A Rare t(3;15;17) in a Patient with Acute Promyelocytic Leukemia: Case Report and Review of the Literature
Abstract
1. Introduction
2. Case Presentation
3. Materials and Methods
3.1. Conventional Cytogenetics
3.2. Molecular Cytogenetics
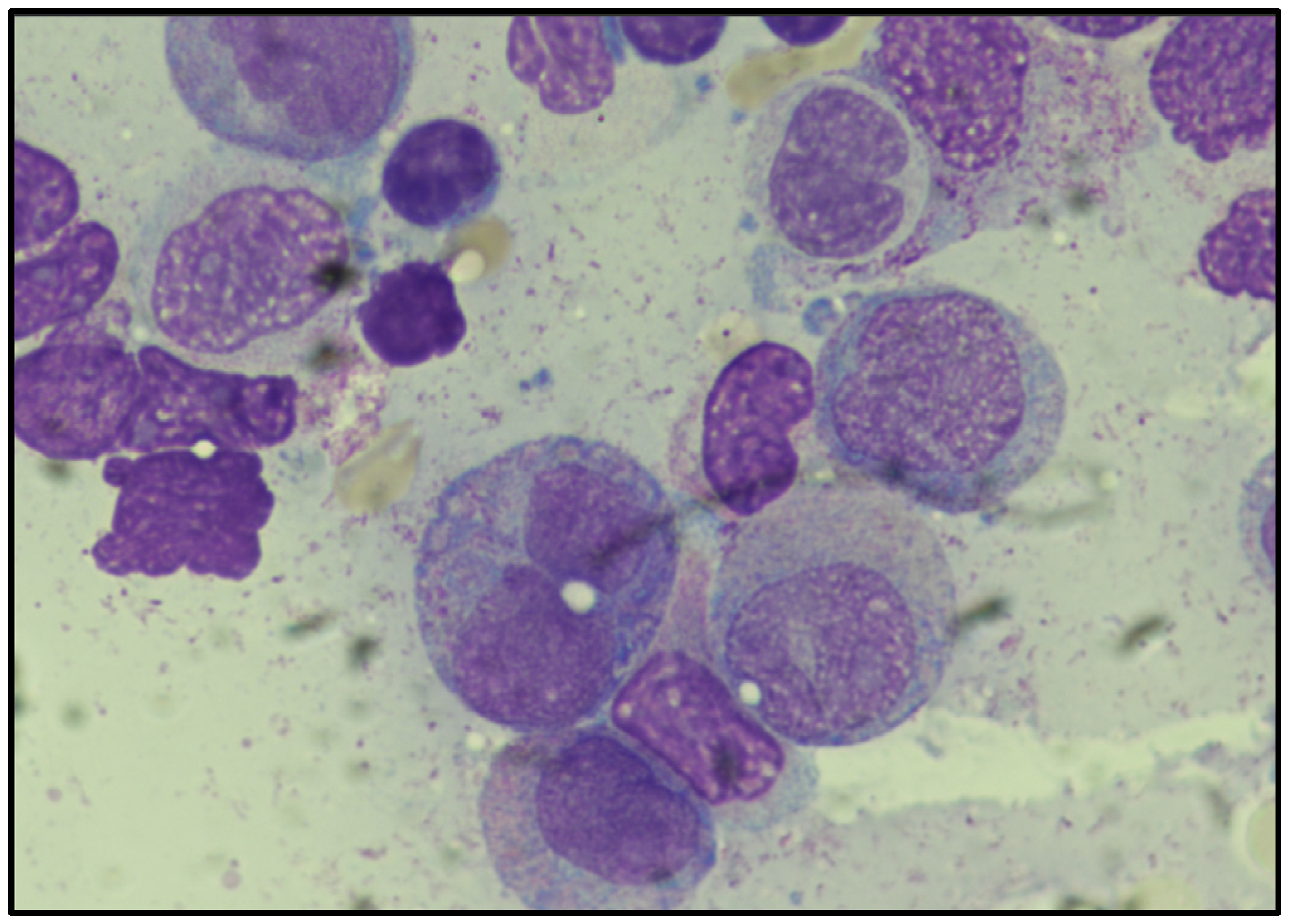
4. Results
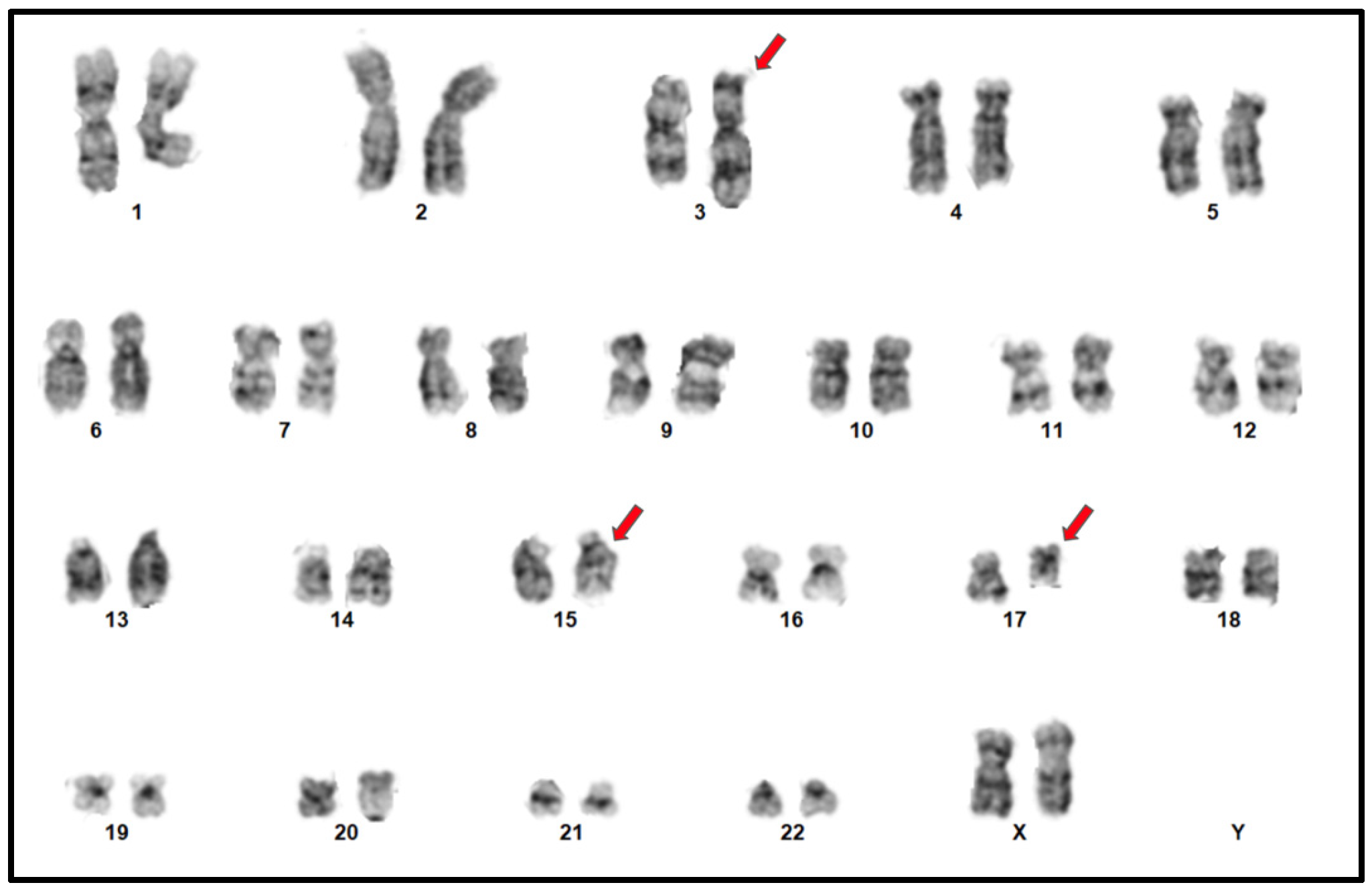
4.1. Conventional Cytogenetics
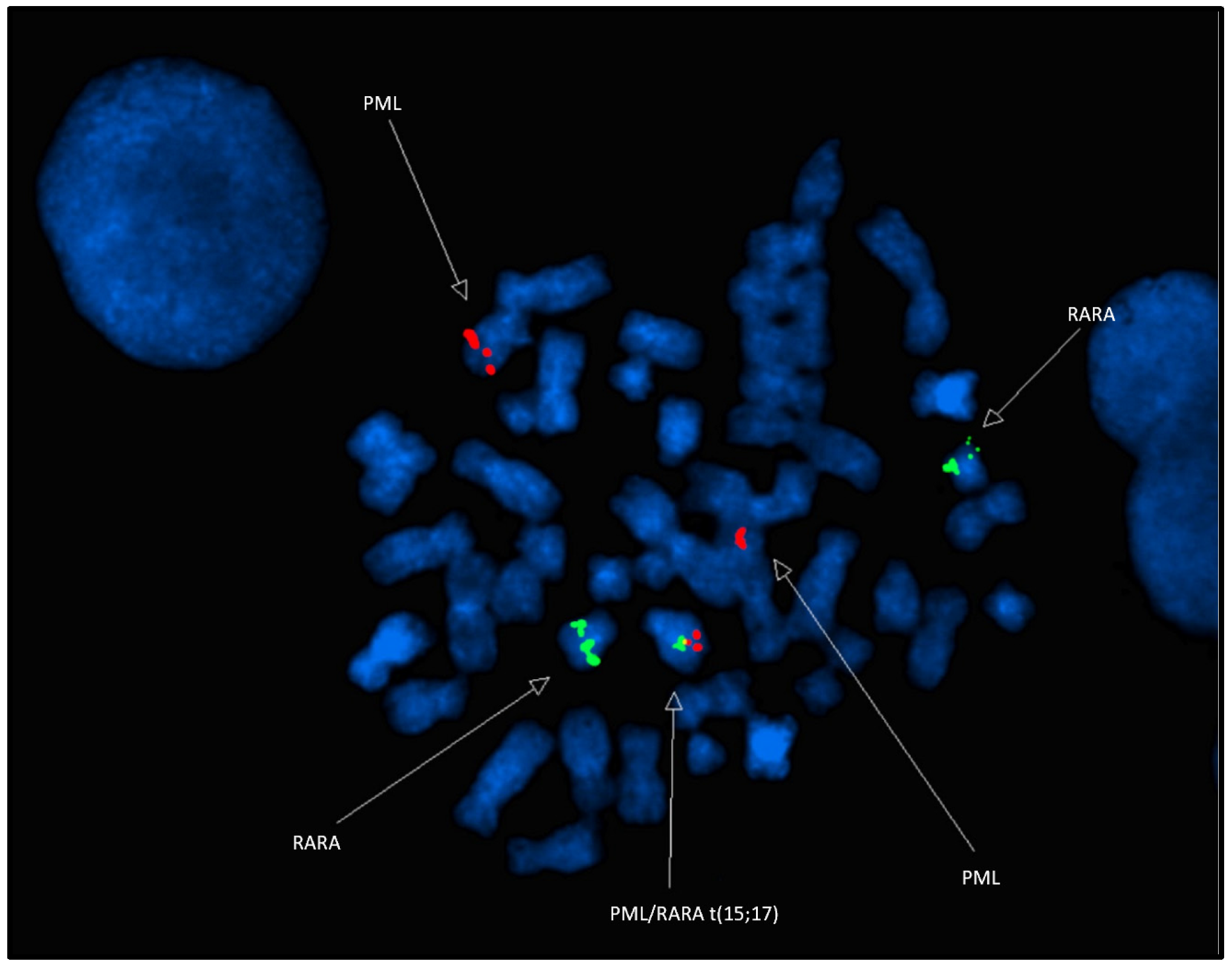
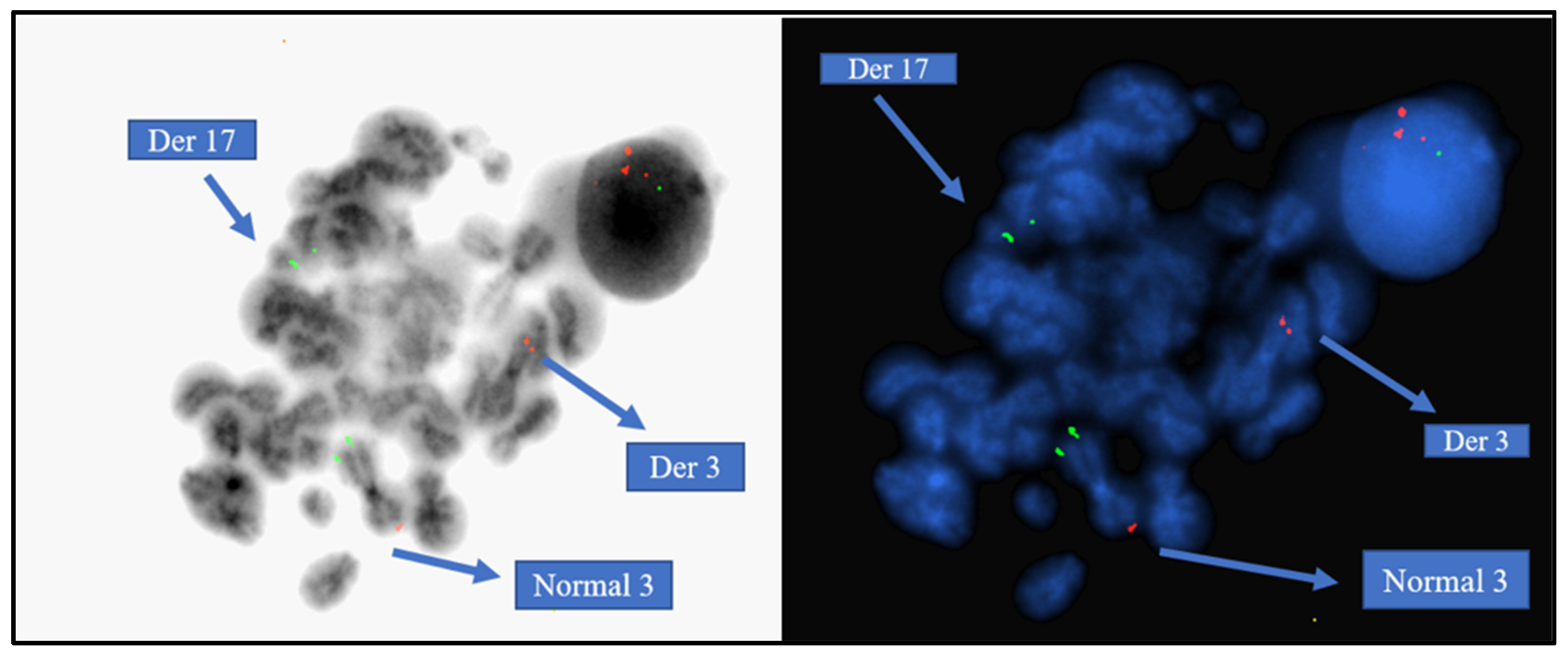
4.2. Molecular Cytogenetics
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryan, M.M. Acute Promyelocytic Leukemia: A Summary. J. Adv. Pract. Oncol. 2018, 9, 178. [Google Scholar] [PubMed]
- Wang, Y.; Ma, J.; Liu, X.; Liu, R.; Xu, L.; Wang, L.; Cen, J.; Chu, X. A complex translocation (3;17;15) in acute promyelocytic leukemia confirmed by fluorescence in situ hybridization. Oncol. Lett. 2016, 12, 4717–4719. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; Chale, R.S.; Abad, A.C.; Schally, A.V. Acute promyelocytic leukemia (APL): A review of the literature. Oncotarget 2020, 11, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Cingam, S.R.; Koshy, N.V. Acute Promyelocytic Leukemia; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Guarnera, L.; Ottone, T.; Fabiani, E.; Divona, M.; Savi, A.; Travaglini, S.; Falconi, G.; Panetta, P.; Rapanotti, M.C.; Voso, M.T. Atypical rearrangements in APL-like acute myeloid leukemias: Molecular characterization and prognosis. Front. Oncol. 2022, 12, 871590. [Google Scholar] [CrossRef]
