Diagnostics and Group Therapy in Patients with Persistent Postural-Perceptual Dizziness and Anxiety Disorder: Biomarkers and Neurofunctional Correlates of Underlying Treatment Effects
Abstract
1. Introduction
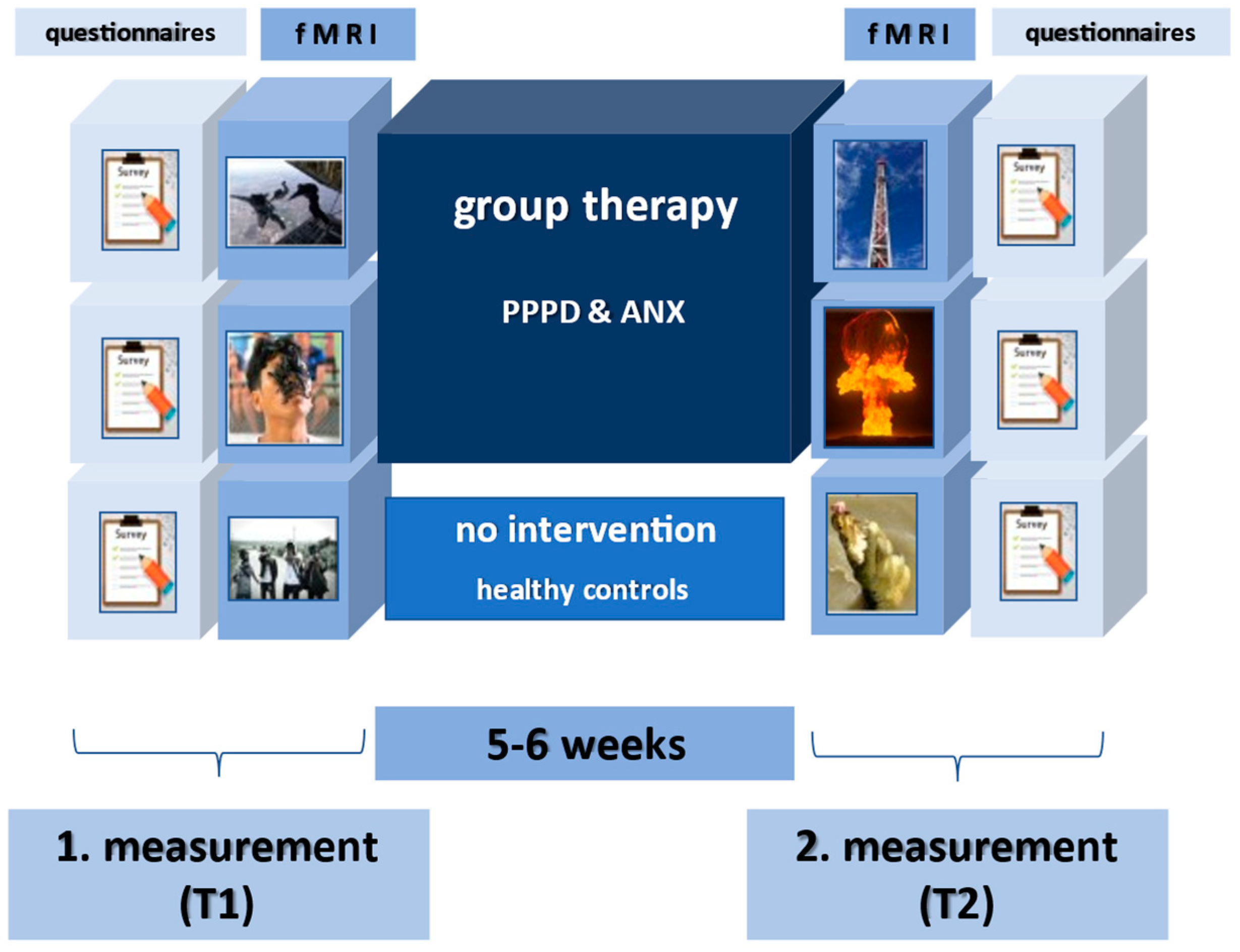
2. Methods
2.1. Sample
2.2. Interventions
2.3. Paradigm
2.4. Psychometric Questionnaires
2.5. MRI and fMRI Data Acquisition, Pre- and Post-Processing and Statistical Analysis
2.6. Analysis of Regions of Interest and Psychometric Data
3. Results
3.1. Comparison of Psychometric Data Between PPPD, ANX and Their Particular Control Group Before and After Therapy
3.2. Questionnaires in a Before-and-After Comparison
3.3. ROI Analysis Before and After Therapy
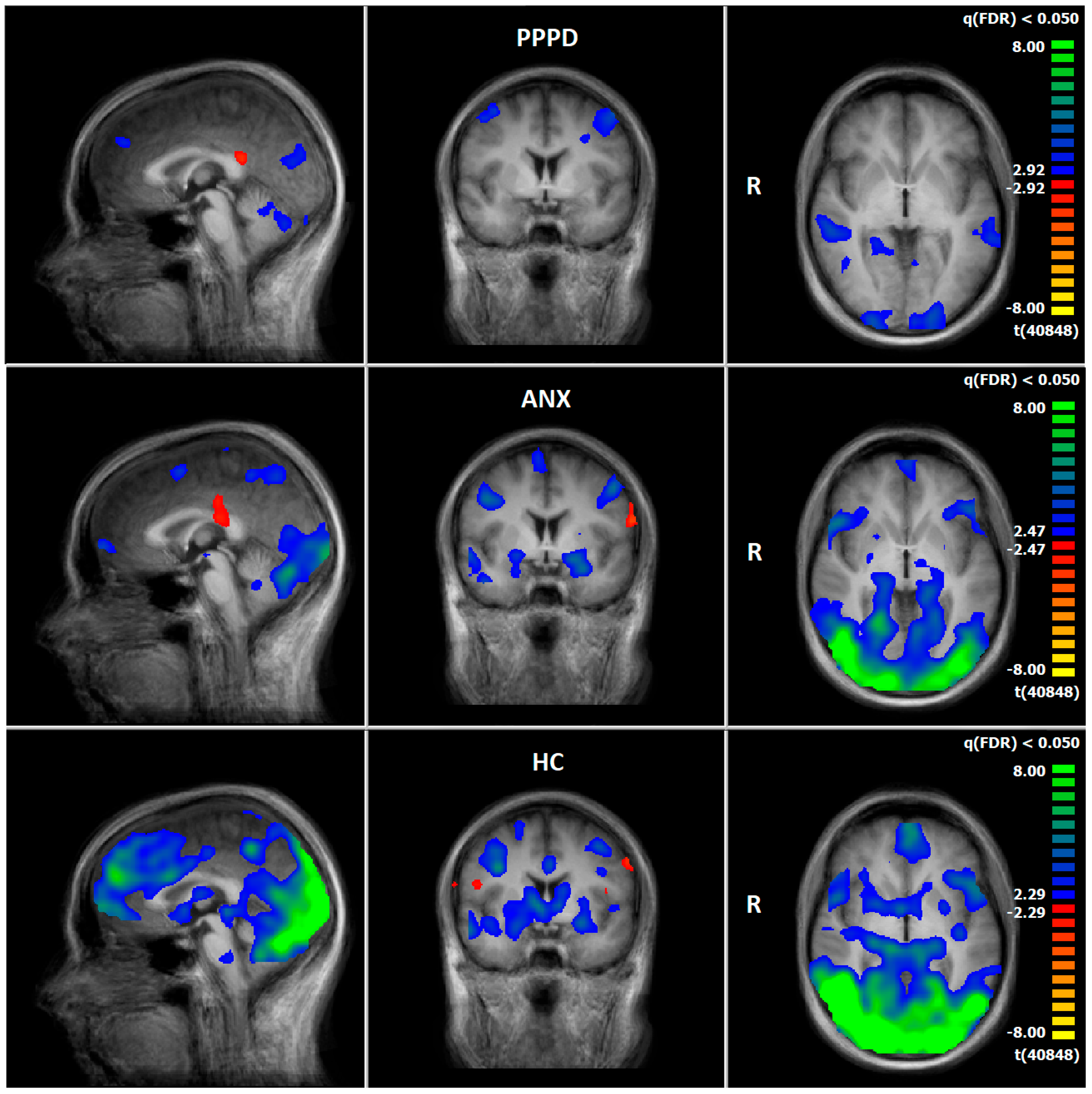
3.4. Comparison of Neuronal Responses Before and After Therapy—PPPD
3.5. Comparison of Neuronal Responses Before and After Therapy—ANX
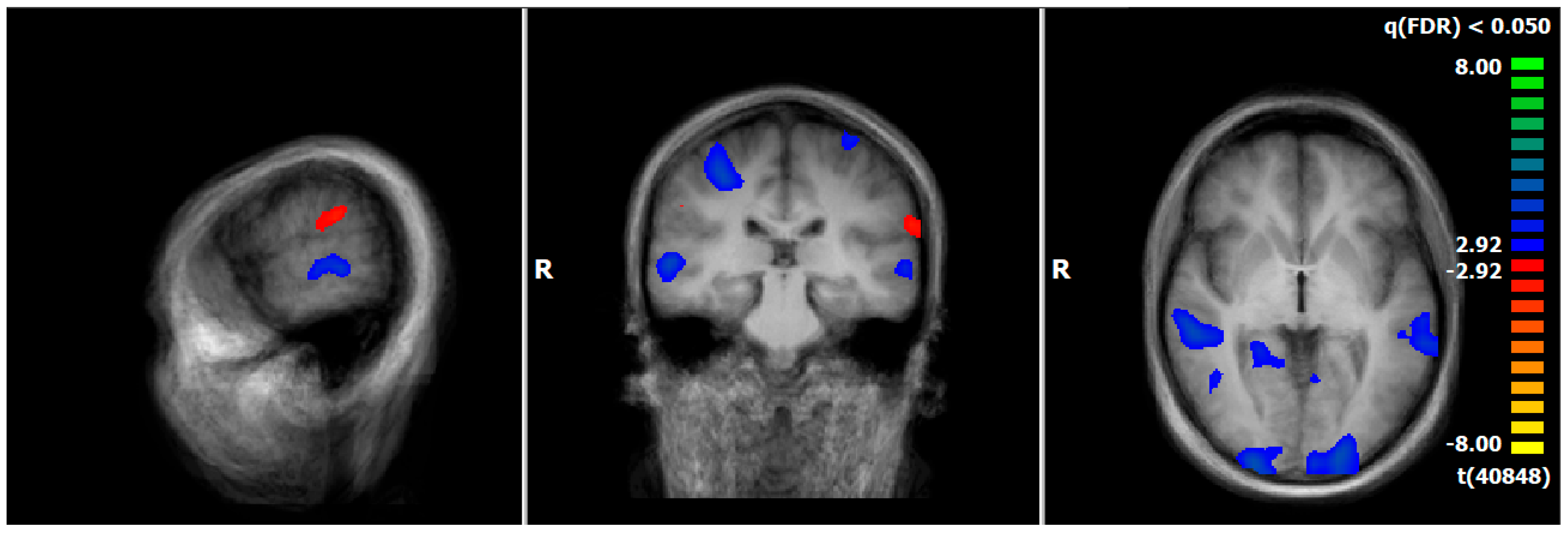
3.6. HC: Pre–Post Comparison of Neuronal Responses Following a 5–6-Week Interval
3.7. Post-Treatment Comparison of Neuronal Responses Between PPPD and ANX
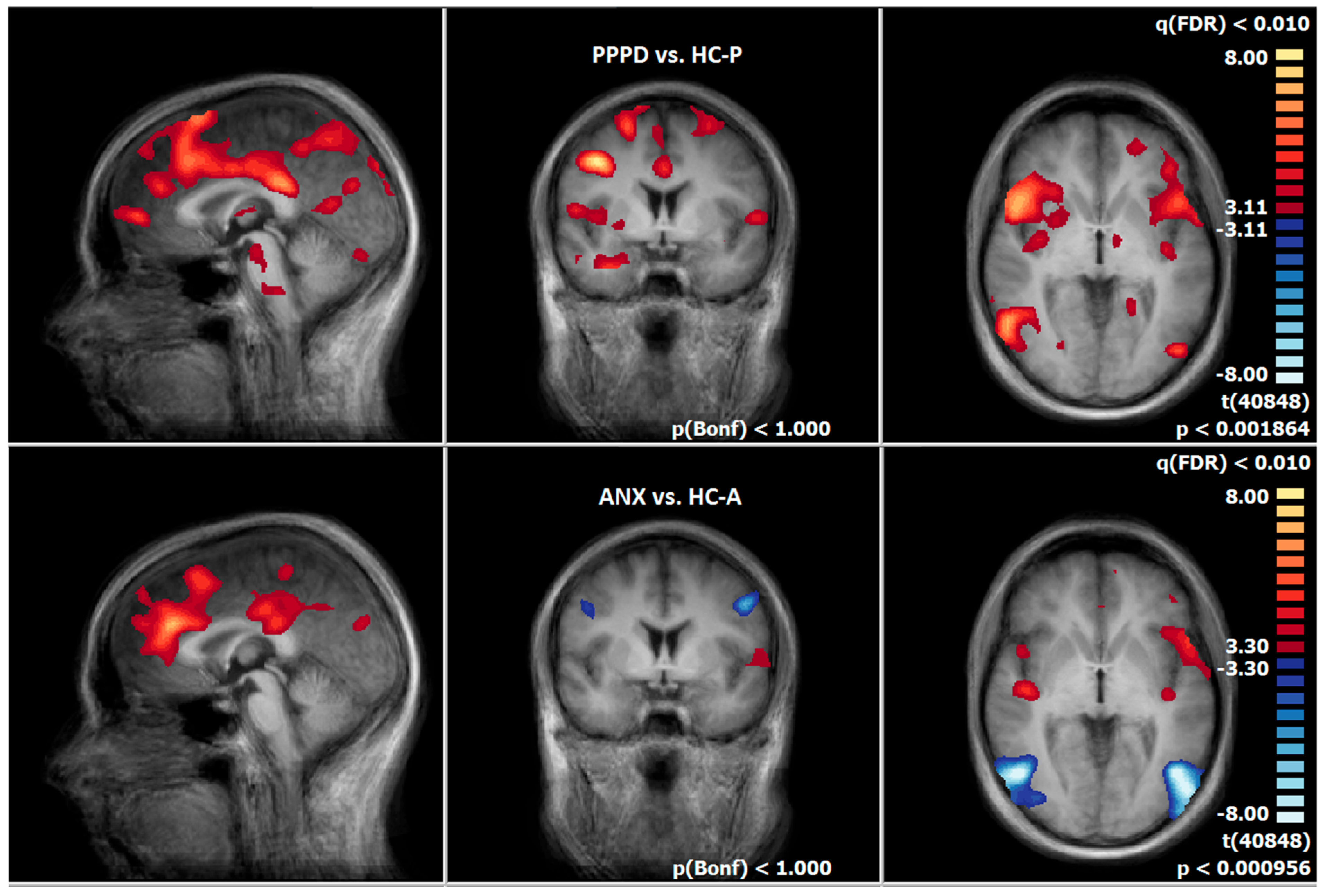
3.8. Post-Treatment Comparison of Neuronal Responses Between PPPD and HC-P
3.9. Post-Treatment Comparison of Neuronal Responses Between ANX and HC-A
4. Discussion
4.1. Clinical Findings from Psychometric Analysis
4.2. Functional Imaging Data
4.2.1. Emotion-Processing Network
4.2.2. Vestibulo-Spatial Network
4.2.3. Visual Network
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maywald, M.; Pogarell, O.; Levai, S.; Paolini, M.; Tschentscher, N.; Rauchmann, B.S.; Krause, D.; Stöcklein, S.; Goerigk, S.; Röll, L.; et al. Neurofunctional differences and similarities between anxiety disorder and persistent postural-perceptual dizziness. NeuroImage Clin. 2023, 37, 103330. [Google Scholar] [CrossRef]
- Staab, J.P. Chronic subjective dizziness. Contin. Lifelong Learn. Neurol. 2012, 18, 1118–1141. [Google Scholar] [CrossRef] [PubMed]
- Habs, M.; Strobl, R.; Grill, E.; Dieterich, M.; Becker-Bense, S. Primary or secondary chronic functional dizziness: Does it make a difference? A DizzyReg study in 356 patients. J. Neurol. 2020, 267, 212–222. [Google Scholar] [CrossRef]
- Staab, J.P.; Ruckenstein, M.J. Expanding the differential diagnosis of chronic dizziness. Arch. Otolaryngol.–Head Neck Surg. 2007, 133, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Schneier, F.R.; Fyer, A.J.; Martin, L.Y.; Ross, D.; Mannuzza, S.; Liebowitz, M.R.; Gorman, J.M.; Klein, D.F. A comparison of phobic subtypes within panic disorder. J. Anxiety Disord. 1991, 5, 65–75. [Google Scholar] [CrossRef]
- Telch, M.J.; Brouillard, M.; Telch, C.F.; Agras, W.S.; Taylor, C.B. Role of cognitive appraisal in panic-related avoidance. Behav. Res. Ther. 1989, 27, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt-Henn, A.; Tschan, R.; Best, C.; Dieterich, M. Somatoform vertigo syndrome. Nervenarzt 2009, 80, 909–917. [Google Scholar] [CrossRef]
- Jacob, R.G.; Furman, J.M.; Durrant, J.D.; Turner, S.M. Panic, agoraphobia, and vestibular dysfunction. Am. J. Psychiatry 1996, 153, 503–512. [Google Scholar]
- Jacob, R.G.; Furman, J.M.; Durrant, J.D.; Turner, S.M. Surface dependence: A balance control strategy in panic disorder with agoraphobia. Psychosom. Med. 1997, 59, 323–330. [Google Scholar] [CrossRef]
- Jacob, R.G.; Redfern, M.S.; Furman, J.M. Space and motion discomfort and abnormal balance control in patients with anxiety disorders. J. Neurol. Neurosurg. Psychiatry 2009, 80, 74–78. [Google Scholar] [CrossRef]
- Feldman, R.; Schreiber, S.; Pick, C.G.; Been, E. Gait, balance, mobility and muscle strength in people with anxiety compared to healthy individuals. Hum. Mov. Sci. 2019, 67, 102513. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Kugler, G.; Schniepp, R.; Wuehr, M.; Huppert, D. Acrophobia impairs visual exploration and balance during standing and walking. Ann. N. Y. Acad. Sci. 2015, 1343, 37–48. [Google Scholar] [CrossRef]
- De Vestel, C.; De Hertogh, W.; Van Rompaey, V.; Vereeck, L. Comparison of Clinical Balance and Visual Dependence Tests in Patients with Chronic Dizziness with and Without Persistent Postural-Perceptual Dizziness: A Cross-Sectional Study. Front. Neurol. 2022, 13, 880714. [Google Scholar] [CrossRef]
