A Systematic Review of Anatomical Variations of the Inferior Thyroid Artery: Clinical and Surgical Considerations
Abstract
1. Introduction
2. Methods
2.1. Protocol
2.2. Eligibility Criteria
2.3. Electronic Search
2.4. Study Selection
2.5. Data Collection Process
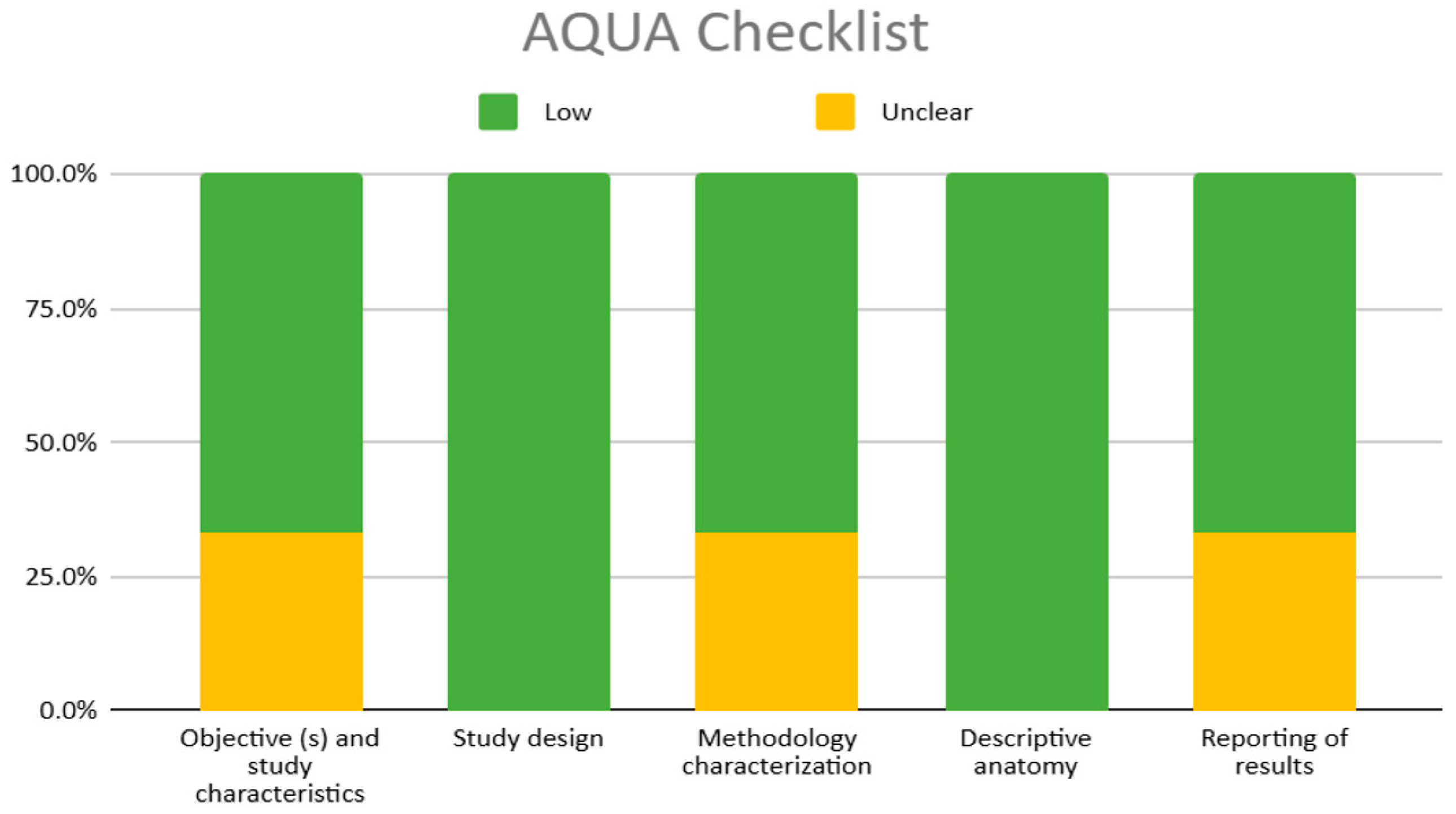
2.6. Methodological Quality Assessment of the Included Studies
3. Results
3.1. Included Articles
3.2. Characteristics of the Included Studies and the Study Population
3.3. Methodological Quality of the Included Studies
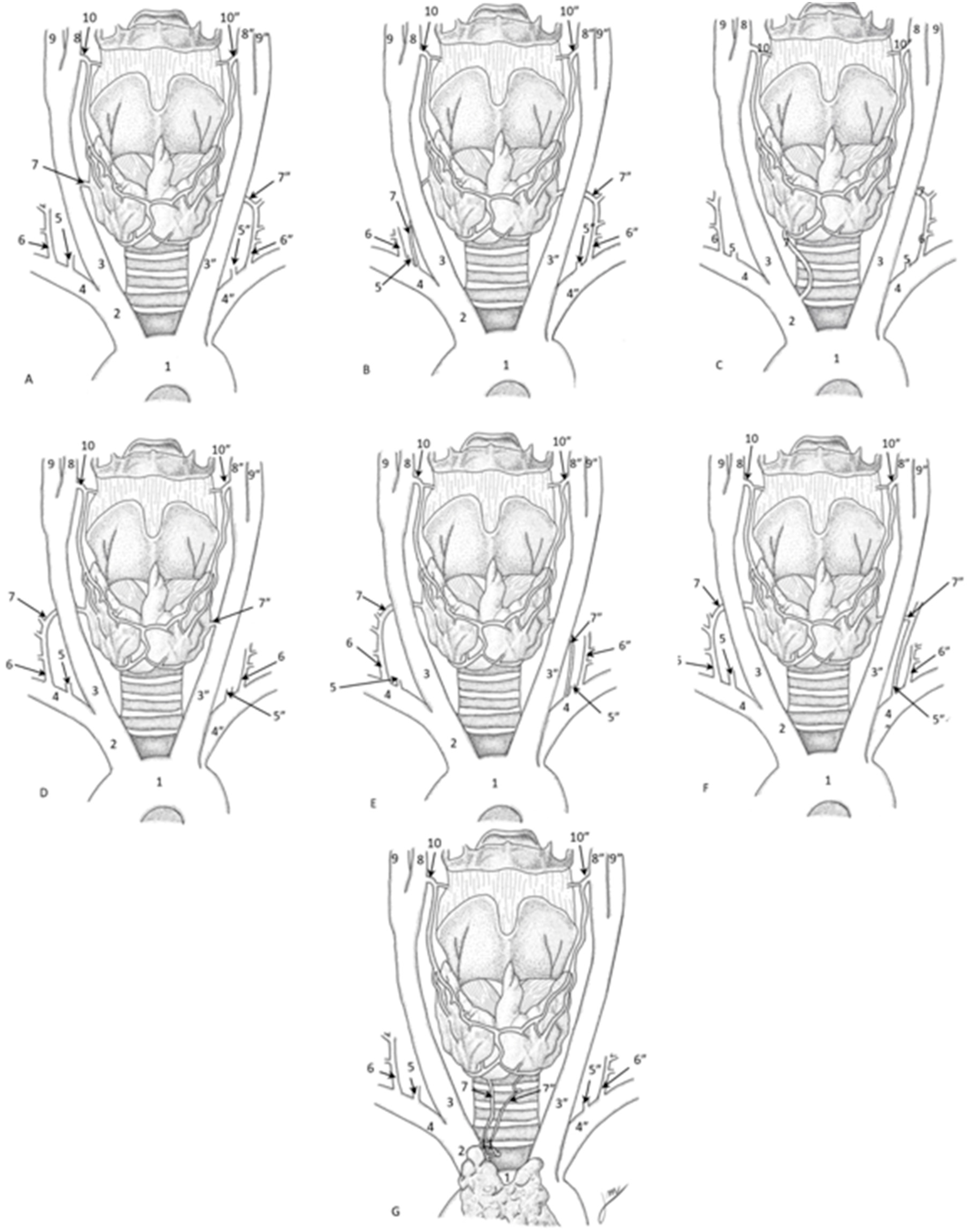
3.4. Inferior Thyroid Artery’s Morphology
3.5. Clinical Considerations
4. Discussion
4.1. Methodological Heterogeneity and Rigor of the Included Studies
4.2. ITA’s Variants Clinical Relevance
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ITA | Inferior Thyroid Artery |
| RLN | Recurrent Laryngeal Nerve |
| STA | Superior Thyroid Artery |
| TCT | Thyrocervical Trunk |
| BCT | Brachiocephalic Trunk |
| SCA | Subclavian Artery |
| CCA | Common Carotid Artery |
| CTA | Computed Tomography Angiography |
| IMA | Thyroidea Ima Artery |
| MTA | Middle Thyroid Artery |
References
- Toni, R.; Casa, C.D.; Castorina, S.; Roti, E.; Ceda, G.; Valenti, G. A meta-analysis of inferior thyroid artery variations in different human ethnic groups and their clinical implications. Ann. Anat. 2005, 187, 371–385. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Lascialfari Bruschi, A.; Pilia, A.M.; Carrino, D.; Guarnieri, G.; Gulisano, M.; Pacini, A.; Paternostro, F. The Thyroid Gland: A Revision Study on Its Vascularization and Surgical Implications. Medicina 2022, 58, 137. [Google Scholar] [CrossRef] [PubMed]
- Bunea, M.C.; Rusali, L.M.; Bratu, I.C.; Tudorache, S.; Bordei, P. Considerations on the origin of the inferior thyroid artery emerging from the subclavian artery determined by CT examination. Surg Radiol Anat 2023, 45, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Noussios, G.; Chatzis, I.; Konstantinidis, S.; Filo, E.; Spyrou, A.; Karavasilis, G.; Katsourakis, A. The Anatomical Relationship of Inferior Thyroid Artery and Recurrent Laryngeal Nerve: A Review of the Literature and Its Clinical Importance. J. Clin. Med. Res. 2020, 12, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Attard, M.; Caronia, A.; Lagalla, R. Color Doppler measurement of blood flow in the inferior thyroid artery in patients with autoimmune thyroid diseases. Eur. J. Radiol. 2000, 36, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2014, 24, 715–759. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Pugazhandhi, B.; D’Souza, A.S.; Saran, S.; Fasil, M.; Srinivasa, R.S. Analysis of the arterial anatomical variations of thyroid gland: Anatomic guide for surgical neck dissection. Bratisl. Lek. Listy 2012, 113, 669–672. