Machine Learning for Coronary Plaque Characterization: A Multimodal Review of OCT, IVUS, and CCTA
Abstract
1. Introduction
2. Materials and Methods
3. Coronary Artery Disease
3.1. Pathophysiology
3.2. Pathological Anatomy
- Fatty streaks: Fatty streaks are formations that typically merge into elongated lesions measuring 1 cm or greater in length. Their composition consists of lipid-filled foamy macrophages, and morphologically, they are minimally raised; therefore, they do not induce substantial hemodynamic alterations [31].
- Atherosclerotic plaque: Intimal thickening and lipid accumulation characterize atherosclerotic plaques [32]. Atherosclerotic plaques are raised, yellow-white lesions ranging from 0.3 to 1.5 cm in diameter. They can coagulate to form larger masses. These tend to be focal lesions, and this characteristic can be explained by local flow irregularities, such as turbulence at the bifurcation points, at the preferred site of plaque formation [33,34]. Atherosclerotic lesions exhibit a structural organization with three primary compositional elements: first, cellular elements such as smooth muscle cells (SMCs), macrophages, and T lymphocytes; second, the extracellular matrix (ECM), consisting of collagen fibres, elastin, and various proteoglycans that ensure structural integrity; and third, lipid accumulations, both in intracellular and extracellular compartments [35]. The structural organization typically shows a superficial fibrous cap characterized by a predominance of SMCs embedded in dense collagenous tissue. Deep within this superficial fibrous cap is a necrotic core containing lipids, cellular debris, foamy cells (macrophages and SMCs embedded in lipids), fibrin deposits, and thrombotic material in different stages of organization. Peripherally, areas of neoangiogenesis are observed, which contribute to plaque growth and instability. Atheromas in advanced stages tend to undergo calcification processes [30,36].
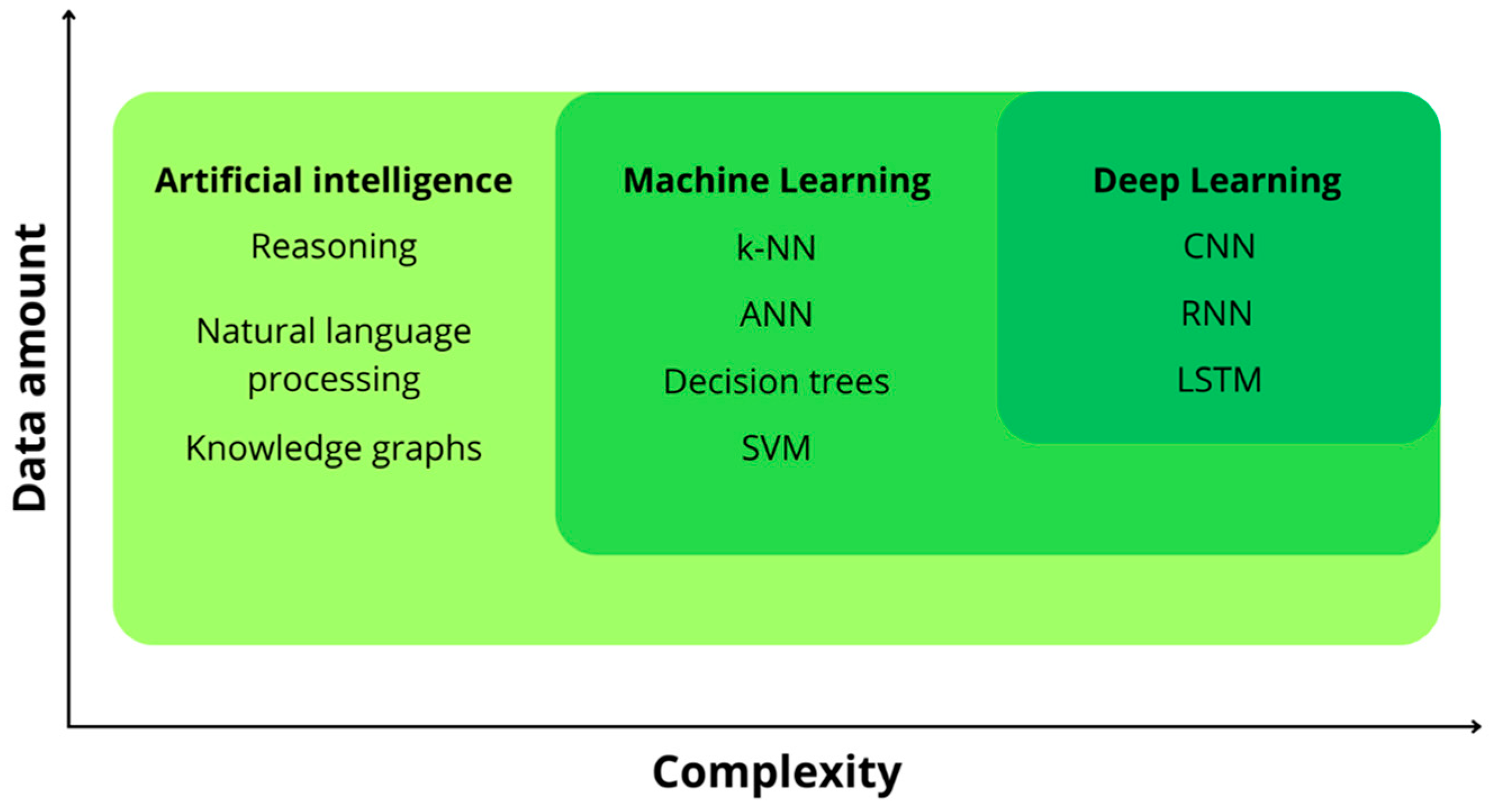
4. Notions of AI
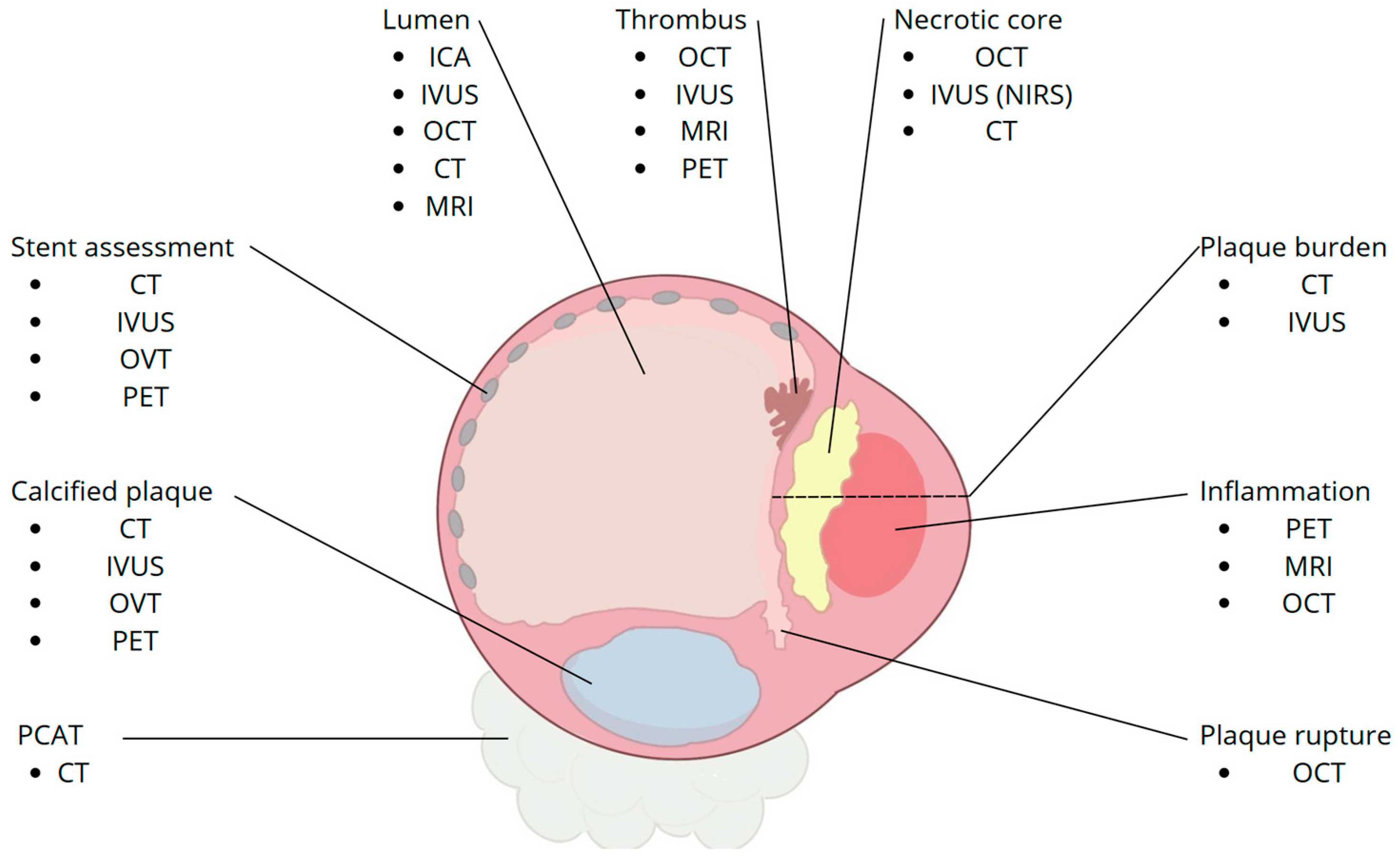
5. High-Risk Plaque (HRP) Features
5.1. OCT
5.2. IVUS
5.3. CCTA
6. Machine Learning Models for Automatic Coronary Arteries Segmentation
6.1. OCT
6.2. IVUS
6.3. CCTA
7. Machine Learning Models for Automatic Detection of Coronary Artery Plaque Vulnerability Features
7.1. OCT
7.2. IVUS