- Matasar, M.J.; Ritchie, E.K.; Consedine, N.; Magai, C.; Neugut, A.I. Incidence rates of acute promyelocytic leukemia among Hispanics, Blacks, Asians, and non-Hispanic Whites in the United States. Eur. J. Cancer Prev. 2006, 15, 367–370. [Google Scholar] [CrossRef]
- Iyer, S.G.; Elias, L.; Stanchina, M.; Watts, J. The treatment of acute promyelocytic leukemia in 2023: Paradigm, advances, and future directions. Front. Oncol. 2023, 18, 12. [Google Scholar] [CrossRef]
- Coombs, C.C.; Tavakkoli, M.; Tallman, M.S. Acute promyelocytic leukemia: Where did we start, where are we now, and the future. Blood Cancer J. 2015, 5, e304. [Google Scholar] [CrossRef]
- PML gene: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/gene/pml/#references (accessed on 19 January 2025).
- RARA gene: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/gene/rara/ (accessed on 19 January 2025).
- ISCN 2024-An International System for Human Cytogenomic Nomenclature (2024). Cytogenet. Genome Res. 2024, 164 (Suppl. 1), 1–224. [CrossRef]
- Appachu, S.; Chintaparthi, O.; Sirsath, N.T.; Kuntejowdahalli, L.C.; Prasanna, K. A complex variant t(3;15)(q26;q13) representing cryptic/masked acute promyelocytic leukaemia with a novel breakpoint of chromosome 15—A case report. Ecancermedicalscience 2013, 7, 340. [Google Scholar]