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Tomaszewski, K.A.; Henry, B.M.; Kumar Ramakrishnan, P.; Roy, J.; Vikse, J.; Loukas, M.; Tubbs, R.S.; Walocha, J.A. Development of the Anatomical Quality Assurance (AQUA) checklist: Guidelines for reporting original anatomical studies. Clin. Anat. 2017, 30, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, J.; Takeda, S.; Walocha, J.A.; Ribatti, D.; Del Sol, M.; Ravi, K.S.; Moryś, J.; Paulsen, F.; Singh, V.; Apaydin, N.; et al. Guidelines Against Discrimination and Bias in Anatomical Research Papers (GDBARP): Recommendations From Anatomical Journal Editors. Clin. Anat. 2025, 38, 613–618. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE guidelines: Consensus-based clinical case reporting guideline development. Headache 2013, 53, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Archibong, V.; Omodan, A.; Omodan, A.; Gashegu, J. A rare anatomical origin of the inferior thyroid artery from the common carotid artery: A case report. Rwanda Med. J. 2023, 80, 63–65. [Google Scholar] [CrossRef]
- Cigali, B.S.; Ulucam, E.; Bozer, C. Accessory inferior thyroid artery and internal thoracic artery originating from the thyrocervical trunk. Anat. Sci. Int. 2008, 83, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Esen, K.; Ozgur, A.; Balci, Y.; Tok, S.; Kara, E. Variations in the origins of the thyroid arteries on CT angiography. Jpn. J. Radiol. 2018, 36, 96–102. [Google Scholar] [CrossRef] [PubMed]
- González-Castillo, A.; Rojas, S.; Ortega, M.; Rodríguez-Baeza, A. Variations in vascular anatomy and unilateral adrenal agenesis in a female cadaver with situs inversus totalis. Surg. Radiol. Anat. SRA 2018, 40, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Jelev, L.; Surchev, L. Lack of inferior thyroid artery. Ann. Anat. Ges. 2001, 183, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Lovasova, K.; Kachlik, D.; Santa, M.; Kluchova, D. Unilateral occurrence of five different thyroid arteries-a need of terminological systematization: A case report. Surg. Radiol. Anat. SRA 2017, 39, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Mariolis-Sapsakos, T.; Kalles, V.; Papapanagiotou, I.; Bonatsos, V.; Orfanos, N.; Kaklamanos, I.G.; Manolis, E. Bilateral aberrant origin of the inferior thyroid artery from the common carotid artery. Surg. Radiol. Anat. 2014, 36, 295–297. [Google Scholar] [CrossRef]
- Moriggl, B.; Sturm, W. Absence of three regular thyroid arteries replaced by an unusual lowest thyroid artery (A. thyroidea ima): A case report. Surg. Radiol. 1996, 18, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Natsis, K.; Didagelos, M.; Noussios, G.; Adamopoulou, A.; Nikolaidou, E.; Paraskevas, G. Combined anomalous origin of a left inferior thyroid artery and a left vertebral artery: A case report. Cases J. 2009, 2, 7400. [Google Scholar] [CrossRef] [PubMed]