7.3. CCTA
8. Multimodality Imaging
9. Discussion
9.1. Pros and Cons of Each Modality
9.2. Clinical Consequences of Technical Constraints
9.3. Emerging Solutions—Beyond Single-Modality Silos
- Advanced deep learning architectures. Spatial–temporal encoder–decoders harness frame-to-frame continuity along OCT pullbacks, pushing Dice coefficients for calcification segmentation above 0.75 and F1-scores towards human concordance [48]. Transformer networks, inherently adept at long-range dependencies, now outperform classic CNNs for erosion detection (AUC 0.94 vs. 0.85) [58]. On CCTA, networks such as PlaqueNet and nnU-Net reach sensitivities > 0.90 for vulnerable plaque identification [56,63].
- Radiomics and explainable AI. High-order texture and shape descriptors extracted from CCTA provide hazard ratios around 2.0 for three-year MACE, eclipsing conventional stenosis metrics [46]. Saliency mapping and Bayesian uncertainty estimates are increasingly embedded in pipelines, granting clinicians a transparent window into model reasoning and confidence intervals—an essential step for regulatory endorsement [9,10,11,12].
- Multimodal fusion. Multimodal fusion is progressively redefining coronary plaque assessment by combining the complementary strengths of intravascular OCT, IVUS, CCTA, and spectroscopic imaging. Early demonstrations with co-registered IVUS–OCT datasets showed that deep learning fusion networks can surpass conventional classifiers in identifying fragile plaque features, largely by balancing OCT’s axial resolution with IVUS’s deeper penetration [67]. The addition of near-infrared spectroscopy brings chemical specificity that further refines lipid/necrotic core discrimination [68], while cross-domain frameworks such as OctPlus highlight how concurrent light–ultrasound inputs reduce modality-specific artifacts and improve annotation reliability [59]. Beyond morphology, the coupling of fused images with finite-element biomechanics has yielded more nuanced risk stratification, emphasizing the role of local wall stress in cap failure pathways [69]. Importantly, CCTA-based fusion models that integrate CT-derived FFR now approach invasive standards for plaque typing and physiological assessment, signalling the possibility of a truly non-invasive, single-session pathway from detection to therapy planning [70].