- Sharma, A.; Yang, J.; Singh, V. Epidemiology and early mortality patterns of acute promyelocytic leukemia in the United States. Ann. Hematol. 2023, 102, 1053–1062. [Google Scholar] [CrossRef]
- Kim, D.H. A three-way complex translocation of (15;15;17)(q24;q14;q21) involving two breakpoints on chromosome 15 in acute promyelocytic leukemia: A case report. Oncol Lett. 2023, 26, 309. [Google Scholar] [CrossRef]
- Gu, S.; Zi, J.; Ma, J.; Ge, Z. Cryptic t(15;17) acute promyelocytic leukemia with a karyotype of add(11)(p15) and t(13,20)—A case report with a literature review. Bosn. J. Basic Med. Sci. 2021, 21, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Bilgesu, A.; Güngör, Ö.; Karaca, E.; Durmaz, B.; Bozer, D.S.; Töbü, M.; Akın, H. A complex t(15;22;17)(q22;q11.2;q21) variant of APL. Cancer Genet. 2024, 286–287, 48–51. [Google Scholar]
- Tirado, C.A.; Golembiewski-Ruiz, V.; Horvatinovich, J.; Moore, J.O.; Buckley, P.J.; Stenzel, T.T.; Goodman, B.K. Cytogenetic and molecular analysis of an unusual case of acute promyelocytic leukemia with a t(15;17;17)(q22;q23;q21). Cancer Genet. Cytogenet. 2003, 145, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, R.; Mendelow, B.; Pinto, M.R.; Morcom, G.; Bezwoda, W. Complex translocations involving chromosomes 15 and 17 in acute promyelocytic leukemia. Br. J. Haematol. 1980, 46, 311–314. [Google Scholar] [CrossRef]