- Chrobok, A.I. Neurofunktionelle Aspekte von Experimentell Induzierter Angst Bei Patienten Mit Phobischem Schwankschwindel. Ph.D. Thesis, Ludwig-Maximilians-Universität, Munich, Germany, 2017. [Google Scholar] [CrossRef]
- de Carvalho, M.R.; Velasques, B.B.; Freire, R.C.; Cagy, M.; Marques, J.B.; Teixeira, S.; Thomaz, R.; Rangé, B.P.; Piedade, R.; Akiskal, H.S. Frontal cortex absolute beta power measurement in Panic Disorder with Agoraphobia patients. J. Affect. Disord. 2015, 184, 176–181. [Google Scholar] [CrossRef]
- Boehme, S.; Ritter, V.; Tefikow, S.; Stangier, U.; Strauss, B.; Miltner, W.H.; Straube, T. Brain activation during anticipatory anxiety in social anxiety disorder. Soc. Cogn. Affect. Neurosci. 2014, 9, 1413–1418. [Google Scholar] [CrossRef]
- Staab, J.P.; Rohe, D.E.; Eggers, S.D.; Shepard, N.T. Anxious, introverted personality traits in patients with chronic subjective dizziness. J. Psychosom. Res. 2014, 76, 80–83. [Google Scholar] [CrossRef]
- Karsten, J.; Penninx, B.W.; Riese, H.; Ormel, J.; Nolen, W.A.; Hartman, C.A. The state effect of depressive and anxiety disorders on big five personality traits. J. Psychiatr. Res. 2012, 46, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Kotov, R.; Gamez, W.; Schmidt, F.; Watson, D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol. Bull. 2010, 136, 768. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, G.; Petrolo, C.; Riccelli, R.; Giofrè, L.; Olivadese, G.; Gioacchini, F.; Scarpa, A.; Cassandro, E.; Passamonti, L. Chronic subjective dizziness: Analysis of underlying personality factors. J. Vestib. Res. 2016, 26, 403–408. [Google Scholar] [CrossRef]
- Zaback, M.; Cleworth, T.W.; Carpenter, M.G.; Adkin, A.L. Personality traits and individual differences predict threat-induced changes in postural control. Hum. Mov. Sci. 2015, 40, 393–409. [Google Scholar] [CrossRef]
- Trinidade, A.; Harman, P.; Stone, J.; Staab, J.P.; Goebel, J.A. Assessment of Potential Risk Factors for the Development of Persistent Postural-Perceptual Dizziness: A Case-Control Pilot Study. Front. Neurol. 2021, 11, 1955. [Google Scholar] [CrossRef] [PubMed]
- Barlow, D.H. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic; Guilford Press: New York, NY, USA, 2004. [Google Scholar]
- Ehlers, A.; Margraf, J. The psychophysiological model of panic attacks. In Fresh Perspectives on Anxiety Disorders; Swets & Zeitlinger: Leiden, The Netherlands, 1989; pp. 1–29. [Google Scholar]
- Brandt, T.; Strupp, M.; Novozhilov, S.; Krafczyk, S. Artificial neural network posturography detects the transition of vestibular neuritis to phobic postural vertigo. J. Neurol. 2012, 259, 182–184. [Google Scholar] [CrossRef]
- Krafczyk, S.; Tietze, S.; Swoboda, W.; Valkovič, P.; Brandt, T. Artificial neural network: A new diagnostic posturographic tool for disorders of stance. Clin. Neurophysiol. 2006, 117, 1692–1698. [Google Scholar] [CrossRef]
- Best, C.; Tschan, R.; Stieber, N.; Beutel, M.E.; Eckhardt-Henn, A.; Dieterich, M. STEADFAST: Psychotherapeutic intervention improves postural strategy of somatoform vertigo and dizziness. Behav. Neurol. 2015, 2015, 456850. [Google Scholar] [CrossRef]
- Limburg, K.; Radziej, K.; Sattel, H.; Henningsen, P.; Dieterich, M.; Probst, T.; Dale, R.; Lahmann, C. A Randomized Controlled Trial Evaluating Integrative Psychotherapeutic Group Treatment Compared to Self-Help Groups in Functional Vertigo/Dizziness. J. Clin. Med. 2021, 10, 2215. [Google Scholar] [CrossRef]
- Goto, F.; Tsutsumi, T.; Ogawa, K. Treatment of chronic subjective dizziness by SSRIs. Nihon Jibiinkoka Gakkai Kaiho 2013, 116, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, J.; Karlberg, M.; Harlacher, U.; Rivano–Fischer, M.; Magnusson, M. Treatment of phobic postural vertigo. J. Neurol. 2006, 253, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, A.E.; Edelman, S.; Cremer, P.D. Cognitive behavior therapy for chronic subjective dizziness: Longer-term gains and predictors of disability. Am. J. Otolaryngol. 2013, 34, 115–120. [Google Scholar] [CrossRef]
- Edelman, S.; Mahoney, A.E.; Cremer, P.D. Cognitive behavior therapy for chronic subjective dizziness: A randomized, controlled trial. Am. J. Otolaryngol. 2012, 33, 395–401. [Google Scholar] [CrossRef]
- Yu, Y.-C.; Xue, H.; Zhang, Y.-X.; Zhou, J. Cognitive behavior therapy as augmentation for sertraline in treating patients with persistent postural-perceptual dizziness. BioMed Res. Int. 2018, 2018, 8518631. [Google Scholar] [CrossRef]