9.4. From Proof-of-Concept to Bedside—An Operational Roadmap
- Step 2: Curated, multicentre repositories. Datasets enriched with complementary ground-truth—histology for cap thickness, OCT for surface detail, IVUS for deep components—offer balanced training and unbiased validation.
- Step 4: External prospective validation. Embedding algorithms in multi-vendor, outcome-driven registries will clarify incremental prognostic value over SYNTAX, CAD-RADS, or calcium scoring.
9.5. Clinical Implications in Light of the PREVENT Trial
10. Limitations
11. Future Directions and Clinical Implications
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OCT | Optical coherence tomography |
| IVUS | Intravascular ultrasound |
| CCTA | Coronary computed tomography angiography |
| CAD | Coronary artery disease |
| ML | Machine learning |
| DL | Deep learning |
| AI | Artificial intelligence |
| HRP | High-risk plaque |
| MACE | Major adverse cardiovascular event(s) |
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Sama, C.; Abdelhaleem, A.; Velu, D.; Ditah Chobufo, M.; Fongwen, N.T.; Budoff, M.J.; Roberts, M.; Balla, S.; Mills, J.D.; Njim, T.N.; et al. Non-Calcified Plaque in Asymptomatic Patients with Zero Coronary Artery Calcium Score: A Systematic Review and Meta-Analysis. J. Cardiovasc. Comput. Tomogr. 2024, 18, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Gallone, G.; Bellettini, M.; Bruno, F.; Scudeler, L.; De Filippo, O.; Iannaccone, M.; Baldetti, L.; Kwon-Koo, B.; Pontone, G.; Depaoli, A.; et al. Coronary Plaque Characteristics Associated with Major Adverse Cardiovascular Events in Atherosclerotic Patients and Lesions—A Systematic Review and Meta-Analysis. Eur. Heart J. 2022, 43, ehac544.1291. [Google Scholar] [CrossRef]
- Nerlekar, N.; Ha, F.J.; Cheshire, C.; Rashid, H.; Cameron, J.D.; Wong, D.T.; Seneviratne, S.; Brown, A.J. Computed Tomographic Coronary Angiography–Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events. Circ. Cardiovasc. Imaging 2018, 11, e006973. [Google Scholar] [CrossRef] [PubMed]
- Ferencik, M.; Hoffmann, U. High-Risk Coronary Plaque on Computed Tomography Angiography. Circ. Cardiovasc. Imaging 2018, 11, e007288. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease. J. Am. Coll. Cardiol. 2023, 82, 833–955. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Barbero, U.; Cerrato, E.; Lipinski, M.J.; Omedè, P.; Montefusco, A.; Taha, S.; Naganuma, T.; Reith, S.; Voros, S.; et al. Accuracy of Intravascular Ultrasound and Optical Coherence Tomography in Identifying Functionally Significant Coronary Stenosis According to Vessel Diameter: A Meta-Analysis of 2,581 Patients and 2,807 Lesions. Am. Heart J. 2015, 169, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Saylik, F.; Hayiroglu, M.I.; Akbulut, T.; Cinar, T. A Comprehensive Network Meta-Analysis: Comparison of Long-Term Outcomes between Intravascular Ultrasound, Optical Coherence Tomography, and Angiography-Guided Stent Implantation. Eur. Heart J. 2023, 44, ehad655.2115. [Google Scholar] [CrossRef]
- Cau, R.; Flanders, A.; Mannelli, L.; Politi, C.; Faa, G.; Suri, J.S.; Saba, L. Artificial Intelligence in Computed Tomography Plaque Characterization: A Review. Eur. J. Radiol. 2021, 140, 109767. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Abdulkareem, M.; Leiner, T. Artificial Intelligence Will Transform Cardiac Imaging—Opportunities and Challenges. Front. Cardiovasc. Med. 2019, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial Intelligence in Cardiovascular Imaging. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Volpato, V.; Cau, R.; Chiesa, M.; Saba, L.; Guglielmo, M.; Senatieri, A.; Chierchia, G.; Pontone, G.; Dell’Aversana, S.; et al. Application of AI in Cardiovascular Multimodality Imaging. Heliyon 2022, 8, e10872. [Google Scholar] [CrossRef] [PubMed]
- Infante, T.; Cavaliere, C.; Punzo, B.; Grimaldi, V.; Salvatore, M.; Napoli, C. Radiogenomics and Artificial Intelligence Approaches Applied to Cardiac Computed Tomography Angiography and Cardiac Magnetic Resonance for Precision Medicine in Coronary Heart Disease: A Systematic Review. Circ. Cardiovasc. Imaging 2021, 14, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Virk, H.U.H.; Bangalore, S.; Wang, Z.; Johnson, K.W.; Pinotti, R.; Zhang, H.; Kaplin, S.; Narasimhan, B.; Kitai, T.; et al. Machine Learning Prediction in Cardiovascular Diseases: A Meta-Analysis. Sci. Rep. 2020, 10, 16057. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.J.H.; Hanif, M.A.; Alradhawi, M.; Ibbetson, J.; Dosanjh, S.S.; Smith, S.L.; Ahmad, M.; Pimenta, D. Machine Learning Applications in Cardiac Computed Tomography: A Composite Systematic Review. Eur. Heart J. Open 2022, 2, oeac018. [Google Scholar] [CrossRef] [PubMed]