- McKinney, C.D.; Golden, W.L.; Gemma, N.W.; Swerdlow, S.H.; Williams, M.E. RARA and PML gene rearrangements in acute promyelocytic leukemia with complex translocations and atypical features. Genes Chromosomes Cancer 1994, 9, 49–56. [Google Scholar] [CrossRef]
- Freeman, C.E.; Mercer, D.D.; Ye, Y.; Van Brunt, J., 3rd; Li, M.M. Cytogenetic and molecular characterization of complex three-way translocations in acute promyelocytic leukemia. Beijing Da Xue Xue Bao Yi Xue Ban 2009, 41, 477–479. [Google Scholar]
- Heim, S.; Kristoffersson, U.; Mandahl, N.; Malm, C.; Mitelman, F. Variant translocation t(3;15)(q21;q22) in a patient with acute promyelocytic leukemia. Leukemia 1988, 2, 65–67. [Google Scholar]
- Chen, Y.; Li, S.; Zhou, C.; Li, C.; Ru, K.; Rao, Q.; Xing, H.; Tian, Z.; Tang, K.; Mi, Y.; et al. TBLR1 fuses to retinoid acid receptor α in a variant t(3;17)(q26;q21) translocation of acute promyelocytic leukemia. Blood 2014, 124, 936–945. [Google Scholar] [CrossRef]
- Cheng, C.K.; Wang, A.Z.; Wong, T.H.Y.; Wan, T.S.K.; Cheung, J.S.; Raghupathy, R.; Chan, N.P.H.; Ng, M.H.L. FNDC3B is another novel partner fused to RARA in the t(3;17)(q26;q21) variant of acute promyelocytic leukemia. Blood 2017, 129, 2705–2709, First Edition. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Yu, W.; Jin, J. Current views on the genetic landscape and management of variant acute promyelocytic leukemia. Biomark. Res. 2021, 9, 33. [Google Scholar] [CrossRef]