- Strauß, B.; Barkowski, S.; Schwartze, D.; Rosendahl, J. Aktueller Stand der Gruppenpsychotherapieforschung. Psychotherapeut 2016, 61, 364–375. [Google Scholar] [CrossRef]
- Pompoli, A.; Furukawa, T.A.; Imai, H.; Tajika, A.; Efthimiou, O.; Salanti, G. Psychological therapies for panic disorder with or without agoraphobia in adults: A network meta-analysis. Cochrane Database Syst. Rev. 2016, 4, CD011004. [Google Scholar] [CrossRef]
- Suica, Z.; Behrendt, F.; Ziller, C.; Gäumann, S.; Schädler, S.; Hilfiker, R.; Parmar, K.; Gerth, H.U.; Bonati, L.H.; Schuster-Amft, C. Comparative effectiveness of non-pharmacological treatments in patients with persistent postural-perceptual dizziness: A systematic review and effect sizes analyses. Front. Neurol. 2024, 15, 1426566. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.G.; Whitney, S.L.; Detweiler-Shostak, G.; Furman, J.M. Vestibular rehabilitation for patients with agoraphobia and vestibular dysfunction: A pilot study. J. Anxiety Disord. 2001, 15, 131–146. [Google Scholar] [CrossRef]
- Schunck, T.; Erb, G.; Mathis, A.; Gilles, C.; Namer, I.J.; Hode, Y.; Demaziere, A.; Luthringer, R.; Macher, J.-P. Functional magnetic resonance imaging characterization of CCK-4-induced panic attack and subsequent anticipatory anxiety. Neuroimage 2006, 31, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Schrammen, E.; Roesmann, K.; Rosenbaum, D.; Redlich, R.; Harenbrock, J.; Dannlowski, U.; Leehr, E.J. Functional neural changes associated with psychotherapy in anxiety disorders–A meta-analysis of longitudinal fMRI studies. Neurosci. Biobehav. Rev. 2022, 142, 104895. [Google Scholar] [CrossRef]
- Santos, V.A.; Carvalho, D.D.; Van Ameringen, M.; Nardi, A.E.; Freire, R.C. Neuroimaging findings as predictors of treatment outcome of psychotherapy in anxiety disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 91, 60–71. [Google Scholar] [CrossRef]
- Bohus, M.; Wolf-Arehult, M. Interaktives Skillstraining für Borderline-Patienten; Auflage, Ed.; Schattauer: Stuttgart, Germany, 2013. [Google Scholar]
- Grawe, K. Neuropsychotherapie; Hogrefe Verlag: Göttingen, Germany, 2004. [Google Scholar]
- Radziej, K.; Schmid-Mühlbauer, G.; Limburg, K.; Lahmann, C. Psychotherapie bei Schwindelerkrankungen–Eine störungsorientierte Gruppenbehandlung für angst-, depressions-und somatoform betonten Schwindel. PPmP-Psychother. Psychosom. Med. Psychol. 2017, 67, 245–251. [Google Scholar] [CrossRef]
- Schaaf, H. Psychotherapie Bei Schwindelerkrankungen; Asanger: Heidelberg, Germany, 2007. [Google Scholar]
- Tschan, R.; Eckhardt-Henn, A.; Scheurich, V.; Best, C.; Dieterich, M.; Beutel, M. Standfest? Erste Ergebnisse der Entwicklung eines kognitiv-verhaltenstherapeutischen Gruppenschulungsprogramms zur Behandlung des somatoformen Schwindels. Psychother. Psychosom. Med. Psychol. 2012, 62, 111–119. [Google Scholar] [CrossRef]
- Alsleben, H.; Weiss, A.; Rufer, M. Psychoedukation—Angst und Panikstörungen; Elsevier: München, Germany, 2004. [Google Scholar]
- Stavemann, H. Sokratische Gesprächsführung. In Verhaltenstherapiemanual; Springer: Berlin/Heidelberg, Germany, 2005; pp. 270–277. [Google Scholar]
- Forsyth, J.P.; Eifert, G.H. The Mindfulness and Acceptance Workbook for Anxiety: A guide to Breaking Free from Anxiety, Phobias, and Worry Using Acceptance and Commitment Therapy; New Harbinger Publications: Oakland, CA, USA, 2016. [Google Scholar]
- Wells, A. Meta-cognition and worry: A cognitive model of generalized anxiety disorder. Behav. Cogn. Psychother. 1995, 23, 301–320. [Google Scholar] [CrossRef]
- Laux, L.; Glanzmann, P.; Schaffner, P.; Spielberger, C. Testmappe mit Handanweisung, Fragebogen STAI-G Form X 1 und Fragebogen STAI-G Form X 2. In Das State-Trait-Angstinventar; Testzentrale: Göttingen, Germany, 1981. [Google Scholar]
- Ehlers, A.; Margraf, J. AKV: Fragebogen zu Körperbezogenen Ängsten, Kognitionen und Vermeidung; Beltz Test: Göttingen, Germany, 2001. [Google Scholar]