- Quer, G.; Arnaout, R.; Henne, M.; Arnaout, R. Machine Learning and the Future of Cardiovascular Care. J. Am. Coll. Cardiol. 2021, 77, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Theroux, P. Pathophysiology of Coronary Artery Disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Boudoulas, K.D.; Triposkiadis, F.; Geleris, P.; Boudoulas, H. Coronary Atherosclerosis: Pathophysiologic Basis for Diagnosis and Management. Prog. Cardiovasc. Dis. 2016, 58, 676–692. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Teng, Z.; Evans, P.C.; Gillard, J.H.; Samady, H.; Bennett, M.R. Role of Biomechanical Forces in the Natural History of Coronary Atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Purushothaman, K.R.; Fuster, V.; Echeverri, D.; Truszczynska, H.; Sharma, S.K.; Badimon, J.J.; O’Connor, W.N. Plaque Neovascularization Is Increased in Ruptured Atherosclerotic Lesions of Human Aorta. Circulation 2004, 110, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Wörner, A.; Urschel, K.; Beronov, K.; Goppelt-Struebe, M.; Verhoeven, E.; Daniel, W.G.; Garlichs, C.D. Carotid Plaque Vulnerability. Stroke 2011, 42, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Varnava, A.M.; Mills, P.G.; Davies, M.J. Relationship Between Coronary Artery Remodeling and Plaque Vulnerability. Circulation 2002, 105, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Terashima, M.; Matsuo, H.; Kinoshita, Y.; Kimura, M.; Tsuchikane, E.; Nasu, K.; Ehara, M.; Asakura, Y.; Katoh, O.; et al. Association of Coronary Plaque Composition and Arterial Remodelling: A Optical Coherence Tomography Study. Atherosclerosis 2012, 221, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Shah, P.K.; Fuster, V. Coronary Plaque Disruption. Circulation 1995, 92, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.H.; Libby, P.; Boden, W.E. Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management—The Plaque Hypothesis. JAMA Cardiol. 2023, 8, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lv, Y.; Bai, X.; Qi, J.; Weng, X.; Liu, S.; Bao, X.; Jia, H.; Yu, B. Plaque Erosion: A Distinctive Pathological Mechanism of Acute Coronary Syndrome. Front. Cardiovasc. Med. 2021, 8, 711453. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons From Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A Definition of Initial, Fatty Streak, and Intermediate Lesions of Atherosclerosis. A Report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994, 89, 2462–2478. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Kramer, M.C.A.; Woudstra, P.; Yahagi, K.; Ladich, E.; Finn, A.V.; de Winter, R.J.; Kolodgie, F.D.; Wight, T.N.; Davis, H.R.; et al. Natural Progression of Atherosclerosis from Pathologic Intimal Thickening to Late Fibroatheroma in Human Coronary Arteries: A Pathology Study. Atherosclerosis 2015, 241, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Morbiducci, U.; Kok, A.M.; Kwak, B.R.; Stone, P.H.; Steinman, D.A.; Wentzel, J.J. Atherosclerosis at Arterial Bifurcations: Evidence for the Role of Haemodynamics and Geometry. Thromb. Haemost. 2016, 115, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Karino, T. Flow Patterns and Spatial Distribution of Atherosclerotic Lesions in Human Coronary Arteries. Circ. Res. 1990, 66, 1045–1066. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, L.; Holm, J.; Skalli, O.; Bondjers, G.; Hansson, G.K. Regional Accumulations of T Cells, Macrophages, and Smooth Muscle Cells in the Human Atherosclerotic Plaque. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1986, 6, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and Growth of Extracellular-Vesicle-Derived Microcalcification in Atherosclerotic Plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.I.; Mitchell, T.M. Machine Learning: Trends, Perspectives, and Prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, T.; Tiwari, T.; Tiwari, S. How Artificial Intelligence, Machine Learning and Deep Learning Are Radically Different? Int. J. Adv. Res. Comput. Sci. Softw. Eng. 2018, 8, 1. [Google Scholar] [CrossRef]
- Leiner, T.; Rueckert, D.; Suinesiaputra, A.; Baeßler, B.; Nezafat, R.; Išgum, I.; Young, A.A. Machine Learning in Cardiovascular Magnetic Resonance: Basic Concepts and Applications. J. Cardiovasc. Magn. Reson. 2019, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Benedek, T.; Mester, A.; Benedek, A.; Rat, N.; Opincariu, D.; Chițu, M. Assessment of Coronary Plaque Vulnerability in Acute Coronary Syndromes Using Optical Coherence Tomography and Intravascular Ultrasound. A Syst. Review. J. Of. Cardiovasc. Emergencies 2016, 2, 173–184. [Google Scholar] [CrossRef]
- Nakazato, R.; Otake, H.; Konishi, A.; Iwasaki, M.; Koo, B.-K.; Fukuya, H.; Shinke, T.; Hirata, K.-I.; Leipsic, J.; Berman, D.S.; et al. Atherosclerotic Plaque Characterization by CT Angiography for Identification of High-Risk Coronary Artery Lesions: A Comparison to Optical Coherence Tomography. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Gatto, L.; Romagnoli, E.; Limbruno, U.; Fineschi, M.; Marco, V.; Albertucci, M.; Tamburino, C.; Crea, F.; Alfonso, F.; et al. In Vivo Vulnerability Grading System of Plaques Causing Acute Coronary Syndromes: An Intravascular Imaging Study. Int. J. Cardiol. 2018, 269, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Maehara, A.; Mintz, G.S.; Weissman, N.J. Advances in Intravascular Imaging. Circ. Cardiovasc. Interv. 2009, 2, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Raffel, O.C.; Merchant, F.M.; Tearney, G.J.; Chia, S.; Gauthier, D.D.; Pomerantsev, E.; Mizuno, K.; Bouma, B.E.; Jang, I.-K. In Vivo Association between Positive Coronary Artery Remodelling and Coronary Plaque Characteristics Assessed by Intravascular Optical Coherence Tomography. Eur. Heart J. 2008, 29, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.C.; Leipsic, J.; Abbara, S.; Achenbach, S.; Berman, D.; Bittencourt, M.; Budoff, M.; Chinnaiyan, K.; Choi, A.D.; Ghoshhajra, B.; et al. CAD-RADSTM 2.0—2022 Coronary Artery Disease—Reporting and Data System An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America Society of Cardiovascular Imaging (NASCI). Radiol. Cardiothorac. Imaging 2022, 4, e220183. [Google Scholar] [CrossRef] [PubMed]
