Synergistic Imaging: Combined Lung Ultrasound and Low-Dose Chest CT for Quantitative Assessment of COVID-19 Severity—A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
- •
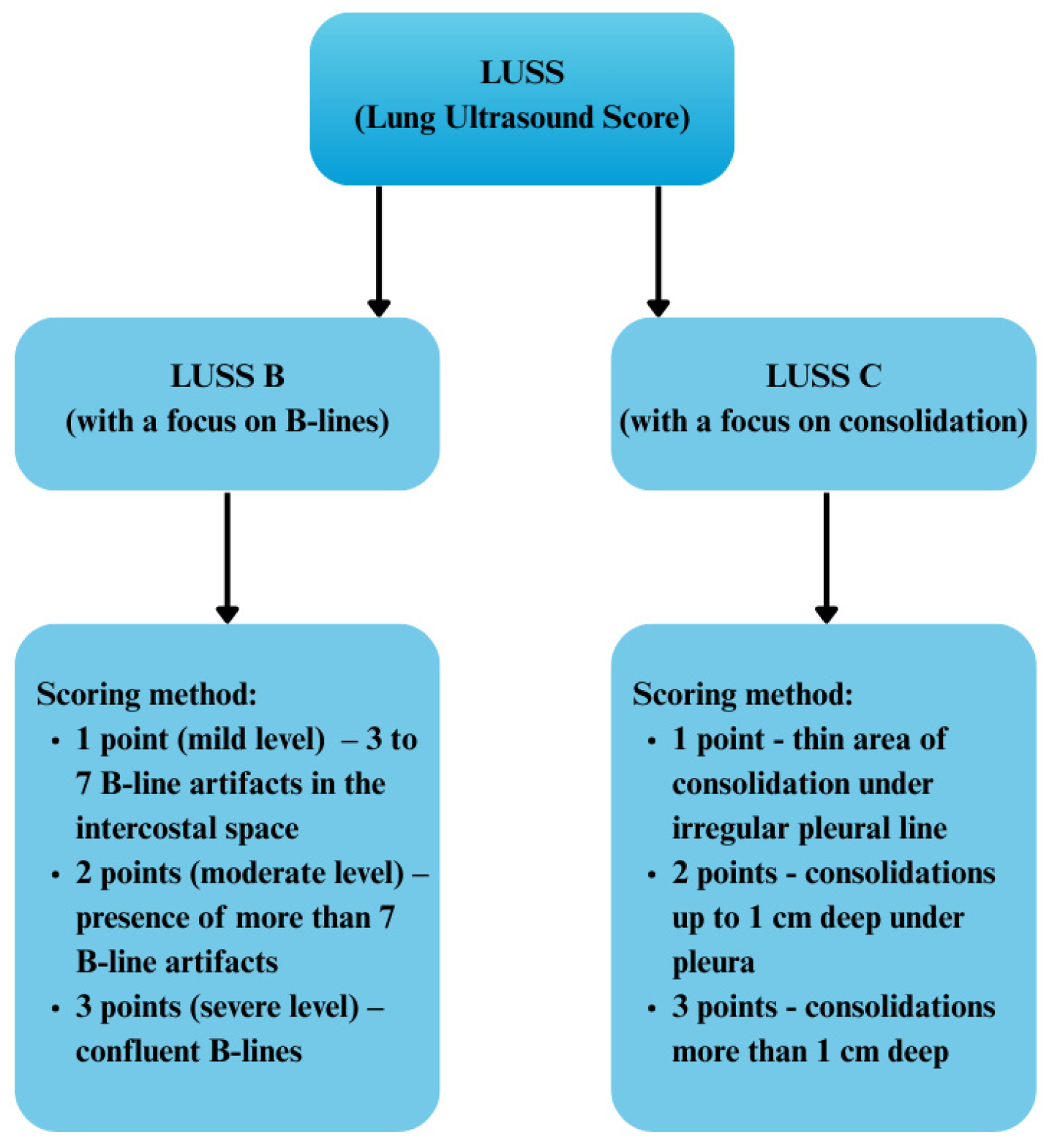
- LUSS B max—no differentiation among severity grades 1, 2, or 3.
- •
- LUSS C max—elimination of grade 1, with analysis restricted solely to grades 2 and 3.