- Tschan, R.; Wiltink, J.; Best, C.; Beutel, M.; Dieterich, M.; Eckhardt-Henn, A. Validation of the German version of the Vertigo Handicap Questionnaire (VHQ) in patients with vestibular vertigo syndromes or somatoform vertigo and dizziness. Psychother. Psychosom. Med. Psychol. 2010, 60, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Tschan, R.; Wiltink, J.; Best, C.; Bense, S.; Dieterich, M.; Beutel, M.E.; Eckhardt-Henn, A. Validation of the German version of the Vertigo Symptom Scale (VSS) in patients with organic or somatoform dizziness and healthy controls. J. Neurol. 2008, 255, 1168–1175. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck depression inventory-II. San Antonio 1996, 78, 490–498. [Google Scholar]
- Moss-Morris, R.; Weinman, J.; Petrie, K.; Horne, R.; Cameron, L.; Buick, D. The Revised Illness Perception Questionnaire (IPQ-R). Psychol. Health 2002, 17, 1–16. [Google Scholar] [CrossRef]
- Gothard, K.M. Multidimensional processing in the amygdala. Nat. Rev. Neurosci. 2020, 21, 565–575. [Google Scholar] [CrossRef]
- Vogt, B.A. Cingulate cortex in the three limbic subsystems. Handb. Clin. Neurol. 2019, 166, 39–51. [Google Scholar]
- Knierim, J.J. The hippocampus. Curr. Biol. 2015, 25, R1116–R1121. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef]
- Morin, A.; Michaud, J. Self-awareness and the left inferior frontal gyrus: Inner speech use during self-related processing. Brain Res. Bull. 2007, 74, 387–396. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and function of the human insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef]
- Ben-Shabat, E.; Matyas, T.A.; Pell, G.S.; Brodtmann, A.; Carey, L.M. The right supramarginal gyrus is important for proprioception in healthy and stroke-affected participants: A functional MRI study. Front. Neurol. 2015, 6, 248. [Google Scholar] [CrossRef]
- Sliwinska, M.W.; Khadilkar, M.; Campbell-Ratcliffe, J.; Quevenco, F.; Devlin, J.T. Early and sustained supramarginal gyrus contributions to phonological processing. Front. Psychol. 2012, 3, 161. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Blanke, O.; Mast, F. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience 2012, 212, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Owen, N.; Nazareth, I.; Luxon, L. Panic disorder with agoraphobia associated with dizziness: Characteristic symptoms and psychosocial sequelae. J. Nerv. Ment. Dis. 2001, 189, 321–327. [Google Scholar] [CrossRef]
- Knowles, K.A.; Olatunji, B.O. Specificity of trait anxiety in anxiety and depression: Meta-analysis of the State-Trait Anxiety Inventory. Clin. Psychol. Rev. 2020, 82, 101928. [Google Scholar] [CrossRef]
- Barlow, D.H.; Sauer-Zavala, S.; Carl, J.R.; Bullis, J.R.; Ellard, K.K. The nature, diagnosis, and treatment of neuroticism: Back to the future. Clin. Psychol. Sci. 2014, 2, 344–365. [Google Scholar] [CrossRef]
- Morath, J.; Moreno-Villanueva, M.; Hamuni, G.; Kolassa, S.; Ruf-Leuschner, M.; Schauer, M.; Elbert, T.; Bürkle, A.; Kolassa, I.-T. Effects of psychotherapy on DNA strand break accumulation originating from traumatic stress. Psychother. Psychosom. 2014, 83, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Schauer, M.; Elbert, T.; Neuner, F. Narrative Expositionstherapie (NET) für Menschen nach Gewalt und Flucht. Psychotherapeut 2017, 62, 306–313. [Google Scholar] [CrossRef]
- Sifneos, P.E. The prevalence of ‘alexithymic’characteristics in psychosomatic patients. Psychother. Psychosom. 1973, 22, 255–262. [Google Scholar] [CrossRef]
- Franz, M.; Schäfer, R. Alexithymie-ein aktuelles Update aus klinischer, neurophysiologischer und entwicklungspsychologischer Sicht. Z. Psychosom. Med. Psychother. 2009, 55, 328–353. [Google Scholar] [CrossRef]
- Franz, M.; Popp, K.; Schaefer, R.; Sitte, W.; Schneider, C.; Hardt, J.; Decker, O.; Braehler, E. Alexithymia in the German general population. Soc. Psychiatry Psychiatr. Epidemiol. 2008, 43, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Maywald, M.; Paolini, M.; Rauchmann, B.S.; Gerz, C.; Heppe, J.L.; Wolf, A.; Lerchenberger, L.; Tominschek, I.; Stöcklein, S.; Reidler, P.; et al. Individual- and Connectivity-Based Real-Time fMRI Neurofeedback to Modulate Emotion-Related Brain Responses in Patients with Depression: A Pilot Study. Brain Sci. 2022, 12, 1714. [Google Scholar] [CrossRef]
- Karch, S.; Maywald, M.; Schwartz, C.; Heil, C.; Neumüller, J.; Keeser, D.; Garcia, S.; Tschentscher, N.; Pogarell, O.; Paolini, M.; et al. Neuronal correlates of intensification and acceptance of symptoms during exposure therapy in patients with obsessive-compulsive disorder. Front. Psychol. 2024, 15, 1256046. [Google Scholar] [CrossRef] [PubMed]