- Kolossváry, M.; Park, J.; Bang, J.-I.; Zhang, J.; Lee, J.M.; Paeng, J.C.; Merkely, B.; Narula, J.; Kubo, T.; Akasaka, T.; et al. Identification of Invasive and Radionuclide Imaging Markers of Coronary Plaque Vulnerability Using Radiomic Analysis of Coronary Computed Tomography Angiography. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.; Guo, X.; Molony, D.; Guo, X.; Samady, H.; Giddens, D.P.; Athanasiou, L.; Nie, R.; Cao, J.; et al. Convolution Neural Networks and Support Vector Machines for Automatic Segmentation of Intracoronary Optical Coherence Tomography. Mol. Cell. Biomech. 2019, 16, 153–161. [Google Scholar] [CrossRef]
- Li, C.; Jia, H.; Tian, J.; He, C.; Lu, F.; Li, K.; Gong, Y.; Hu, S.; Yu, B.; Wang, Z. Comprehensive Assessment of Coronary Calcification in Intravascular OCT Using a Spatial-Temporal Encoder-Decoder Network. IEEE Trans. Med. Imaging 2022, 41, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Gharaibeh, Y.; Kolluru, C.; Zimin, V.N.; Dallan, L.A.P.; Kim, J.N.; Bezerra, H.G.; Wilson, D.L. Segmentation of Coronary Calcified Plaque in Intravascular OCT Images Using a Two-Step Deep Learning Approach. IEEE Access 2020, 8, 225581–225593. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Mintz, G.S.; Dohi, T.; Li, W.; Shang, A.; Fall, K.; Sato, T.; Sugizaki, Y.; Chatzizisis, Y.S.; Moses, J.W.; et al. Accuracy of IVUS-Based Machine Learning Segmentation Assessment of Coronary Artery Dimensions and Balloon Sizing. JACC Adv. 2023, 2, 100564. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xia, Y.; Zhang, Y. Supervised Machine Learning for Coronary Artery Lumen Segmentation in Intravascular Ultrasound Images. Int. J. Numer. Method. Biomed. Eng. 2020, 36, e3348. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, R.; Huang, X.; Kilic, Y.; Ramasamy, A.; Jain, A.; Ozkor, M.; Tufaro, V.; Safi, H.; Erdogan, E.; Serruys, P.W.; et al. Advanced Deep Learning Methodology for Accurate, Real-Time Segmentation of High-Resolution Intravascular Ultrasound Images. Int. J. Cardiol. 2021, 339, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Xu, S.; Dai, D.; Zhang, Y.; Zhang, C.; Li, Z. A Novel Multi-Attention, Multi-Scale 3D Deep Network for Coronary Artery Segmentation. Med. Image Anal. 2023, 85, 102745. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Antón, B.; Villa, M.I.; Pendón-Minguillón, S.; Paramés-Estévez, S.; Otero-Cacho, A.; López-Otero, D.; Díaz-Fernández, B.; Bastos-Fernández, M.; González-Juanatey, J.R.; Muñuzuri, A.P. Unsupervised Clustering Based Coronary Artery Segmentation. BioData Min. 2025, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Nannini, G.; Saitta, S.; Baggiano, A.; Maragna, R.; Mushtaq, S.; Pontone, G.; Redaelli, A. A Fully Automated Deep Learning Approach for Coronary Artery Segmentation and Comprehensive Characterization. APL Bioeng. 2024, 8, 016103. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Tian, C.; Chen, S.; Deng, Y.; Liao, X.; Wang, Q.; Si, W. PlaqueNet: Deep Learning Enabled Coronary Artery Plaque Segmentation from Coronary Computed Tomography Angiography. Vis. Comput. Ind. Biomed. Art. 2024, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, J.; Yin, Y.; Li, Z. Automated Classification of Coronary Plaque Calcification in OCT Pullbacks with 3D Deep Neural Networks. J. Biomed. Opt. 2020, 25, 095003. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Araki, M.; Nakajima, A.; Lee, H.; Fuster, V.; Ye, J.C.; Jang, I.K. Enhanced Diagnosis of Plaque Erosion by Deep Learning in Patients with Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2022, 15, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ninomiya, K.; Tu, S.; Masuda, S.; Dijkstra, J.; Chu, M.; Ding, D.; Hynes, S.O.; O’Leary, N.; Wijns, W.; et al. Calcified Plaque Detected on OCT with Deep Learning and Cross-Validated with Optical and Ultrasound Signals: A Complementary Appraisal and Preamble to Combined IVUS-OCT Catheter. Front. Photonics 2022, 3, 1019552. [Google Scholar] [CrossRef]
- Cho, H.; Kang, S.J.; Min, H.S.; Lee, J.G.; Kim, W.J.; Kang, S.H.; Kang, D.Y.; Lee, P.H.; Ahn, J.M.; Park, D.W.; et al. Intravascular Ultrasound-Based Deep Learning for Plaque Characterization in Coronary Artery Disease. Atherosclerosis 2021, 324, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Shen, T.Y.; Chen, C.C.; Chang, W.T.; Lee, P.Y.; Huang, C.C.J. Automatic Detection of Atherosclerotic Plaque and Calcification from Intravascular Ultrasound Images by Using Deep Convolutional Neural Networks. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, T.; Wang, Y.N.; Schoepf, U.J.; Bidwell, S.L.; Qiao, H.; Feng, Y.; Xu, C.; Xu, H.; Xie, G.; et al. A Coronary CT Angiography Radiomics Model to Identify Vulnerable Plaque and Predict Cardiovascular Events. Radiology 2023, 307, e221693. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Deep Learning-Based Quantitative Image Analysis for Detecting Coronary Artery Stenosis, Calcification, and Vulnerable Plaque in Coronary Computed Tomography Angiography. Eur. Heart J. 2023, 44, ehad655.145. [Google Scholar] [CrossRef]