- Wen, L.; Xu, Y.; Yao, L.; Wang, N.; Wang, Q.; Liu, T.; Pan, J.; Cen, J.; Zhou, H.; Miao, M.; et al. Clinical and molecular features of acute promyelocytic leukemia with variant retinoid acid receptor fusions. Haematologica 2019, 104, e195–e199. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Liu, S.; Chen, Y.; Xing, H.; Tang, K.; Tian, Z.; Xu, Y.; Rao, Q.; Wang, M.; et al. A novel fusion protein TBLR1-RARα acts as an oncogene to induce murine promyelocytic leukemia: Identification and treatment strategies. Cell Death Dis. 2021, 12, 607. [Google Scholar] [CrossRef]
- Chong, M.L.; Cheng, H.; Xu, P.; You, H.; Wang, M.; Wang, L.; Ho, H.H. TFG-RARA: A novel fusion gene in acute promyelocytic leukemia that is responsive to all-trans retinoic acid. Leuk. Res. 2018, 74, 51–54. [Google Scholar] [CrossRef]
| Patient % | Reference Range % | |
|---|---|---|
| Blasts + Promyelocytes | 89.0 | 0–3 |
| Myelocytes | 2.0 | 8–17 |
| Metamyelocytes | 1.0 | 10–25 |
| Band/PML Neutrophils | 4.0 | 16–27 |
| Eosinophils | 1.0 | 1–6 |
| Lymphocytes | 0.0 | 10–23 |
| Plasma Cells | 0.0 | 0–2 |
| Monocytes | 0.0 | 0–3 |
| Nucleated RBC | 2.0 | 14–30 |
| Reference | Karyotype | Presence of Classic t(15;17) | Age | Treatment |
|---|---|---|---|---|
| Bernstein et al., 1980 [18] | 46, XY, t(3;15;17)(p21;q25?6?;q21?2?); 4p+ | No | N/A | N/A |
| McKinney et al., 1994 [19] | 46, XX, t(3;17;15)(p21;q21;q22) [12] | No | 51 y/o | Ara-C, daunorubicin, and trans-retinoic acid |
