Apocrine Breast Carcinoma with Thanatosomes (Hyaline Globules)
Abstract
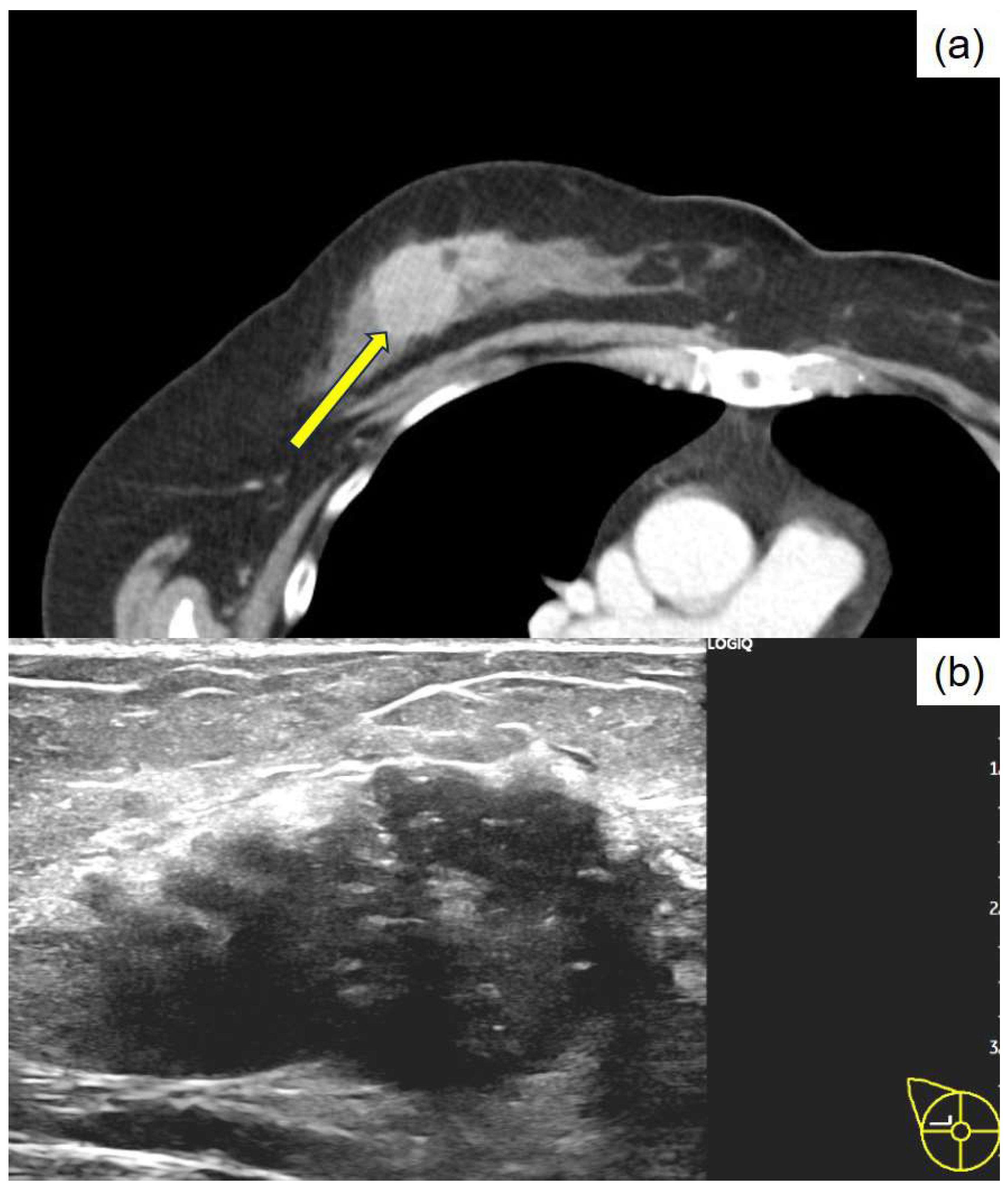





Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HG | Hyaline globule |
| HER2 | Human epidermal growth factor receptor type 2 |
References
- Papadimitriou, J.C.; Drachenberg, C.B.; Brenner, D.S.; Newkirk, C.; Trump, B.F.; Silverberg, S.G. “Thanatosomes”: A unifying morphogenetic concept for tumor hyaline globules related to apoptosis. Hum. Pathol. 2000, 31, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Dikov, D.I.; Auriault, M.L.; Boivin, J.F.; Sarafian, V.S.; Papadimitriou, J.C. Hyaline globules (thanatosomes) in gastrointestinal epithelium. Pathophysiologic correlations. Am. J. Clin. Pathol. 2007, 127, 792–799. [Google Scholar] [CrossRef] [PubMed]
- D’Alfonso, T.M.; Ginter, P.S.; Salvatore, S.P.; Antonio, L.B.; Hoda, S.A. Phylloides tumor with numerous thanatosomes (“death bodies”): A report of two cases and a study of thanatosomes in breast tumors. Int. J. Surg. Pathol. 2014, 22, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ozerdem, U.; Wells, J.; Hoda, S.A. Hyaline globules in mammary myofibroblastoma: A case report. Int. J. Surg. Pathol. 2015, 23, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Panicker, N.K.; Buch, A.C.; Patel, A.R. Breast carcinoma with numerous large “thanatosomes”. J. Cancer Res. Ther. 2015, 11, 980–982. [Google Scholar] [PubMed]
- Bezić, J. Invasive breast carcinoma with hyaline globules (“thanatosomes”). Breast Dis. 2020, 39, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.C.; Sandhya, B.N.; Swami, S.Y. Invasive lobular carcinoma of breast with hyaline globules (Thanatosomes). Int. J. Clin. Diagn. Pathol. 2021, 4, 171–172. [Google Scholar]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Koreyasu, R.; Kamimura, K.; Tsutsumi, Y. Pancreatic intraductal papillary mucinous neoplasm with hyaline globules (Thanatosomes): Report of two cases. Int. Med. Case Rep. J. 2021, 14, 393–399. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachibana, M.; Nozawa, M.; Isono, T.; Tsukamoto, K.; Kamimura, K. Apocrine Breast Carcinoma with Thanatosomes (Hyaline Globules). Diagnostics 2025, 15, 1768. https://doi.org/10.3390/diagnostics15141768
Tachibana M, Nozawa M, Isono T, Tsukamoto K, Kamimura K. Apocrine Breast Carcinoma with Thanatosomes (Hyaline Globules). Diagnostics. 2025; 15(14):1768. https://doi.org/10.3390/diagnostics15141768
Chicago/Turabian StyleTachibana, Mitsuhiro, Masashi Nozawa, Tadahiro Isono, Kei Tsukamoto, and Kazuyasu Kamimura. 2025. "Apocrine Breast Carcinoma with Thanatosomes (Hyaline Globules)" Diagnostics 15, no. 14: 1768. https://doi.org/10.3390/diagnostics15141768
APA StyleTachibana, M., Nozawa, M., Isono, T., Tsukamoto, K., & Kamimura, K. (2025). Apocrine Breast Carcinoma with Thanatosomes (Hyaline Globules). Diagnostics, 15(14), 1768. https://doi.org/10.3390/diagnostics15141768