- •
- The LDCT protocol was required to meet the minimum criteria defined by the NCCN guidelines:
- ➢
- Radiation dose for individuals with a BMI ≤ 30 did not exceed 3 mSv
- ➢
- X-ray tube voltage: 100–120 kVp
- ➢
- X-ray tube current: ≤40 mAs
- ➢
- Detector collimation: ≤1.5 mm
- ➢
- Reconstruction slice thickness: ≤1 mm
- ➢
- Acquisition time: ≤15 s
- •
- The scanning range of the LDCT examination extended from the lung apices to the costophrenic angles. The imaging was performed during deep inspiration, without the administration of intravenous or oral contrast agents. In cases where breath-holding was not feasible, scanning was conducted during shallow, quiet breathing.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aboshosha, A. AI based medical imagery diagnosis for COVID-19 disease examination and remedy. Sci. Rep. 2025, 15, 1607. [Google Scholar] [CrossRef]
- Kovács, A.; Palásti, P.; Veréb, D.; Bozsik, B.; Palkó, A.; Kincses, Z.T. The sensitivity and specificity of chest CT in the diagnosis of COVID-19. Eur. Radiol. 2021, 31, 2819–2824. [Google Scholar] [CrossRef]
- Garg, M.; Devkota, S.; Prabhakar, N.; Debi, U.; Kaur, M.; Sehgal, I.S.; Sandhu, M.S. Ultra-low dose CT chest in acute COVID-19 pneumonia: A pilot study from India. Diagnostics 2023, 13, 351. [Google Scholar] [CrossRef]
- Campagnano, S.; Angelini, F.; Fonsi, G.B.; Novelli, S.; Drudi, F.M. Diagnostic imaging in COVID-19 pneumonia: A literature review. J. Ultrasound 2021, 24, 383–395. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, C. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef]
- Rizzetto, F.; Perillo, N.; Artioli, D.; Travaglini, F.; Cuccia, A.; Zannoni, S.; Niguarda COVID-19 Working Group. Correlation between lung ultrasound and chest CT patterns with estimation of pulmonary burden in COVID-19 patients. Eur. J. Radiol. 2021, 138, 109650. [Google Scholar] [CrossRef]
- Wang, M.; Luo, X.; Wang, L.; Estill, J.; Lv, M.; Zhu, Y.; Tian, J. A comparison of lung ultrasound and computed tomography in the diagnosis of patients with COVID-19: A systematic review and meta-analysis. Diagnostics 2021, 11, 1351. [Google Scholar] [CrossRef]
- Tung-Chen, Y.; Martí de Gracia, M.; Díez-Tascón, A.; Alonso-Gonzalez, R.; Agudo-Fernandez, S.; Parra-Gordo, M.L.; Llamas-Fuentes, R. Correlation between chest computed tomography and lung ultrasonography in patients with coronavirus disease COVID-19. Ultrasound Med. Biol. 2020, 46, 2918–2926. [Google Scholar] [CrossRef]
- Portale, G.; Ciolina, F.; Arcari, L.; Giraldi, G.D.L.; Danti, M.; Pietropaolo, L.; Sighieri, C. Lung ultrasound in COVID-19: Clinical correlates and comparison with chest computed tomography. SN Compr. Clin. Med. 2021, 3, 2075–2081. [Google Scholar] [CrossRef]
- Zieleskiewicz, L.; Markarian, T.; Lopez, A.; Taguet, C.; Mohammedi, N.; Boucekine, M.; AZUREA Network. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020, 46, 1707–1713. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Demi, L. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19: A simple, quantitative, reproducible method. J. Ultrasound Med. 2020, 39, 1413–1419. [Google Scholar] [CrossRef]
- Tana, C.; Ricci, F.; Coppola, M.G.; Mantini, C.; Lauretani, F.; Campanozzi, D.; Mucci, L. Prognostic significance of chest imaging by LUS and CT in COVID-19 inpatients: The ECOVID multicenter study. Respiration 2022, 101, 122–131. [Google Scholar] [CrossRef]
- Jackson, K.; Butler, R.; Aujayeb, A. Lung ultrasound in the COVID-19 pandemic. Postgrad. Med. J. 2021, 97, 34–39. [Google Scholar] [CrossRef]