| McKinney et al., 1994 [19] | 46, XX, t(3;17;15)(p21;q21;q22) [12] | No | 63 y/o | Ara-C, daunorubicin, and mitoxantrone |
| Freeman et al., 2009 [20] | 46, XY, t(3;15;17)(p12;q21;q22) [12] | Yes | 78 y/o | Idarubicin, Ara-C, and ATRA |
| Wang et al., 2016 [2] | 46, XY, der(3) t(3;17;15)(q25;q21;q24), der(15)t(3;17;15),der(17),der(17)(q11q12) | Yes | 33 y/o | ATO, daunorubicin, dexamethasone, intrathecal methotrexate, and cytosine arabinoside * patient was intolerant to ATRA |
| Present Case | 46,XX,der(3)(3pter→q29::17q21→qter),der(15)(15pter→q22-24::17q21→17qter),det(17)(p13→q21::3q29→qter) | Yes | 48 y/o | All-trans retinoic acid (ATRA) and arsenic trioxide (ATO) |
| Reference | Karyotype | Presence of Classic t(15;17) | Age | Treatment | Outcome |
|---|---|---|---|---|---|
| Heim et al., 1988 [21] | t(3;15)(q21;q22) | No | - | - | - |
| Appachu et al., 2013 [22] | 46, XY, t(3;15)(q26;q13) | No | 15 y/o | ATRA, ATO | Complete remission |
| Chen et al., 2014 [23] | 46, XY, t(3;17)(q26;q21),t(7;17)(q11.2;q21) [15]/46,XY | No | 63 y/o | ATRA, cytarabine, mitoxantrone, ATO, MTZ | Passed away 1 month after relapse |
| Cheng et al., 2017 [24] | 45, XY, t(3;17)(q26;q21) [8]/46,XY | No | 36 y/o | ATRA, 7 + 3 induction therapy with cytarabine | Complete remission |