- Schnell, K.; Herpertz, S.C. Effects of dialectic-behavioral-therapy on the neural correlates of affective hyperarousal in borderline personality disorder. J. Psychiatr. Res. 2007, 41, 837–847. [Google Scholar] [CrossRef]
- Kircher, T.; Arolt, V.; Jansen, A.; Pyka, M.; Reinhardt, I.; Kellermann, T.; Konrad, C.; Lueken, U.; Gloster, A.T.; Gerlach, A.L. Effect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorder. Biol. Psychiatry 2013, 73, 93–101. [Google Scholar] [CrossRef]
- Avery, J.A.; Drevets, W.C.; Moseman, S.E.; Bodurka, J.; Barcalow, J.C.; Simmons, W.K. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatry 2014, 76, 258–266. [Google Scholar] [CrossRef]
- Yang, Y.; Kircher, T.; Straube, B. The neural correlates of cognitive behavioral therapy: Recent progress in the investigation of patients with panic disorder. Behav. Res. Ther. 2014, 62, 88–96. [Google Scholar] [CrossRef]
- Zhao, X.; Xi, Q.; Wang, P.; Li, C.; He, H. Altered activity and functional connectivity of superior temporal gyri in anxiety disorders: A functional magnetic resonance imaging study. Korean J. Radiol. 2014, 15, 523. [Google Scholar] [CrossRef] [PubMed]
- Chavanne, A.V.; Robinson, O.J. The overlapping neurobiology of induced and pathological anxiety: A meta-analysis of functional neural activation. Am. J. Psychiatry 2021, 178, 156–164. [Google Scholar] [CrossRef]
- Zhao, Z.; Yao, S.; Li, K.; Sindermann, C.; Zhou, F.; Zhao, W.; Li, J.; Lührs, M.; Goebel, R.; Kendrick, K.M. Real-time functional connectivity-informed neurofeedback of amygdala-frontal pathways reduces anxiety. Psychother. Psychosom. 2019, 88, 5–15. [Google Scholar] [CrossRef]
- Reinecke, A.; Thilo, K.; Filippini, N.; Croft, A.; Harmer, C.J. Predicting rapid response to cognitive-behavioural treatment for panic disorder: The role of hippocampus, insula, and dorsolateral prefrontal cortex. Behav. Res. Ther. 2014, 62, 120–128. [Google Scholar] [CrossRef] [PubMed]
- McTeague, L.M.; Rosenberg, B.M.; Lopez, J.W.; Carreon, D.M.; Huemer, J.; Jiang, Y.; Chick, C.F.; Eickhoff, S.B.; Etkin, A. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am. J. Psychiatry 2020, 177, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain A J. Neurol. 2014, 137, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Kamourieh, S.; Beckmann, C.F.; Sharp, D.J. Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 2011, 31, 3217–3224. [Google Scholar] [CrossRef]
- Biggs, E.E.; Timmers, I.; Meulders, A.; Vlaeyen, J.W.; Goebel, R.; Kaas, A.L. The neural correlates of pain-related fear: A meta-analysis comparing fear conditioning studies using painful and non-painful stimuli. Neurosci. Biobehav. Rev. 2020, 119, 52–65. [Google Scholar] [CrossRef]
- Buchheim, A.; Viviani, R.; Kessler, H.; Kächele, H.; Cierpka, M.; Roth, G.; George, C.; Kernberg, O.F.; Bruns, G.; Taubner, S. Changes in prefrontal-limbic function in major depression after 15 months of long-term psychotherapy. PLoS ONE 2012, 7, e33745. [Google Scholar] [CrossRef]
- Schiller, D.; Freeman, J.B.; Mitchell, J.P.; Uleman, J.S.; Phelps, E.A. A neural mechanism of first impressions. Nat. Neurosci. 2009, 12, 508–514. [Google Scholar] [CrossRef]
- Sakai, Y.; Kumano, H.; Nishikawa, M.; Sakano, Y.; Kaiya, H.; Imabayashi, E.; Ohnishi, T.; Matsuda, H.; Yasuda, A.; Sato, A. Changes in cerebral glucose utilization in patients with panic disorder treated with cognitive–behavioral therapy. Neuroimage 2006, 33, 218–226. [Google Scholar] [CrossRef]
- Lopez, C.; Blanke, O. The thalamocortical vestibular system in animals and humans. Brain Res. Rev. 2011, 67, 119–146. [Google Scholar] [CrossRef]
- Dieterich, M.; Brandt, T. Thalamic infarctions: Differential effects on vestibular function in the roll plane (35 patients). Neurology 1993, 43, 1732. [Google Scholar] [CrossRef]
- Popp, P.; Zu Eulenburg, P.; Stephan, T.; Bögle, R.; Habs, M.; Henningsen, P.; Feuerecker, R.; Dieterich, M. Cortical alterations in phobic postural vertigo–a multimodal imaging approach. Ann. Clin. Transl. Neurol. 2018, 5, 717–729. [Google Scholar] [CrossRef]