- Allinovi, M.; Parise, A.; Giacalone, M.; Amerio, A.; Delsante, M.; Odone, A.; Mangia, A. Lung ultrasound may support diagnosis and monitoring of COVID-19 pneumonia. Ultrasound Med. Biol. 2020, 46, 2908–2917. [Google Scholar] [CrossRef]
- Brahier, T.; Meuwly, J.Y.; Pantet, O.; Brochu Vez, M.J.; Gerhard Donnet, H.; Hartley, M.A.; Boillat-Blanco, N. Lung ultrasonography for risk stratification in patients with coronavirus disease 2019 (COVID-19): A prospective observational cohort study. Clin. Infect. Dis. 2021, 73, e4189–e4196. [Google Scholar] [CrossRef]
- Heldeweg, M.L.A.; Lopez Matta, J.E.; Haaksma, M.E.; Smit, J.M.; Elzo Kraemer, C.V.; de Grooth, H.J.; Tuinman, P.R. Lung ultrasound and computed tomography to monitor COVID-19 pneumonia in critically ill patients: A two-center prospective cohort study. Intensive Care Med. Exp. 2021, 9, 1. [Google Scholar] [CrossRef]
- Zhang, R.; Ouyang, H.; Fu, L.; Wang, S.; Han, J.; Huang, K.; Fu, Z. CT features of SARS-CoV-2 pneumonia according to clinical presentation: A retrospective analysis of 120 consecutive patients from Wuhan city. Eur. Radiol. 2020, 30, 4417–4426. [Google Scholar] [CrossRef]
- Fukumoto, W.; Nakamura, Y.; Yoshimura, K.; Sueoka, T.; Tatsugami, F.; Kitamura, N.; Awai, K. Triaging of COVID-19 patients using low dose chest CT: Incidence and factor analysis of lung involvement on CT images. J. Infect. Chemother. 2022, 28, 797–801. [Google Scholar] [CrossRef]
- Bonadia, N.; Carnicelli, A.; Piano, A.; Buonsenso, D.; Gilardi, E.; Kadhim, C.; Franceschi, F. Lung ultrasound findings are associated with mortality and need for intensive care admission in COVID-19 patients evaluated in the emergency department. Ultrasound Med. Biol. 2020, 46, 2927–2937. [Google Scholar] [CrossRef]
- Buonsenso, D.; Raffaelli, F.; Tamburrini, E.; Biasucci, D.G.; Salvi, S.; Smargiassi, A.; Moro, F. Clinical role of lung ultrasound for the diagnosis and monitoring of COVID-19 pneumonia in pregnant women. Ultrasound Obstet. Gynecol. 2020, 56, 106–109. [Google Scholar] [CrossRef]
- De Rose, C.; Inchingolo, R.; Smargiassi, A.; Zampino, G.; Valentini, P.; Buonsenso, D. How to perform pediatric lung ultrasound examinations in the time of COVID-19. J. Ultrasound Med. 2020, 39, 2081. [Google Scholar] [CrossRef]
- Bai, H.X.; Wang, R.; Xiong, Z.; Hsieh, B.; Chang, K.; Halsey, K.; Liao, W.H. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT. Radiology 2020, 296, E156–E165. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Q.; Huang, C.; Shi, C.; Wang, L.; Shi, N.; Shi, Y. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics 2020, 10, 5613–5622. [Google Scholar] [CrossRef]
- Li, M.; Lei, P.; Zeng, B.; Li, Z.; Yu, P.; Fan, B.; Liu, H. Coronavirus disease (COVID-19): Spectrum of CT findings and temporal progression of the disease. Acad. Radiol. 2020, 27, 603–608. [Google Scholar] [CrossRef]
- Shiri, I.; Akhavanallaf, A.; Sanaat, A.; Salimi, Y.; Askari, D.; Mansouri, Z.; Zaidi, H. Ultra-low-dose chest CT imaging of COVID-19 patients using a deep residual neural network. Eur. Radiol. 2021, 31, 1420–1431. [Google Scholar] [CrossRef]
- Calvo-Cebrián, A.; Alonso-Roca, R.; Rodriguez-Contreras, F.J.; Rodríguez-Pascual, M.D.L.N.; Calderín-Morales, M.D.P. Usefulness of lung ultrasound examinations performed by primary care physicians in patients with suspected COVID-19. J. Ultrasound Med. 2021, 40, 741–750. [Google Scholar] [CrossRef]