| Present Case | 46,XX,der(3)(3pter→q29::17q21→qter),der(15)(15pter→q22-24::17q21→17qter),det(17)(p13→q21::3q29→qter) | Yes | 48 y/o | All-trans retinoic acid (ATRA) and Arsenic trioxide (ATO) | Complete remission |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Chen, C.E.; Ahmed, T.; Rocha, J.; Materum, P.; Cherukuri, S.; Gallagher, L.; Fernicola, P.; Ponce, R.; Lee, H.; et al. A Rare t(3;15;17) in a Patient with Acute Promyelocytic Leukemia: Case Report and Review of the Literature. Diagnostics 2025, 15, 1901. https://doi.org/10.3390/diagnostics15151901
Shi L, Chen CE, Ahmed T, Rocha J, Materum P, Cherukuri S, Gallagher L, Fernicola P, Ponce R, Lee H, et al. A Rare t(3;15;17) in a Patient with Acute Promyelocytic Leukemia: Case Report and Review of the Literature. Diagnostics. 2025; 15(15):1901. https://doi.org/10.3390/diagnostics15151901
Chicago/Turabian StyleShi, Linda, Chu En Chen, Tahmeena Ahmed, Jacob Rocha, Pons Materum, Sashank Cherukuri, Leah Gallagher, Paula Fernicola, Roxana Ponce, Htien Lee, and et al. 2025. "A Rare t(3;15;17) in a Patient with Acute Promyelocytic Leukemia: Case Report and Review of the Literature" Diagnostics 15, no. 15: 1901. https://doi.org/10.3390/diagnostics15151901
APA StyleShi, L., Chen, C. E., Ahmed, T., Rocha, J., Materum, P., Cherukuri, S., Gallagher, L., Fernicola, P., Ponce, R., Lee, H., Giordano, C., Evans, G., Tian, C., & Tirado, C. A. (2025). A Rare t(3;15;17) in a Patient with Acute Promyelocytic Leukemia: Case Report and Review of the Literature. Diagnostics, 15(15), 1901. https://doi.org/10.3390/diagnostics15151901