- McGeoch, P.D.; Brang, D.; Ramachandran, V.J.M.H. Apraxia, metaphor and mirror neurons. Med. Hypotheses 2007, 69, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Becker-Bense, S.; Willoch, F.; Stephan, T.; Brendel, M.; Yakushev, I.; Habs, M.; Ziegler, S.; Herz, M.; Schwaiger, M.; Dieterich, M.J.P.o. Direct comparison of activation maps during galvanic vestibular stimulation: A hybrid H2 [15 O] PET—BOLD MRI activation study. PLoS ONE 2020, 15, e0233262. [Google Scholar] [CrossRef]
- Kahane, P.; Hoffmann, D.; Minotti, L.; Berthoz, A. Reappraisal of the human vestibular cortex by cortical electrical stimulation study. Ann. Neurol. 2003, 54, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Stephan, T.; Deutschländer, A.; Nolte, A.; Schneider, E.; Wiesmann, M.; Brandt, T.; Dieterich, M. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage 2005, 26, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, V.; Boegle, R.; Keeser, D.; Kierig, E.; Ertl-Wagner, B.; Brandt, T.; Dieterich, M.J.N. Handedness-dependent functional organizational patterns within the bilateral vestibular cortical network revealed by fMRI connectivity based parcellation. Neuroimage 2018, 178, 224–237. [Google Scholar] [CrossRef]
- Schlindwein, P.; Bense, S.; Prange, K.; Lochmann, M.; Brandt, T.; Bartenstein, P.; Dieterich, M.J.K.N. Zerebraler Glukosemechanismus des vestibulären und visuellen Kortex bei akuter Neuritis vestibularis: PET-Studie. Klin. Neurophysiol. 2003, 34, 130. [Google Scholar] [CrossRef]
- Indovina, I.; Riccelli, R.; Chiarella, G.; Petrolo, C.; Augimeri, A.; Giofrè, L.; Lacquaniti, F.; Staab, J.P.; Passamonti, L. Role of the insula and vestibular system in patients with chronic subjective dizziness: An fMRI study using sound-evoked vestibular stimulation. Front. Behav. Neurosci. 2015, 9, 334. [Google Scholar] [CrossRef]
- Pelphrey, K.A.; Morris, J.P.; Michelich, C.R.; Allison, T.; McCarthy, G. Functional Anatomy of Biological Motion Perception in Posterior Temporal Cortex: An fMRI Study of Eye, Mouth and Hand Movements. Cereb. Cortex 2005, 15, 1866–1876. [Google Scholar] [CrossRef]
- Narumoto, J.; Okada, T.; Sadato, N.; Fukui, K.; Yonekura, Y. Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Cogn. Brain Res. 2001, 12, 225–231. [Google Scholar] [CrossRef]
- Olson, I.R.; Plotzker, A.; Ezzyat, Y. The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain 2007, 130, 1718–1731. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Si, L.; Cui, B.; Ling, X.; Shen, B.; Yang, X. Altered spontaneous functional activity of the right precuneus and cuneus in patients with persistent postural-perceptual dizziness. Brain Imaging Behav. 2019, 14, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Si, L.; Cui, B.; Ling, X.; Shen, B.; Yang, X. Altered intra-and inter-network functional connectivity in patients with persistent postural-perceptual dizziness. NeuroImage Clin. 2020, 26, 102216. [Google Scholar] [CrossRef]
- Kjaer, T.W.; Nowak, M.; Lou, H.C. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage 2002, 17, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Ray, R.D.; Cooper, J.C.; Robertson, E.R.; Chopra, S.; Gabrieli, J.D.; Gross, J.J. For better or for worse: Neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage 2004, 23, 483–499. [Google Scholar] [CrossRef]
- Barsky, A.J.; Goodson, J.D.; Lane, R.S.; Cleary, P.D. The amplification of somatic symptoms. Psychosom. Med. 1988, 50, 510–519. [Google Scholar] [CrossRef]
- Lahmann, C.; Henningsen, P.; Dinkel, A. Somatoforme und funktionelle Störungen. Der Nervenarzt 2010, 81, 1383–1396. [Google Scholar] [CrossRef]
- Wuehr, M.; Brandt, T.; Schniepp, R. Distracting attention in phobic postural vertigo normalizes leg muscle activity and balance. Neurology 2017, 88, 284–288. [Google Scholar] [CrossRef]
- Riccelli, R.; Passamonti, L.; Toschi, N.; Nigro, S.; Chiarella, G.; Petrolo, C.; Lacquaniti, F.; Staab, J.P.; Indovina, I. Altered insular and occipital responses to simulated vertical self-motion in patients with persistent postural-perceptual dizziness. Front. Neurol. 2017, 8, 529. [Google Scholar] [CrossRef]
- Indovina, I.; Passamonti, L.; Mucci, V.; Chiarella, G.; Lacquaniti, F.; Staab, J.P. Brain correlates of persistent postural-perceptual dizziness: A review of neuroimaging studies. J. Clin. Med. 2021, 10, 4274. [Google Scholar] [CrossRef]
- Van Ombergen, A.; Heine, L.; Jillings, S.; Roberts, R.E.; Jeurissen, B.; Van Rompaey, V.; Mucci, V.; Vanhecke, S.; Sijbers, J.; Vanhevel, F. Altered functional brain connectivity in patients with visually induced dizziness. NeuroImage Clin. 2017, 14, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [PubMed]
