tissue disease | 3 (15) |
| Systemic sclerosis | 1 (5) |
| Rheumatoid arthritis | 1 (5) |
| Systemic lupus erythematosus | 1 (5) |
| Severity | |
| SOFA Score | 4 (3–7) |
| Criteria for ARDS | 19 (95) |
| Mild | 5 (25) |
| Moderate | 11 (55) |
| Severe | 3 (15) |
| Ventilatory support | |
| HFNO | 3 (15) |
| Invasive ventilation | 16 (80) |
| Veno-venous ECMO | 1 (5) |
| Ventilatory parameters | |
| PaO2:FiO2 | 174 (148–198) |
| PEP (cmH2O) | 8 (8–10) |
| Driving pressure (cmH20) | 19 (17–20) |
| Static compliance (mL/cmH20) | 21 (18–24) |
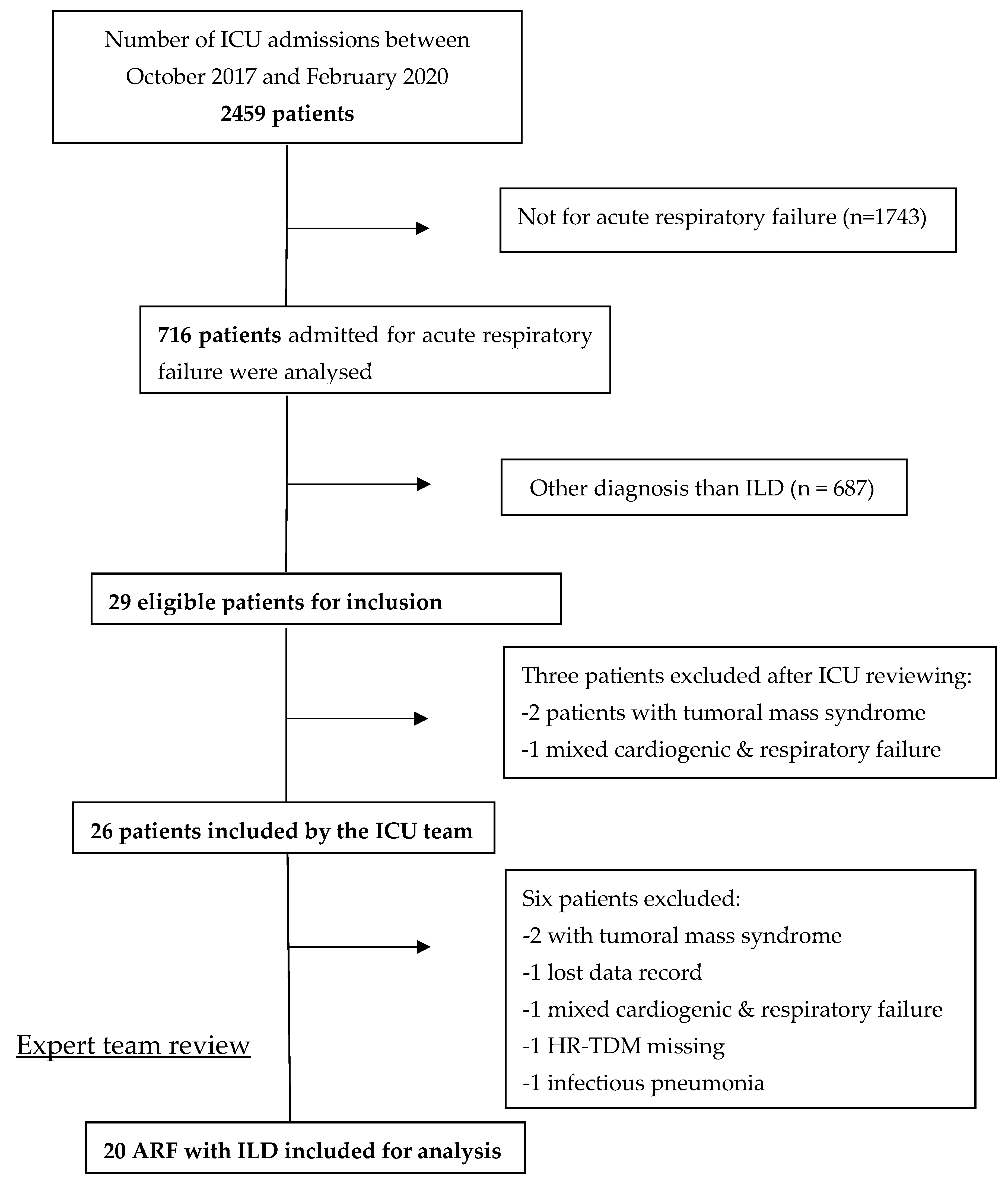
| Total ICU Admission | 2459 |
| ILD initially diagnosed by the ICU team | 26 |
| Confirmed ILD among ICU team’s selection (%) | 20/26 (77) |
| Confirmed ILD among all ICU admissions (%) | 20/2459 (~1) |
| Confirmed ILD among ARF patients (%) | 20/687 (~3) |
| ILD | Related Main HRCT Pattern | n = 20 (%) |
|---|---|---|
| Idiopathic ILD | 7 (35) | |
| Idiopathic non-specific interstitial pneumonia | NSIP | 3 (15) |
| Cryptogenic organising pneumonia | OP | 1 (5) |
| Idiopathic pulmonary fibrosis | UIP | 3 (15) |
| Auto-immune-related ILD | 7 (35) | |
| CTD associated ILD | 6 (30) | |
| Systemic lupus erythematosus | Shrinking lung syndrome | 1 (5) |
| Rheumatoid arthritis | UIP | 1 (5) |
| Systemic sclerosis | NSIP | 2 (10) |
| Anti-synthetase syndrome (anti-Jo1 syndrome) | NSIP | 1 (5) |
| aMDA-5-associated amyopathic dermatomyositis | NSIP | 1 (5) |
| Interstitial pneumonia with auto-immune features | NSIP | 1 (5) |
| Exposure-related | 3 (15) | |
| Hypersensitivity pneumonitis | HP | 1 (5) |
| Drug-induced (docetaxel) | NSIP | 1 (5) |
| Radiation-induced lung injury | NSIP | 1 (5) |
| Others | 3 (15) | |
| Carcinomatous lymphangitis | Crazy paving | 3 (15) |