- Ngo Nyeki, A.R.; Peloni, G.; Karenovics, W.; Triponez, F.; Sadowski, S.M. Aberrant origin of the inferior thyroid artery from the common carotid artery: A rare anatomical variation. Gland. Surg. 2016, 5, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Novakov, S.S.; Delchev, S.D. Two cases of variations in inferior thyroid arterial pattern and their clinical implications. Folia Morphol. 2023, 82, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Özgüner, G.; Sulak, O. Arterial supply to the thyroid gland and the relationship between the recurrent laryngeal nerve and the inferior thyroid artery in human fetal cadavers. Clin. Anat. 2014, 27, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.H.; Colborn, G.L. Absence of the left inferior thyroid artery: Clinical implications. Clin. Anat. 2003, 16, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Westrych, K.; Ruzik, K.; Zielinska, N.; Paulsen, F.; Georgiev, G.P.; Olewnik, Ł.; Łabętowicz, P. Common trunk of the internal thoracic artery, inferior thyroid artery and thyrocervical trunk from the subclavian artery: A rare arterial variant. Surg. Radiol. Anat. SRA 2022, 44, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, X.; Luo, J. A Rare Case of Anatomical Variation of Inferior Thyroid Artery Originated From the Brachiocephalic Trunk During Retrosternal Goiter Excision. Ear Nose Throat J. 2024, 1455613241239529. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Celik, H.H.; Durgun, B.; Atasever, A.; Ilgi, S. Arteria thyroidea ima arising from the brachiocephalic trunk with bilateral absence of inferior thyroid arteries: A case report. Surg. Radiol. Anat. SRA 1993, 15, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Yohannan, D.G.; Rajan, R.; Chandran, A.B.; Krishnapillai, R. An unusual origin and course of the thyroidea ima artery, with absence of inferior thyroid artery bilaterally. Surg. Radiol. Anat. SRA 2019, 41, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute: Adelaide, South Australia, Australia, 2017. [Google Scholar]
- Centeno Malfaz, F.; Salamanca Zarzuela, B. Mbriología Básica Cardíaca [Basic cardiac embryology]. Pediatría Integral 2001, 29, 438–442. [Google Scholar]
- Gilbert, S.F. Lateral Plate Mesoderm. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9982/ (accessed on 4 April 2025).
- Khalid, N.; Bordoni, B. Embryology, Great Vessel. [Updated 2023 May 1]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545254/ (accessed on 4 April 2025).
- Ghabriel, M.N.; Bhatia, K.; Henneberg, M. Anatomical variations in the branches of the human aortic arch: A recent study of a South Australian population. Folia Morphol. 2005, 64, 217–224. Available online: www.fm.viamedica.pl (accessed on 4 April 2024).