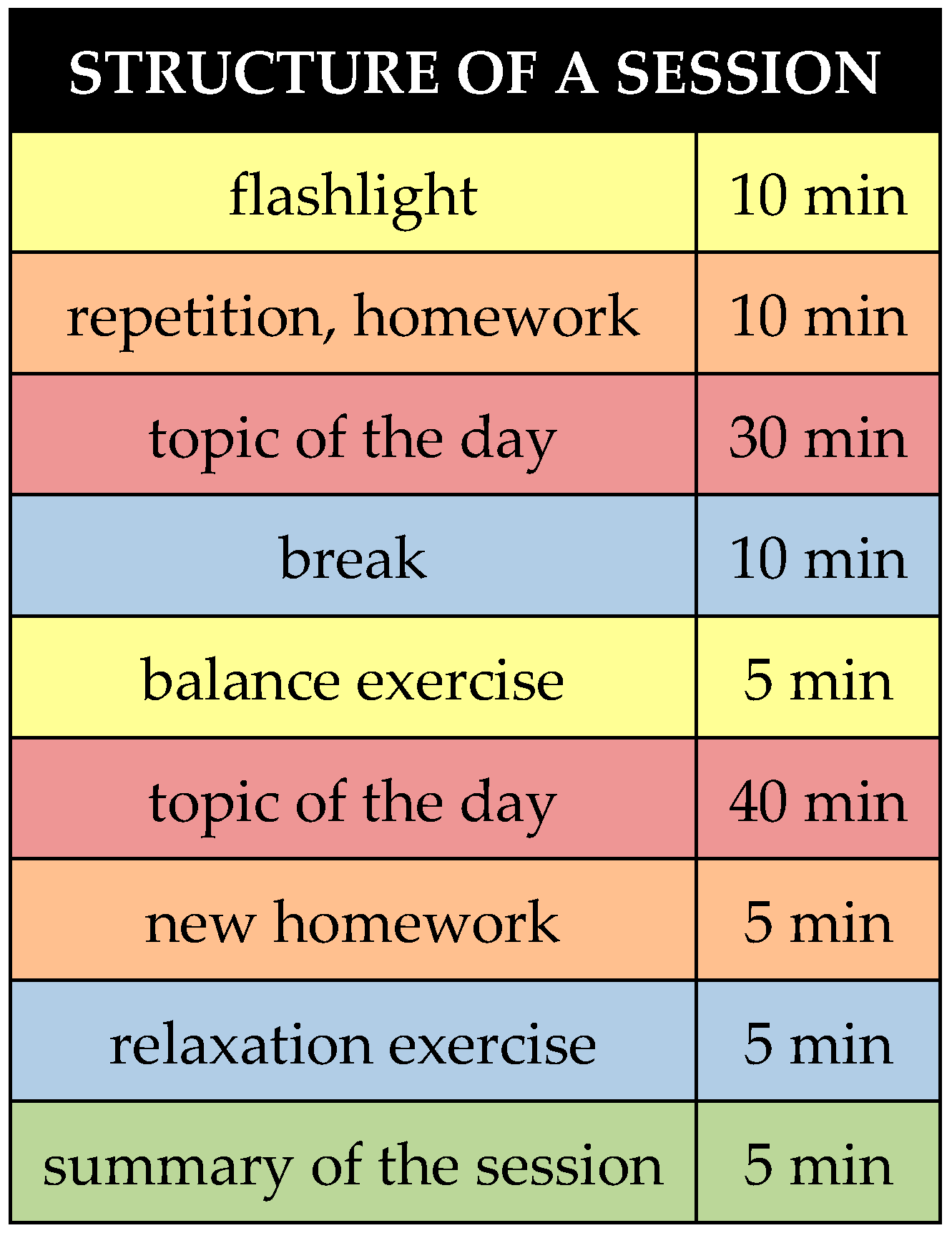

| 1. session | Topic: Introduction to the topic of anxiety and vertigo, and mindfulness Symptoms, origins, causes, principles of mindfulness theory and exercises. |
| 2. session | Topic: Explanatory models on the topic of anxiety and dizziness When anxiety/dizziness is useful/becomes a problem, vulnerability stress model, bio-psycho-social conditioning, bio-psycho-social disease model, vicious circle of anxiety/dizziness, primary/secondary illness gain. |
| 3. session | Topic: Strategies to deal with thoughts Recognizing anxiety thoughts, changing anxiety thoughts, PMR. |
| 4. session | Topic: Strategies for dealing with emotions Recognizing and understanding emotions, emotion management, mindfulness-based acceptance and commitment strategies. |
| 5. session | Topic: Exposure with reaction prevention Recognizing and understanding avoidance behaviours, anxiety hierarchy, anticipatory anxiety, provocation exercises (in sensu, in vivo, in a group and individual setting). |
| 6–8. session | Topic: individual therapy in a group setting Individual history of symptom development and deriving new strategies. |
| 9. session | Topic: resources Activation of strengths, three pillars model of self-esteem, reframing techniques, resource exercises, gratitude diary. |
| 10. session | Topic: dealing with setbacks Relapse prevention, emergency kit, early warning symptoms, saying goodbye. |
| Questionnaire | Scale | Abbreviation |
|---|---|---|
| State Trait Anxiety Inventory [50] Subscales:
| 1 (not at all) to 4 (very much) | STAI STAI-T STAI_S |
| Fragebogen zu körperbezogenen Ängsten, Kognitionen & Vermeidung [51] Subscales:
| 1 (not at all) to 5 (extreme) | AKV ACQ BSQ MI |
| Vertigo Handicap Questionnaire [52]—Physical and psychosocial impairment due to dizziness | 0 (never) to 4 (always) | VHQ |
| Vertigo Symptom Scale [53] Subscales:
| 0 (never) to 4 (very often) | VSS VSS-AA VSS-VER |
| Beck Depressions Inventory II [54] | 0 to 4 | BDI-II |
| Toronto Alexithymia Scale Subscales:
| 1 (strongly disagree) to 5 (strongly agree) | TAS TAS-DIF TAS-DDF |
| Illness Perception Questionnaire [55] Subscales:
| 1 (not true at all) to 5 (completely true) | IPQ IPQ-T a/c IPQ-TC IPQ-CON IPQ-PC IPQ-CC IPQ-COH IPQ-ER |
| Patients | fMRI-Pre | fMRI-Post | Q-Pre | Q-Post |
|---|---|---|---|---|
| PPPD | 10 | 11 | 14 | 11 |
| ANX | 13 | 11 | 20 | 16 |
| total | 24 | 22 | 34 | 27 |
| Healthy Controls | ||||
| HC-P | 9 | 8 | 13 | 10 |
| HC-A | 15 | 15 | 16 | 16 |
| Total | 24 | 23 | 29 | 26 |
| ROI | Abbreviation | Relevance |
|---|---|---|
| Amygdala | AMY | Emotion processing, threat detection, emotional memory, fear learning and conditioning [56] |
| Cingulate gyrus | CG | Emotion processing/regulation, behavioural regulation, learning, cognitive processing [57] |
| Hippocampus | HIP | Emotion memory, new memories (declarative and episodic), spatial navigation [58] |
| Inferior frontal gyrus | IFG | Language, inhibition and cognitive control, social cognition [59,60], decreased after CBT in patients with AD [40] |
| Insula | INS | Interoception, emotion processing/regulation, autonomic regulation, cognitive control, taste and motivation [61] |
| Supramarginal gyrus | SMG | Integrates multiple sensory modalities, including proprioceptive (body position), auditory, visual, vestibular and somatosensory information, empathy and social cognition, phonological processing [61,62,63,64] |
| Questionnaire | HC-P | PPPD | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | ||||||
| M | SD | M | SD | M | SD | M | SD | ||
| STAI-S | 34.40 | 9.79 | 33.10 | 9.11 | 47.55 | 11.29 | 45.27 | 8.51 | <0.01 * |
| STAI-T | 45.70 | 3.53 | 46.10 | 2.13 | 49.00 | 3.13 | 49.91 | 1.45 | 0.02 * |
| ACQ | 1.26 | 0.25 | 1.15 | 0.15 | 1.74 | 0.51 | 1.44 | 0.24 | 0.04 * |
| BSQ | 1.71 | 0.71 | 1.29 | 0.31 | 2.38 | 0.70 | 1.98 | 0.53 | 0.01 * |
| MI-A | 1.32 | 0.33 | 1.16 | 0.23 | 1.69 | 0.78 | 1.36 | 0.42 | 0.25 |
| M-B | 1.04 | 0.12 | 1.02 | 0.03 | 1.46 | 0.63 | 1.19 | 0.28 | 0.10 |
| VSS-AA | 7.40 | 5.17 | 4.60 | 3.92 | 25.91 | 10.52 | 24.64 | 10.92 | <0.01 * |
| VSS-VER | 2.40 | 2.12 | 1.00 | 0.82 | 27.64 | 17.40 | 25.73 | 18.80 | <0.01 * |