- Nouvenne, A.; Ticinesi, A.; Parise, A.; Prati, B.; Esposito, M.; Cocchi, V.; Meschi, T. Point-of-care chest ultrasonography as a diagnostic resource for COVID-19 outbreak in nursing homes. J. Am. Med. Dir. Assoc. 2020, 21, 919–923. [Google Scholar] [CrossRef]
- Jari, R.; Alfuraih, A.M.; McLaughlan, J.R. The diagnostic performance of lung ultrasound for detecting COVID-19 in emergency departments: A systematic review and meta-analysis. J. Clin. Ultrasound 2022, 50, 618–627. [Google Scholar] [CrossRef]
- Gargani, L.; Soliman-Aboumarie, H.; Volpicelli, G.; Corradi, F.; Pastore, M.C.; Cameli, M. Why, when, and how to use lung ultrasound during the COVID-19 pandemic: Enthusiasm and caution. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 941–948. [Google Scholar] [CrossRef]
- ACEP Board of Directors. Ultrasound guidelines: Emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann. Emerg. Med. 2017, 69, e27–e54. [Google Scholar] [CrossRef]






| CTSS | Extent of Lobe Involvement |
|---|---|
| 1 point | <5% |
| 2 points | 5–25% |
| 3 points | 26–50% |
| 4 points | 51–75% |
| 5 points | >75% |
| Formula | |
|---|---|
| LUSS B | CTSS = 0.18(±0.05) × LUSS B + 6.43(±0.75) ± 4.38 (F = 14.6, p = 0.0002) |
| LUSS C | (F = 7.9, p = 0.006) |
| Correlation | Spearman’s Correlation Coefficient (r) | Spearman’s p-Value | Pearson’s Correlation Coefficient (r) | Pearson’s p-Value | Statistical Significance |
|---|---|---|---|---|---|
| CTSS vs. LUSS C max | 0.2 | 0.03 | 0.18 | 0.04 | Yes |
| CTSS vs. LUSS B max | 0.1 | 0.24 | 0.12 | 0.17 | No |
| p—for Death | p—for ICU | |
|---|---|---|
| LUSS C max | 0.185 | 0.895 |
| LUSS B max | 0.351 | 0.638 |
| Correlation | Pearson’s Correlation Coefficient (r) | Pearson’s p-Value | Spearman’s Correlation Coefficient (r) | Spearman’s p-Value | Kendall’s Correlation Coefficient (τ) | Kendall’s p-Value |
|---|---|---|---|---|---|---|
| CTSS vs. LUSS B | 0.32 | 0 | 0.33 | 0 | 0.25 | 0 |
| CTSS vs. LUSS C | 0.24 | 0.006 | 0.227 | 0.003 | 0.197 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górecki, A.; Piech, P.; Kołodziejczyk, K.; Jankowska, A.; Szostak, Z.; Bronikowska, A.; Borowski, B.; Staśkiewicz, G. Synergistic Imaging: Combined Lung Ultrasound and Low-Dose Chest CT for Quantitative Assessment of COVID-19 Severity—A Prospective Observational Study. Diagnostics 2025, 15, 1875. https://doi.org/10.3390/diagnostics15151875
Górecki A, Piech P, Kołodziejczyk K, Jankowska A, Szostak Z, Bronikowska A, Borowski B, Staśkiewicz G. Synergistic Imaging: Combined Lung Ultrasound and Low-Dose Chest CT for Quantitative Assessment of COVID-19 Severity—A Prospective Observational Study. Diagnostics. 2025; 15(15):1875. https://doi.org/10.3390/diagnostics15151875
Chicago/Turabian StyleGórecki, Andrzej, Piotr Piech, Karolina Kołodziejczyk, Ada Jankowska, Zuzanna Szostak, Anna Bronikowska, Bartosz Borowski, and Grzegorz Staśkiewicz. 2025. "Synergistic Imaging: Combined Lung Ultrasound and Low-Dose Chest CT for Quantitative Assessment of COVID-19 Severity—A Prospective Observational Study" Diagnostics 15, no. 15: 1875. https://doi.org/10.3390/diagnostics15151875
APA StyleGórecki, A., Piech, P., Kołodziejczyk, K., Jankowska, A., Szostak, Z., Bronikowska, A., Borowski, B., & Staśkiewicz, G. (2025). Synergistic Imaging: Combined Lung Ultrasound and Low-Dose Chest CT for Quantitative Assessment of COVID-19 Severity—A Prospective Observational Study. Diagnostics, 15(15), 1875. https://doi.org/10.3390/diagnostics15151875