- Kau, T.; Sinzig, M.; Gasser, J.; Lesnik, G.; Rabitsch, E.; Celedin, S.; Eicher, W.; Illiasch, H.; Hausegger, K.A. Aortic development and anomalies. Semin. Interv. Radiol. 2007, 24, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Tae, K.; Ji, Y.B.; Song, C.M.; Ryu, J. Robotic and Endoscopic Thyroid Surgery: Evolution and Advances. Clin. Exp. Otorhinolaryngol. 2019, 12, 1–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, R.; Chen, Y.; Deng, X.; Qiao, D.; Li, X.; Yang, H. Comparison of bilateral axillo-breast approach robotic thyroidectomy and open thyroidectomy for papillary thyroid carcinoma. J. Robot. Surg. 2023, 17, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Rajabian, A.; Walsh, M.; Quraishi, N.A. Berry’s Ligament and the Inferior Thyroid Artery as reliable anatomical landmarks for the Recurrent Laryngeal Nerve (RLN): A fresh-cadaveric study of the cervical spine. The RLN relevant to spine. Spine J. 2017, 17, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, G.; Paschopoulos, I.; Duparc, F.; Tsakotos, G.; Tsiouris, C.; Olewnik, Ł.; Georgiev, G.; Zielinska, N.; Piagkou, M. The superior thyroid artery origin pattern: A systematic review with meta-analysis. Surg. Radiol. Anat. SRA 2024, 46, 1549–1560. [Google Scholar] [CrossRef] [PubMed]

| Domains | Questions |
|---|---|
| Domain 1: objective(s) and subject characteristics. | (1) Was (Were) the objective(s) of the study clearly defined? (yes, no, or unclear) |
| (2) Was (Were) the chosen subject sample(s) and size appropriate for the objective(s) of the study? (yes, no, or unclear) | |
| (3) Are the baseline and demographic characteristics of the subjects (age, sex, ethnicity, healthy or diseased, etc.) appropriate and clearly defined? (yes, no, or unclear) | |
| (4) Could the method of subject selection have in any way introduced bias into the study? (yes, no, or unclear) | |
| Domain 2: study design. | (5) Does the study design appropriately address the research question(s)? (yes, no, or unclear) |
| (6) Were the materials used in the study appropriate for the given objective(s) of the study? (yes, no, or unclear) | |
| (7) Were the methods used in the study appropriate for the given objective(s) of the study? (yes, no, or unclear) | |
| (8) Was the study design, including methods/techniques applied in the study, widely accepted or standard in the literature? If “no”, are the novel features of the study design clearly described? | |
| (9) Could the study design have in any way introduced bias into the study? (yes, no, or unclear) | |
| Domain 3: methodology characterization. | (10) Are the methods/techniques applied in the study described in enough detail for them to be reproduced? (yes, no, or unclear) |
| (11) Was the specialty and the experience of the individual(s) performing each part of the study (such as cadaveric dissection or image assessment) clearly stated? (yes, no, or unclear) | |
| (12) Are all the materials and methods used in the study clearly described, including details of manufacturers, suppliers, etc.? (yes, no, or unclear) | |
| (13) Were appropriate measures taken to reduce inter- and intra-observer variability? (yes, no, or unclear) | |
| (14) Do the images presented in the study indicate an accurate reflection of the methods/techniques (imaging, cadaveric, intraoperative, etc.) applied in the study? (yes, no, or unclear) | |
| (15) Could the characterization of methods have in any way introduced bias into the study? (yes, no, or unclear) | |
| Domain 4: descriptive anatomy. | (16) Were the anatomical definition(s) (normal anatomy, variations, classifications, etc.) clearly and accurately described? (yes, no, or unclear) |
| (17) Were the outcomes and parameters assessed in the study (variation, length, diameter, etc.) appropriate and clearly defined? (yes, no, or unclear) | |
| (18) Were the figures (images, illustrations, diagrams, etc.) presented in the study clear and understandable? (yes, no, or unclear) | |
| (19) Were any ambiguous anatomical observations (i.e., those likely to be classified as “others”) clearly described/depicted? (yes, no, or unclear) | |
| (20) Could the description of anatomy have in any way introduced bias into the study? (yes, no, or unclear) | |
| Domain 5: reporting of results. | (21) Was the statistical analysis appropriate? (yes, no, or unclear) |
| (22) Are the reported results as presented in the study clear and comprehensible, and are the reported values consistent throughout the manuscript? (yes, no, unclear) | |
| (23) Do the reported numbers or results always correspond to the number of subjects in the study? If not, do the authors clearly explain the reason(s) for subject exclusion? (yes, no, or unclear) | |
| (24) Are all potential confounders reported in the study, and subsequently measured and evaluated, if appropriate? (yes, no, or unclear) | |
| (25) Could the reporting of results have in any way introduced bias into the study? (yes, no, or unclear) |
| Questions | Yes | No | Unclear | Not Applicable |
|---|---|---|---|---|
| 1. Were the patient’s demographic characteristics clearly described? | ||||
| 2. Was the patient’s history clearly described and presented as a timeline? | ||||
| 3. Was the patient’s current clinical condition at the time of presentation clearly described? | ||||
| 4. Were the diagnostic tests or assessment methods and results clearly described? | ||||
| 5. Were the intervention(s) or treatment procedure clearly described? | ||||
| 6. Was the clinical condition following the intervention clearly described? | ||||
| 7. Were adverse events (harms) or unanticipated events identified and described? | ||||
| 8. Does the case report provide learned lessons? | ||||
| ||||
| ||||
| ||||
| ||||
| ||||
| Author, Year | Total Number of Subjects | Age or Mean Age (SD) and/or Range | Ethicity | Country | Type of Study | Study Technique | Sex Sample |