| ILD Patient with IMV | Patient with Auto-Immune-Related ILD | ILD Patient with Steroid Administration | |
|---|---|---|---|
| Number of patients | 16 | 7 | 6 |
| Deceased in the ICU | 13 | 4 | 0 |
| Mortality rate (%) | 81 | 57 | 0 |
| Confirmed ILD | Overall ICU patients | p-value | |
| Number of patients | 20 | 2459 | |
| Deceased in the ICU | 13 | 632 | |
| Mortality rate (%) | 65 | 26 | <0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, D.; Bermudez, J.; Leone, M.; Di Bisceglie, M.; Montini, F. Characteristics and Outcomes of Diffuse Interstitial Pneumonias Discovered in the ICU: A Retrospective Monocentric Study—The “IPIC” (Interstitial Pneumonia in Intensive Care) Study. Diagnostics 2025, 15, 1995. https://doi.org/10.3390/diagnostics15161995
Eckert D, Bermudez J, Leone M, Di Bisceglie M, Montini F. Characteristics and Outcomes of Diffuse Interstitial Pneumonias Discovered in the ICU: A Retrospective Monocentric Study—The “IPIC” (Interstitial Pneumonia in Intensive Care) Study. Diagnostics. 2025; 15(16):1995. https://doi.org/10.3390/diagnostics15161995
Chicago/Turabian StyleEckert, Damien, Julien Bermudez, Marc Leone, Mathieu Di Bisceglie, and Florent Montini. 2025. "Characteristics and Outcomes of Diffuse Interstitial Pneumonias Discovered in the ICU: A Retrospective Monocentric Study—The “IPIC” (Interstitial Pneumonia in Intensive Care) Study" Diagnostics 15, no. 16: 1995. https://doi.org/10.3390/diagnostics15161995
APA StyleEckert, D., Bermudez, J., Leone, M., Di Bisceglie, M., & Montini, F. (2025). Characteristics and Outcomes of Diffuse Interstitial Pneumonias Discovered in the ICU: A Retrospective Monocentric Study—The “IPIC” (Interstitial Pneumonia in Intensive Care) Study. Diagnostics, 15(16), 1995. https://doi.org/10.3390/diagnostics15161995