- Buckler, A.J.; Gotto, A.M.; Rajeev, A.; Nicolaou, A.; Sakamoto, A.; St Pierre, S.; Phillips, M.; Virmani, R.; Villines, T.C. Atherosclerosis Risk Classification with Computed Tomography Angiography: A Radiologic-Pathologic Validation Study. Atherosclerosis 2023, 366, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Al’Aref, S.J.; Singh, G.; Choi, J.W.; Xu, Z.; Maliakal, G.; van Rosendael, A.R.; Lee, B.C.; Fatima, Z.; Andreini, D.; Bax, J.J.; et al. A Boosted Ensemble Algorithm for Determination of Plaque Stability in High-Risk Patients on Coronary CTA. JACC Cardiovasc. Imaging 2020, 13, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Koo, B.K.; Hoshino, M.; Lee, J.M.; Murai, T.; Park, J.; Zhang, J.; Hwang, D.; Shin, E.S.; Doh, J.H.; et al. CT Angiographic and Plaque Predictors of Functionally Significant Coronary Disease and Outcome Using Machine Learning. JACC Cardiovasc. Imaging 2021, 14, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Kang, S.J.; Kim, G.; Lee, J.G.; Min, H.S.; Cho, H.; Kang, D.Y.; Lee, P.H.; Ahn, J.M.; Park, D.W.; et al. Prediction of Coronary Thin-Cap Fibroatheroma by Intravascular Ultrasound-Based Machine Learning. Atherosclerosis 2019, 288, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, R.; Parasa, R.; Broersen, A.; Johnson, T.; Garg, M.; Prati, F.; Çap, M.; Yap, N.A.L.; Karaduman, M.; Busk, C.A.G.R.; et al. Examination of the Performance of Machine Learning-Based Automated Coronary Plaque Characterization by near-Infrared Spectroscopy–Intravascular Ultrasound and Optical Coherence Tomography with Histology. Eur. Heart J. Digit. Health 2025, 6, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Wang, L.; Maehara, A.; Matsumura, M.; Guo, X.; Samady, H.; Giddens, D.P.; Zheng, J.; Mintz, G.S.; Tang, D. Combining IVUS + OCT Data, Biomechanical Models and Machine Learning Method for Accurate Coronary Plaque Morphology Quantification and Cap Thickness and Stress/Strain Index Predictions. J. Funct. Biomater. 2023, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Z.; Chen, T.; Liu, S.; Tan, J.; Sun, Y.; Feng, L.; Zhang, D.; Ma, L.; Liu, H.; et al. Artificial Intelligence Driven Plaque Characterization and Functional Assessment from CCTA Using OCT-Based Automation: A Prospective Study. Int. J. Cardiol. 2025, 428, 133140. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Stuckey, T.D.; Nakazawa, G.; Ahmadi, A.; Matsumura, M.; Petersen, K.; Mirza, S.; Ng, N.; Mullen, S.; Schaap, M.; et al. Prospective Deep Learning-Based Quantitative Assessment of Coronary Plaque by Computed Tomography Angiography Compared with Intravascular Ultrasound: The REVEALPLAQUE Study. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Pinna, A.; Faa, G.; Balestrieri, A.; Politi, C.; Coghe, F.; Suri, J.S. The Evolution from Qualitative to Quantitative Analysis in Medical Sciences: A Historical Perspective and the Impact of AI. Imaging 2025, 17, 15–25. [Google Scholar] [CrossRef]
- Park, S.J.; Ahn, J.M.; Kang, D.Y.; Yun, S.C.; Ahn, Y.K.; Kim, W.J.; Nam, C.W.; Jeong, J.O.; Chae, I.H.; Shiomi, H.; et al. Preventive Percutaneous Coronary Intervention versus Optimal Medical Therapy Alone for the Treatment of Vulnerable Atherosclerotic Coronary Plaques (PREVENT): A Multicentre, Open-Label, Randomised Controlled Trial. Lancet 2024, 403, 1753–1765. [Google Scholar] [CrossRef] [PubMed]


| AHA Classification | Virmani et al. Classification | Key Differences |
|---|---|---|
| Type I—Initial lesion | Intimal xanthoma | Both describe early foam cell accumulation. Virmani uses a standard pathological term. |
| Type II—Fatty streak | Intimal xanthoma | Virmani emphasizes its potential regression and uses more precise terminology. |
| Type III—Intermediate lesion | Pathological intimal thickening | Describes lesions with extracellular lipids but no necrosis; emphasizes morphologic criteria. |
| Type IV—Atheroma | Fibrous cap atheroma | Focuses on the presence of a necrotic core and a well-formed fibrous cap. |
| Type V (Va, Vb, Vc)—Fibroatheroma/calcified/fibrotic | Fibrous cap atheroma/fibrocalcific plaque | Virmani simplifies subdivisions with descriptive terms based on morphology and stability. |
| Type VI—Complicated lesion | Plaque rupture/erosion/calcified nodule | Virmani differentiates mechanisms leading to thrombosis instead of grouping all under “complicated”. |
| Model/Family | Advantages | Disadvantages | Adaptability |
|---|---|---|---|
| Random Forest/SVM | Easy to interpret; train quickly on small datasets | Limited performance on raw imaging; needs feature engineering | High—retune hyperparameters only |
| 2D-CNN | High accuracy on single-frame OCT/IVUS; mature libraries | Requires datasets > 103 images; needs GPU | Medium—fine-tune with a small site-specific set |