|---|---|---|---|---|---|---|---|
| Buena et al., 2023 [3] | 108 | 66.5 ± 17.5 years 51.5 ± 30.5 years | Caucasian | Romania | Case report | Computed tomography angiographies (CTA) | 48 males 60 females |
| Cigali et al., 2008 [13] | 1 | 86 years | Caucasian | Turkey | Case report | Cadaveric The anatomical dissection | 1 Male |
| Esen et al., 2018 [14] | 640 | 62.4 ± 13.8 years 59.1 ± 15.7 years | Not specified | Japan | Cross-sectional study | CT Angiography images. | 379 Males 261 Females |
| González-Castillo et al., 2018 [15] | 1 | 91 years | Not specified | Spain | Case report | Cadaveric The anatomical dissection | 1 Female |
| Jelev and Surchev, 2001 [16] | 1 | 67 years | Not specified | Bulgary | Case report | Cadaveric | 1 male |
| Lovasava et al., 2017 [17] | 1 | 72 years | Not specified | Slovak Republic | Case report | Cadaveric | 1 Female |
| Mariolis-Sapsakos et al., 2014 [18] | 1 | 56 | Not specified | Greece | Case report | Thyroidectomy | 1 Male |
| Moriggl and Sturm 1996 [19] | 1 | 89 years old | Not specified | Austria | Case report | Cadaveric | Female |
| Natsis et al., 2009 [20] | 1 | 72 years old | Caucasian | Greece | Case report | Cadaveric | male |
| Ngo Nyeki et al., 2016 [21] | 1 | 46 | Not specified | Switzerland | Case report | Thyroidectomy | 1 Male |
| Novakov and Delchev, 2023 [22] | 2 | 61 85 | Not specified | Bulgary | Case report | Cadaveric | 1 Male 1 Female |
| Özgüner G and Sulak O. 2014 [23] | 200 | 9 to 40 weeks | Not specified | Turkey | Cross-sectional study | Cadaveric | 100 Males 100 Females |
| Ray et al., 2012 [7] | 25 | 50–60 year | not specified | India | Case report | Cadaveric | 13 Males 12 Females |
| Sherman andand Colborn, 2003 [24] | 1 | Not specified | Not specified | USA | Case report | Cadaveric | 1 Male |
| V. Archibong et al., 2023 [12] | 1 | 54 years | Not specified | Ruanda | Case report | Cadaveric | 1 male |
| Westrych et al., 2022 [25] | 1 | 74 years | not specified | Poland | case report | Cadaveric | 1 Female |
| Yang et al., 2024 [26] | 1 | 60 years | not specified | China | Case report | Thyroidectomy | 1 Female |
| Yilmaz et al., 1993 [27] | 130 | Not specified | Not specified | Turkey | Case report | Cadaveric | Not specified |
| Yohannan et al., 2019 [28] | 1 | 60 | South asian | India | Case report | Cadaveric | 1 Male |
| Author | JBI Q1 | JBI Q2 | JBI Q3 | JBI Q4 | JBI Q5 | JBI Q6 | JBI Q7 | JBI Q8 | Bias Risk |
|---|---|---|---|---|---|---|---|---|---|
| Novakov and Delchev, 2023 [22] |  | N/A | N/A |  | N/A | N/A | N/A |  | High |
| Sherman and Colborn, 2003 [24] |  | N/A | N/A |  | N/A | N/A | N/A |  | High |
| Yang et al., 2024 [26] |  |  |  |  |  |  | N/A |  | Low |
| Westrych et al., 2022 [25] |  | N/A | N/A |  | N/A | N/A | N/A |  | High |
| Yohannan et al., 2019 [28] |  | N/A | N/A |  | N/A | N/A | N/A |  | High |
| Yilmaz et al., 1993 [27] |  | N/A | N/A |  | N/A | N/A | N/A |  | High |
| Bunea et al., 2023 [3] |  | N/A |  |  |  |  |  |  | Moderate |
| González-Castillo et al., 2018 [15] |  | N/A | N/A |  |  | N/A |  |  | High |
| Ngo Nyeki et al., 2016 [21] |  |  |  |  |  |  |  |  | Low |
| Cigali et al., 2008 [13] |  | N/A | N/A |  |  | N/A |  |  | High |
| Natsis et al., 2009 [20] |  |  | N/A |  |  | N/A |  |  | High |
| Moriggl and Sturn, 1996 [19] |  |  | N/A |  |  | N/A |  |  | High |
| Jelev and Surchev, 2001 [16] |  | N/A | N/A |  |  | N/A |  |  | High |
| Mariolis- Sapsakos et al., 2014 [18] |  |  |  |  |  |  |  |  | Low |
| V. Archibong et al., 2023 [12] |  |  | N/A |  |  | N/A |  |  | High |
| Lovasova et al., 2017 [17] |  |  | N/A |  |  | N/A |  |  | Moderate |
| Author, Year | Absence of ITA | Differences in Origin | Additional Arteries |
|---|---|---|---|
| Archibong et al., 2023 [12] | - | - | An additional one from the left CCA |
| Bunea et al., 2023 [3] | - | ITA originating from the right and left SCA, left vertebral artery, and CCA | - |
| Cigali et al., 2008 [13] | - | - | Accessory inferior thyroid artery from the suprascapular artery |
| Esen et al., 2018 [14] | Multiple cases of absence without specified compensation | ITA originating from the right and left SCA, left vertebral artery, and CCA | - |
| González-Castillo et al., 2018 [15] | Absence of right IA in a female with situs inversus totalis, compensated by two left ITAs | - | - |
| Jelev and Surchev, 2001 [16] | Absence on the right side in a male, compensated by a large STA | - | - |
| Lovasova et al., 2017 [17] | - | ITA originating from the BCT | Three additional arteries on the right side, including MTA and aberrant accessory thyroid artery |
| Mariolis-Sapsakos et al., 2014 [18] | - | ITA originating from the right and left SCA, left vertebral artery, and CCA | - |
| Moriggl and Saturn, 1996 [19] | Bilateral absence in a female, compensated by a branch of the left internal thoracic artery | - | - |
| Novakov and Delchev, 2023 [22] | Bilateral absence in a female, compensated by large STAs and an ITA from the BCT | - | Right thymothyroid artery supplying both thyroid and thymus |
| Natsis et al., 2009 [20] | Bilateral absence of the ITA compensated by the left STA | ITA originating from the right and left SCA, left vertebral artery, and CCA | - |
| Ngo Nyeki et al., 2016 [21] | - | ITA originating from the right and left SCA, left vertebral artery, and CCA | - |
| Özgüner and Sulak, 2014 [23] | - | ITA originating from the right and left SCA, left vertebral artery, and CCA | - |
| Ray et al., 2012 [7] | - | ITA originating from the right CCA supplying the left thyroid lobe | - |
| Sherman and Colborn, 2003 [24] | Absence on the left side in a male, with compensation by right ITA branches | - | - |
| Westrych et al., 2022 [25] | - | - | Common trunk for ITA, internal thoracic artery, and thyrocervical trunk |
| Yang et al., 2024 [26] | - | ITA originating from the right and left SCA, left vertebral artery, and CCA | - |
| Yilmaz et al., 1993 [27] | Bilateral absence in an unspecified case, compensated by an ITA from the left BCT | - | - |
| Yohannan et al., 2019 [28] | Bilateral absence in a male, compensated by an ITA from the subclavian artery | - | - |
| Author, Year | Inferior Thyroid Artery Variation and Other Variations | Clinical Consideration |
|---|---|---|
| Buena et al., 2023 [3] | 108 ITAs, 31.48% (n = 34) originated directly from the subclavian artery (SCA), while 68.52% (n = 74) arose from the thyrocervical trunk (TCT). Laterally, right-sided ITAs demonstrated SCA origins in 31.25% (20/64) of cases versus 31.82% (14/44) on the left. Gender analysis revealed males exhibited higher SCA origins (37.50%, 18/48) compared to females (26.67%, 16/60), with right-sided male ITAs more frequently originating from the SCA (55.56%, 10/18) than left-sided (44.44%, 8/18). TCT origins predominated across both sexes (males: 62.50%, females: 73.33%), particularly on the right side in females (63.64%, 28/44). These variations are consistent with documented anatomical diversity in cervical vasculature. | - |
| Cigali et al., 2008 [13] | An unusual unilateral variation was observed where the left ITA originated anomalously. Alongside the standard inferior and STA, a third thyroid artery (identified as an accessory ITA) arose from the left suprascapular artery. This accessory artery descended ~1 cm vertically before branching medially, coursing anterior to the carotid sheath, phrenic nerve, and internal thoracic artery, and terminating at the left inferior thyroid pole. The inferior thyroid artery maintained its typical branching pattern, giving rise to the ascending cervical artery. This variant highlights rare collateral thyroid vasculature, which could influence surgical planning due to its proximity to critical neurovascular structures | An accurate knowledge of the normal anatomy of the thyroid gland vessels, particularly of their patterns of variation, is important in parathyroid localization studies, and in neck surgery procedures. especially in tracheostomy. |
| Esen et al., 2018 [14] | The right and left superior thyroid arteries arose from the external carotid artery in 413 (64.5%) and 254 (39.7%) patients, from the bifurcation of the common carotid artery in 131 (20.5%) and 148 (23.1%) patients, and from the common carotid artery in 90 (14.1%) and 226 (35.3%) patients, respectively. We could not observe the right and the left superior arteries in 6 (0.9%) and 12 (1.9%) of the patients, respectively. However, the right and left inferior thyroid arteries thyroid were not identified in 14 (2.2%) and 45 (7%) of the patients, respectively. The thyroidea ima artery was detected in 2.3% of the patients. | The high variability of the origin and the occur rence rate of the TIA can lead to bleeding during surgery or tracheostomy |
| González-Castillo et al., 2018 [15] | A description is made of how situs inversus totalis occurs in the abdomen and thorax, therefore, not so much detail is given on the inferior thyroid artery. It is noted that a double left inferior thyroid artery and agenesis of the left adrenal gland were observed—two variants that have not been previously reported in association with situs inversus.. | The thyroid primordium is initially irrigated by a dense arterial network, including lateral branches from the subclavian arteries. Post-development, this network typically regresses, leaving the four principal thyroid arteries as the dominant supply. Persistent embryonic vessels, such as an accessory inferior thyroid artery arising from the thyrocervical trunk, may remain. Inferior thyroid artery ligation is critical during thyroidectomy; undetected variants risk intraoperative hemorrhage. |
| Jelev and Surchev, 2001 [16] | In the male specimen, a right thymothyroid artery originated 48 mm proximal to the carotid bifurcation (CCA diameter: 8 mm), dividing into three thyroid branches (middle branch: 63 mm length, 2.5 mm diameter) and a thymic branch (1.5 mm diameter), while the left ITA atypically arose from the CCA (50 mm from bifurcation; diameter: 2.5 mm) with superior (10 mm, 1.5 mm) and inferior (16 mm, 0.8 mm) branches. In the female, bilateral ITA absence was compensated by hypertrophied STAs and a TIA from the brachiocephalic trunk (28 mm from origin; 4 mm diameter), bifurcating into thymic and ITA branches (right: 3 mm trunk; left: 2 mm ITA with isthmic branch). These variations, including accessory thymothyroid arteries and TIA-mediated compensation, highlight embryological persistence of aortic arch derivatives and necessitate preoperative vascular mapping to avoid hemorrhage during cervical surgeries. | The thyroid gland’s rich vascularization makes the dissection of its vessels a crucial aspect of thyroid surgeries. While the STAs tend to have stable anatomical positions, the inferior thyroid arteries exhibit significant variability. The recurrent laryngeal nerve’s proximity to the inferior thyroid artery is particularly concerning during thyroidectomies, as this area is considered highly vulnerable due to complex nerve-artery relationships. Thus, understanding the anatomical variations of the ITA is essential for minimizing surgical complications. |