| 3D/2.5D CNN (U-Net, SegNet) | Ensures spatial consistency; Dice ≈ 0.9 for segmentation | High RAM usage; overfitting risk on small cohorts | Good after pre-training on multicentre databases |
| Transformer | Captures long-range context; AUC > 0.9 for erosion detection | GPU/TPU intensive; needs > 104 examples | Emerging—promising with transfer learning |
| GAN | Powerful for data augmentation; real-time IVUS inference | Training instability; sensitive hyper-parameter tuning | High for augmenting new cohorts |
| Ensemble/Hybrid | Improves multicentre robustness | Complex pipeline; harder to debug | High—retrain component models only |
| Feature Type | OCT | IVUS | CCTA |
|---|---|---|---|
| Thin-cap fibroatheroma | High resolution (10–20 μm) for accurate measurement | Limited resolution (100–200 μm) for cap thickness | Indirect detection through napkin-ring sign |
| Lipid content | High accuracy in detecting lipid-rich plaques | Moderate accuracy, improved with VH-IVUS | Low attenuation plaque as a surrogate marker |
| Fibrous cap thickness | Precise measurement capability | Limited by resolution | Not directly measurable |
| Macrophage infiltration | Capable of detecting and quantifying | Limited capability | Not directly detectable |
| Positive remodelling | Detectable, but limited field of view | Excellent capability | Detectable and quantifiable |
| Spotty calcification | High-resolution detection | Detectable | Detectable, size-limited definition |
| Microchannels | Unique capability to detect | No mention found | No mention found |
| Authors | Year | N | Imaging Modality | Variables | ML Models | Performance Metrics | Validation Method | Key Results |
|---|---|---|---|---|---|---|---|---|
| Zhang C., et al. [47] | 2019 | 5 | OCT, IVUS | Lumen border, plaque components (lipid, fibrous, background) | CNN (U-Net), SVM | Classification accuracy (CNN: 95.8%, SVM: 71.9%) | 11-fold cross-validation | CNN provided superior segmentation performance; significantly reduced manual effort |
| Li C., et al. [48] | 2022 | 45 * | IVOCT | Calcification area, depth, angle, thickness, volume, calcification score | 2.5D U-Net, DenseNet | Dice coefficient (0.756 ± 0.222), F1-score (0.883 ± 0.008), Precision (0.964 ± 0.002) | Comparison with human-level inter-observer agreement | Accurate and efficient segmentation and classification of calcification with near-human agreement |
| Lee J., et al. [49] | 2020 | 68 † | IVOCT | Major calcification lesions | 3D CNN + SegNet | F1-score (0.781), Sensitivity (86.2%), Precision (75.8%) | Comparison with one-step approach; reproducibility tests | Two-step deep learning improved performance and reproducibility, enabling real-time planning |
| Matsumura M., et al. [50] | 2023 | 326 ‡ | IVUS | Lumen and vessel dimensions, stent area, balloon size | U-Net | IoU (0.92–0.94), DSC (0.96–0.97), Correlation (0.991–0.993) | Expert comparison, independent test set | High correlation with experts; 92.4% agreement on balloon size, >85% agreement on lumen/stent area |
| Cui H., et al. [51] | 2020 | 3 § | IVUS | Lumen area and contour | Gradient Boosting with handcrafted features | Jaccard similarity (96.8%), Mean error distance (0.55) | IVUS Challenge benchmark + manual annotation comparison | Outperformed other ML methods; highly accurate lumen segmentation with minimal error |
| Bajaj R., et al. [52] | 2021 | 65 ¶ | IVUS (NIRS-IVUS) | EEM and lumen borders, plaque area | Pix2Pix GAN + ResNet | Mean difference ≤ 0.23 mm2; DSC (0.96–0.98), IOU (0.92–0.96) | Compared to two expert analysts; Williams Index (0.75–1.06) | Real-time DL segmentation as accurate as experts; robust even in complex images |
| Dong C., et al. [53] | 2023 | 119 | CCTA | Coronary artery anatomy | CAS-Net (multi-attention, multi-scale 3D network) | DSC: improvement of at least 4% over U-Net3D | Comparison with three public datasets + self-collected data | Outperformed 14 state-of-the-art methods; strong generalization and accuracy |
| Serrano-Antón B., et al. [54] | 2025 | 32 | CCTA | Coronary arteries | Ward Clustering (3Axis, Perp), VGG-19, ResNet-50, EfficientNet-b2, U-Net++, 3D U-Net, Swin UNETR | Dice (0.88 test, 0.83 lesion), IoU, Precision, Recall | Manual annotation comparison on 10 test and 22 lesion cases | 3Axis clustering comparable to advanced DL models; Swin UNETR best Dice (0.8978) but more complex |
| Nannini G., et al. [55] | 2024 | 324 ** | CCTA | Coronary artery segmentation, CAC, tortuosity | 2.5D U-Net + 3D U-Net cascaded | DSC (0.895), Surface distance (0.47 mm), Precision (93.5%) | Manual annotations, test set inference | Accurate segmentation of stenotic regions, robust CAC and CorT extraction |
| Wang L., et al. [56] | 2024 | NS †† | CCTA | Coronary plaques | PlaqueNet (AResNet + DASPP-BICECA + BINet) | IoU: 87.37%, Dice: 93.26%, Mean Dice: 96.63% | Comparison with 3 other models | Highest performance among tested models; sensitive and precise plaque detection |
| Authors | Year | N | Imaging Modality | Variables | ML Models | Performance Metrics | Validation Method | Key Results |
|---|---|---|---|---|---|---|---|---|
| He C., et al. [57] | 2020 | 24 * | OCT | Calcified vs. non-calcified plaque | ResNet-3D, ResNet-2D | F1-score (up to 96%) | 10 runs with cross-validation, split into train/validation/test | ResNet-3D outperformed ResNet-2D; 3D model achieved up to 96% F1-score |