| Lovasava et al., 2017 [17] | The thyroid gland is typically supplied by the STA and ITA, with occasional anatomical variations such as the TIA, an inconstant vessel originating from the brachiocephalic trunk or aortic arch. Vascular anomalies like the TIA, which ascends to the thyroid isthmus, and rare branches such as MTAs or AITAs arising from the common carotid artery, highlight the diversity in thyroid vasculature. These variations, alongside broader vascular anomalies (e.g., lingual artery course deviations or persistent embryonic arteries), emphasize the importance of preoperative imaging to avoid complications during neck surgeries. | The risk of blood vessel damage during the surgery can be minimized by keeping in mind all the anatomical variations and development anomalies. In the area of the neck, the anterior cervical region is the most important one from clinical point of view and the area of the thyroid gland must be evaluated precisely before any surgical procedures. |
| Mariolis-Sapsakos et al., 2014 [18] | The lower lobe of the thyroid was found to be supplied by left and right inferior thyroid arteries, which originated bilaterally from the common carotid artery. | Rare variation during procedures in the neck area, and prior knowledge will be beneficial to limit the incidence of possible complications |
| Moriggl and Sturm 1996 [19] | Both inferior thyroid arteries and the left superior thyroid artery were absent. The right superior thyroid artery originated from the terminal right common carotid artery (RCA) to supply the isthmus. The left internal thoracic artery (ITA) exhibited an enlarged proximal segment, emitting ascending cervical and suprascapular arteries before bifurcating at the first intercostal space: a dominant lowest thyroid artery ascending to the thyroid’s inferior margin and a standard ITA continuation. The lowest thyroid artery bifurcated midline into twin branches (equal caliber) that traversed the trachea anteriorly to perfuse the thyroid lobes. | All diagnostic and/or surgical procesdures involving supraesternal fossa, tracheostomy in particular, require a carfeul approach because of a possibly existing lower thyroid artery. Once cut or injured it can cause extensive and uncontrollable bleeding. This is especially true if this vessel is huge. Finally, description of arterial variation, especially if they are of rare occurrence, is important for interpretation within the scope of modern imaging techniques. |
| Natsis et al., 2009 [20] | Left vertebral artery originated from the root of the left subclavian artery, close to the aortic arch. Following its route upwards to the neck we came across another arterial branch that arose from it having and upward to the midline course, that was the inferior thyroid artery. | It should be known by neck surgeons in order to avoid implication during thyroidectomy while trying to ligate the regional vessels. Vascular interventionalist and angiographers should also bear in mind this variation during inferior thyroid artery catherization either diagnostic or therapeutic, in case of an aneurysm or a rupture at the thyroid region. |
| Ngo Nyeki et al., 2016 [21] | The right inferior thyroid artery (ITA) was originating directly from the right common carotid artery, right ITA moved towards the lower third of the right thyroid lobe where it divided into three branches. The contralateral ITA originated from the thyrocervical trunk and the superior thyroid arteries from the external carotid arteries, as expected. | Two major risks from a surgical perspective: the first one is hemorrhagic by injuring this artery when on aberrant course; the second risk is increased injury to the recurrent laryngeal nerves (RLN). |
| Novakov and Delchev, 2023 [22] | In the male specimen, a right thymothyroid artery was noted to originate 48 mm from the carotid bifurcation and branched into thyroid and thymic branches. The left inferior thyroid artery (ITA) arose unusually from the common carotid artery, with distinct superior and inferior branches. In the female specimen, the absence of bilateral ITAs was compensated by enlarged superior thyroid arteries and a thyroidea ima artery from the brachiocephalic trunk. These anatomical variations highlight the importance of preoperative vascular mapping, such as CT angiography, to reduce surgical risks during thyroidectomies or mediastinal procedures. | - |
| Özgüner G and Sulak O. 2014 [23] | The origins of the ITA were as follows: thyrocervical trunk in 97.5% of cases on the right and 96.5% on the left; CCA in 2.5% of cases on the right and 2.5% on the left. The ITA was absent on the left side in two cases. The location of the ITA in relation to the entrance of the thyroid lobe was determined and three different types were detected: 1. The ITA entered the thyroid lobe from the subpolar; right 79 (39.5%), left 78 (39%). 2. “The ITA entered the thyroid lobe from the lower one-third aspect.”; right 101 (50.5%), left 102 (51%). 3. The ITA entered the thyroid lobe from the one-third middle side; right 20 (10%), left 18 (9%). | The relationship of the RLN to the ITA is highly variable, and the variability of the ITA and its position relative to the RLN makes it a poor surgical landmark. |
| Ray et al., 2012 [7] | Origin branching pattern: In the present study, ITA arose as a single trunk from TCT in 5 males and 7 females and as multiple branches it was observed in 8 males and 5 females. The incidence of multiplication is more in the males. Branching patter: The mean number of branches from STA and ITA was found to be three Lenght: Longer arteries were observed on left side. | Preservation of all the branches is important during thyroidectomy while ligating the regional vessels (31). The variation should also be borne in mind during ITA catheterization for diagnostic or therapeutic purposes in case of an aneurysm. |
| Sherman and Colborn, 2003 [24] | The case report describes a cadaver with an atypically small ITA that followed its normal trajectory but branched into several small vessels, ultimately not supplying the thyroid gland. In contrast, the right inferior thyroid artery divided into two branches that provided blood to the inferior pole of the right lobe, the thyroid isthmus, and the inferior pole of the left lobe. This incomplete development of the ITA disrupted the typical anatomical relationship with the left recurrent laryngeal nerve, potentially increasing the risk of nerve injury during surgical procedures. | As an alternative surgical approach, ligation of the tertiary branches of the inferior thyroid artery at the surface of the thyroid gland, with subsequent identification of the recurrent laryngeal nerve, can aid in protecting the nerve |
| V. Archibong et al., 2023 [12] | The ITA usually originates from the thyrocervical trunk in the majority of the human population (90.5%), or from the subclavian artery in a few populations of humans (7.5%). It is quite rare to find the ITA originating from the CCA. The case was a male who had two inferior thyroid arteries on the left side, with one originating from the TCT and the other taking a rare anatomical origin from the CCA. The ITA on the right side was one and from the normal source. | Documenting anatomical arterial patterns is crucial for increasing awareness among surgeons and imaging specialists, thereby minimizing the risk of iatrogenic complications during thyroid surgeries. The ITA’s close relationship with the recurrent laryngeal nerve, which is vital for vocal cord mobility and respiratory functions, underscores the importance of visualizing this anatomical relationship to ensure nerve preservation during surgical procedures. |
| Westrych et al., 2022 [25] | The ITHA originated from the common trunk. The ITHA enters the thyroid gland and anastomoses with the contralateral ITHA and ipsilateral superior thyroid artery. | ITHA: Thyroidectomy is a common procedure in general and endocrine surgery in which ligation of the ITHA is performed. Therefore, the correct location of the ITHA is key to a well-performed surgery Common trunk: “This variation may result in the failure of coronary bypass surgery due to a coronary steal associated with a common ITA-ITHA-TCT trunk, highlighting the importance of considering its potential causes.” |
| Yang et al., 2024 [26] | The inferior thyroid artery, which exhibited a diameter of approximately 0.5 cm and originated directly from the BCT. The distance between inferior thyroid artery and aortic arch was about 1.5 cm. The inferior thyroid artery originating from the BCT was approximately 2 cm long and divided into two branches to supply blood for the thyroid gland. | - |
| Yilmaz et al., 1993 [27] | ITA: there was found the absence of both inferior thyroid arteries. IMA: An IMA artery arose from the brachiocephalic trunk as a short, wide vessel. It bifurcated almost immediately into two branches, reaching the bases of both the right and left thyroid gland lobes. Subclavian Artery: The first part of the left subclavian artery is anomalous due to the absence of the thyrocervical trunk, costocervical trunk, and vertebral artery. Vertebral artery: The left vertebral artery arises from the aortic arch between the left common carotid artery and the left subclavian artery. | Authors note: “An accurate knowledge of the normal anatomy of the thyroid gland vessels, and particularly of their patterns of variation, is important for parathyroid localisation studies, in neck surgery procedures and especially in tracheostomy” |
| Yohannan et al., 2019 [28] | ITA: There was absence of the inferior thyroid artery on the right and left side. IMA: The ima artery originated from the subclavian artery, close to the origin of the vertebral artery. The ima artery was seen to take an anterior course between the common carotid artery medially and the internal jugular vein and the vagus nerve laterally. The artery then traced a path medially, superficial to the common carotid artery to reach the lower pole of the right lobe of the thyroid | The thyroidea ima artery’s anterior tracheal position increases hemorrhage risk during lower neck procedures (e.g., tracheostomy, laryngeal transplantation). Though typically superficial and oblique in the neck, its terminal branches may interface with tracheal structures. “Surgeons must remain highly aware of this anatomical variant to prevent inadvertent vascular injury.” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruna-Mejias, A.; Pérez-Farías, C.; Prieto-Heredia, T.; Vergara-Vargas, F.; Martínez-Cid, J.; Sanchis-Gimeno, J.; Afandi-Rebolledo, S.; Valdés-Orrego, I.; Nova-Baeza, P.; Suazo-Santibáñez, A.; et al. A Systematic Review of Anatomical Variations of the Inferior Thyroid Artery: Clinical and Surgical Considerations. Diagnostics 2025, 15, 1858. https://doi.org/10.3390/diagnostics15151858
Bruna-Mejias A, Pérez-Farías C, Prieto-Heredia T, Vergara-Vargas F, Martínez-Cid J, Sanchis-Gimeno J, Afandi-Rebolledo S, Valdés-Orrego I, Nova-Baeza P, Suazo-Santibáñez A, et al. A Systematic Review of Anatomical Variations of the Inferior Thyroid Artery: Clinical and Surgical Considerations. Diagnostics. 2025; 15(15):1858. https://doi.org/10.3390/diagnostics15151858
Chicago/Turabian StyleBruna-Mejias, Alejandro, Carla Pérez-Farías, Tamara Prieto-Heredia, Fernando Vergara-Vargas, Josefina Martínez-Cid, Juan Sanchis-Gimeno, Sary Afandi-Rebolledo, Iván Valdés-Orrego, Pablo Nova-Baeza, Alejandra Suazo-Santibáñez, and et al. 2025. "A Systematic Review of Anatomical Variations of the Inferior Thyroid Artery: Clinical and Surgical Considerations" Diagnostics 15, no. 15: 1858. https://doi.org/10.3390/diagnostics15151858
APA StyleBruna-Mejias, A., Pérez-Farías, C., Prieto-Heredia, T., Vergara-Vargas, F., Martínez-Cid, J., Sanchis-Gimeno, J., Afandi-Rebolledo, S., Valdés-Orrego, I., Nova-Baeza, P., Suazo-Santibáñez, A., Valenzuela-Fuenzalida, J. J., & Orellana-Donoso, M. (2025). A Systematic Review of Anatomical Variations of the Inferior Thyroid Artery: Clinical and Surgical Considerations. Diagnostics, 15(15), 1858. https://doi.org/10.3390/diagnostics15151858