| Park J., et al. [58] | 2022 | 873 † | OCT | Plaque erosion | CNN, transformer | AUC, Sensitivity, Specificity, PPV, NPV | Internal and external validation | Transformer model outperformed CNN (AUC 0.94 vs. 0.85 externally) |
| Li C., et al. [48] | 2022 | 45 ‡ | IVOCT | Calcification segmentation and classification | Spatial–temporal encoder–decoder + DenseNet | Dice coefficient, Precision, F1-score | Comparative study vs. other methods | F1-score improved from 0.791 to 0.883; Dice from 0.615 to 0.756 |
| Huang J., et al. [59] | 2022 | 15 ¶ | OCT + IVUS | Calcified plaques (pure, hybrid) | OCT-DL (OctPlus), optical and ultrasound validation | Kappa statistics, ICC for arc measurements | Cross-validation with IVUS and optical signals | Substantial agreement (kappa > 0.69); ICC up to 0.81 |
| Cho H., et al. [60] | 2021 | 598 †† | IVUS | Attenuated plaque, calcified plaque | EfficientNet (ensemble of 5 models) | Dice, Accuracy, Sensitivity, Specificity | 5-fold cross-validation, test on separate set | Frame-level accuracy: 93% (attenuation), 96% (calcification); Dice up to 0.84 |
| Li YC., et al. [61] | 2021 | 18 ‡‡ | IVUS | Media–adventitia border, lumen, calcified plaque | Cascaded modified U-Nets | Dice, Precision, Sensitivity, Specificity, AP | Leave-one-subject-out cross-validation | Calcification AP up to 0.73; Dice for media/lumen > 0.90; superior to VH-IVUS in artifact conditions |
| Chen Q.., et al. [62] | 2023 | 933 §§ | CCTA + IVUS (reference) | Vulnerable plaques, MACE risk | XGBoost (radiomic signature) | AUC, Hazard ratio | Internal and external test sets, Cox regression for MACE | AUC: 0.81–0.77 (train to external test); HR for MACE: 2.01; validated in multicenter cohort |
| Kim D. [63] | 2023 | 3747 *** | CCTA | Stenosis, calcification, vulnerable plaques | 2D nnU-Net segmentation | Correlation (QSI vs. QCA), Sensitivity, Specificity | Expert annotation and independent test | Sensitivity 0.929, Specificity 0.910 for VP detection |
| Buckler AJ., et al. [64] | 2023 | 53 ‡‡‡ | CCTA + Histopathology | Plaque phenotype (stable/unstable/minimal) | ML classifier with ResNet-18 architecture | AUC, Kappa | Radiologic-pathologic validation | AUC: 0.97 (unstable), 0.95 (stable); Kappa = 0.82 |
| Al’Aref SJ., et al. [65] | 2020 | 468 §§§ | CCTA + ICA | Culprit lesion prediction | Boosted ensemble (XGBoost) | AUC | 10-fold cross-validation, test set | AUC: 0.77 (ML) vs. 0.60–0.67 (traditional); specificity: 89.3% |
| Yang S., et al. [66] | 2021 | 1013 (vessels) | CCTA | Boruta + hierarchical clustering | HR | Prospective registry with FFR | HR 5.43 when ≥4/6 features |
| Author | Year | N | Imaging Modality | Clinical/Technical End-Point | ML Models | Performance Metrics | Validation Method |
|---|---|---|---|---|---|---|---|
| Bae Y., et al. [67] | 2019 | 517 lesions/40 908 frames | IVUS → OCT (label) | Prediction of OCT-TCFA | ANN, SVM, NB | Accuracy 82%, AUC 0.82 | 5-fold CV + independent test set |
| Bajaj R., et al. [68] | 2025 | 131 NIRS-IVUS + 184 OCT/histology pairs | NIRS-IVUS + OCT + histology | Plaque component quantification | Dedicated ML classifiers | Accuracy 83% (NIRS-IVUS), CCC 0.88 | Cadaveric histology reference |
| Huang J., et al. [59] | 2022 | 15 pts/72 slices | OCT-DL + IVUS + optical props. | Calcified plaque detection | OctPlus DL | κ 0.77; ICC 0.81; agreement > 90% | Cross-validation with IVUS and optics |
| Lv R., et al. [69] | 2023 | 10 pts/228 slices | IVUS + OCT + biomechanics | Prediction of vulnerability index change | Random Forest | Accuracy 90.3%, AUC 0.877 | 5-fold CV, baseline vs. follow-up |
| Han J., et al. [70] | 2025 | 100 pts | CCTA + OCT (+QFR) | Plaque type and CT-FFR | Multi-stage CNN | AUC 0.98 (>50% stenosis); ICC 0.91 (MLD) | Hold-out test; comparison with manual OCT and QFR |
| Narula J., et al. [71] | 2024 | 237 pts | CCTA ↔ IVUS | Plaque volume agreement | AI-QCPA DL | TPV r 0.91; slope 0.99 | Prospective, 15-centre |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, A.; Boi, A.; Mannelli, L.; Balestrieri, A.; Sanfilippo, R.; Suri, J.; Saba, L. Machine Learning for Coronary Plaque Characterization: A Multimodal Review of OCT, IVUS, and CCTA. Diagnostics 2025, 15, 1822. https://doi.org/10.3390/diagnostics15141822
Pinna A, Boi A, Mannelli L, Balestrieri A, Sanfilippo R, Suri J, Saba L. Machine Learning for Coronary Plaque Characterization: A Multimodal Review of OCT, IVUS, and CCTA. Diagnostics. 2025; 15(14):1822. https://doi.org/10.3390/diagnostics15141822
Chicago/Turabian StylePinna, Alessandro, Alberto Boi, Lorenzo Mannelli, Antonella Balestrieri, Roberto Sanfilippo, Jasjit Suri, and Luca Saba. 2025. "Machine Learning for Coronary Plaque Characterization: A Multimodal Review of OCT, IVUS, and CCTA" Diagnostics 15, no. 14: 1822. https://doi.org/10.3390/diagnostics15141822
APA StylePinna, A., Boi, A., Mannelli, L., Balestrieri, A., Sanfilippo, R., Suri, J., & Saba, L. (2025). Machine Learning for Coronary Plaque Characterization: A Multimodal Review of OCT, IVUS, and CCTA. Diagnostics, 15(14), 1822. https://doi.org/10.3390/diagnostics15141822