| VHQ | 11.50 | 14.58 | 10.70 | 12.53 | 43.45 | 20.05 | 34.82 | 19.98 | <0.01 * |
| BDI-II | 3.10 | 3.63 | 2.80 | 3.49 | 14.00 | 7.18 | 11.09 | 5.38 | <0.01 * |
| TAS-total | 53.40 | 8.36 | 52.90 | 5.49 | 59.82 | 9.14 | 59.27 | 9.81 | 0.49 |
| TAS-DIF | 13.60 | 4.14 | 13.50 | 2.88 | 20.55 | 5.92 | 20.00 | 6.80 | 0.02 * |
| TAS-DDF | 12.20 | 3.16 | 11.20 | 3.33 | 13.09 | 3.75 | 13.00 | 4.29 | 1.00 |
| IPQ-TL a/c | 13.00 | 3.74 | 12.10 | 4.07 | 16.73 | 3.95 | 14.73 | 2.90 | 0.16 |
| IPQ-TL c | 9.90 | 2.60 | 9.70 | 3.40 | 13.00 | 2.72 | 12.64 | 2.25 | 0.04 * |
| IPQ-CON | 12.30 | 4.27 | 12.60 | 4.30 | 16.73 | 2.97 | 15.09 | 3.59 | 0.17 |
| IPQ-PC | 13.20 | 1.81 | 13.30 | 2.67 | 12.82 | 2.71 | 15.18 | 4.29 | 1.00 |
| IPQ-CC | 13.70 | 2.83 | 14.40 | 2.91 | 13.73 | 1.74 | 14.45 | 3.91 | 1.00 |
| IPQ-COH | 16.50 | 2.99 | 18.20 | 3.91 | 15.18 | 5.31 | 16.55 | 4.87 | 1.00 |
| IPQ-ER | 12.20 | 4.02 | 13.20 | 4.44 | 18.18 | 5.72 | 16.36 | 4.43 | 0.06 |
| Questionnaire | HC-A | ANX | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | ||||||
| M | SD | M | SD | M | SD | M | SD | ||
| STAI-S | 33.38 | 8.56 | 29.31 | 7.29 | 47.06 | 11.20 | 37.94 | 10.21 | <0.01 * |
| STAI-T | 46.81 | 3.35 | 45.56 | 3.63 | 50.19 | 3.90 | 49.75 | 2.79 | <0.01 * |
| ACQ | 1.27 | 0.32 | 1.12 | 0.16 | 1.92 | 0.72 | 1.56 | 0.41 | 0.02 * |
| BSQ | 1.66 | 0.64 | 1.28 | 0.27 | 2.36 | 0.84 | 1.80 | 0.68 | 0.01 * |
| MI-A | 1.44 | 0.60 | 1.26 | 0.43 | 2.31 | 0.96 | 1.87 | 1.00 | 0.03 * |
| MI-B | 1.09 | 0.11 | 1.05 | 0.10 | 1.90 | 0.73 | 1.38 | 0.56 | 0.01 * |
| VSS-AA | 8.31 | 9.96 | 8.31 | 9.70 | 24.06 | 11.42 | 18.19 | 6.78 | <0.01 * |
| VSS-VER | 3.88 | 4.46 | 3.38 | 3.74 | 13.00 | 12.16 | 7.75 | 8.33 | 0.08 |
| BDI-II | 3.25 | 3.89 | 1.88 | 3.56 | 13.13 | 10.31 | 8.00 | 7.46 | <0.01 * |
| TAS-total | 50.88 | 9.38 | 50.88 | 9.22 | 63.25 | 9.67 | 62.25 | 7.65 | <0.01 * |
| TAS-DIF | 14.13 | 4.10 | 13.38 | 5.23 | 21.63 | 6.62 | 20.25 | 5.29 | <0.01 * |
| TAS-DDF | 10.50 | 3.386 | 10.56 | 3.52 | 15.00 | 3.93 | 15.12 | 4.00 | <0.01 * |
| IPQ-TL a/c | 12.62 | 5.23 | 12.69 | 4.63 | 16.25 | 2.41 | 15.44 | 3.98 | 0.04 * |
| IPQ-TL c | 10.62 | 2.99 | 10.31 | 3.18 | 11.94 | 2.67 | 11.81 | 2.14 | 0.65 |
| IPQ-CON | 12.31 | 4.24 | 12.75 | 4.93 | 16.87 | 3.40 | 14.63 | 4.90 | 0.07 |
| IPQ-PC | 14.50 | 3.74 | 14.44 | 3.12 | 12.69 | 2.85 | 15.50 | 1.97 | 1.00 |
| IPQ-CC | 14.06 | 3.86 | 14.19 | 3.21 | 13.69 | 1.70 | 15.00 | 1.79 | 1.00 |
| IPQ-COH | 17.06 | 4.95 | 17.37 | 3.93 | 13.69 | 3.91 | 17.94 | 3.97 | 1.00 |
| IPQ-ER | 12.81 | 4.87 | 12.69 | 3.75 | 17.81 | 4.76 | 15.31 | 3.74 | <0.05 * |
| Questionnaire | PPPD | ANX | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | ||||||
| M | SD | M | SD | M | SD | M | SD | ||
| STAI-S | 47.55 | 11.29 | 45.27 | 8.51 | 47.06 | 11.20 | 37.94 | 10.21 | 1.00 |
| STAI-T | 49.00 | 3.13 | 49.91 | 1.45 | 50.19 | 3.90 | 49.75 | 2.79 | 1.00 |
| ACQ | 1.74 | 0.51 | 1.44 | 0.24 | 1.92 | 0.72 | 1.56 | 0.41 | 0.82 |
| BCQ | 2.38 | 0.70 | 1.98 | 0.53 | 2.36 | 0.84 | 1.80 | 0.68 | 1.00 |
| MI-A | 1.69 | 0.78 | 1.36 | 0.42 | 2.31 | 0.96 | 1.87 | 1.00 | 0.12 |
| MI-B | 1.46 | 0.63 | 1.19 | 0.28 | 1.90 | 0.73 | 1.38 | 0.56 | 0.22 |
| VSS-AA | 25.91 | 10.52 | 24.64 | 10.92 | 24.06 | 11.42 | 18.19 | 6.78 | 0.70 |
| VSS-VER | 27.64 | 17.40 | 25.73 | 18.80 | 13.00 | 12.16 | 7.75 | 8.33 | 0.07 |
| BDI-II | 14.00 | 7.18 | 11.09 | 5.38 | 13.13 | 10.31 | 8.00 | 7.46 | 0.86 |
| TAS-total | 59.82 | 9.14 | 59.27 | 9.81 | 63.25 | 9.67 | 62.25 | 7.65 | 1.00 |
| TAS-DIF | 20.55 | 5.92 | 20.00 | 6.80 | 21.63 | 6.62 | 20.25 | 5.29 | 1.00 |
| TAS-DDF | 13.09 | 3.75 | 13.00 | 4.29 | 15.00 | 3.93 | 15.12 | 4.00 | 0.85 |
| IPQ-TL a/c | 16.73 | 3.95 | 14.73 | 2.90 | 16.25 | 2.41 | 15.44 | 3.98 | 1.00 |
| IPQ-TL c | 13.00 | 2.72 | 12.64 | 2.25 | 11.94 | 2.67 | 11.81 | 2.14 | 1.00 |
| IPQ-CON | 16.73 | 2.97 | 15.09 | 3.59 | 16.87 | 3.40 | 14.63 | 4.90 | 1.00 |
| IPQ-PC | 12.82 | 2.71 | 15.18 | 4.29 | 12.69 | 2.85 | 15.50 | 1.97 | 1.00 |
| IPQ-CC | 13.73 | 1.74 | 14.45 | 3.91 | 13.69 | 1.70 | 15.00 | 1.79 | 1.00 |
| IPQ-COH | 15.18 | 5.31 | 16.55 | 4.87 | 13.69 | 3.91 | 17.94 | 3.97 | 1.00 |
| IPQ-ER | 18.18 | 5.72 | 16.36 | 4.43 | 17.81 | 4.76 | 15.31 | 3.74 | 1.00 |
| Questionnaire | PPPD | ANX | p-Value | ||
|---|---|---|---|---|---|
| T2 | T2 | ||||
| M | SD | M | SD | ||
| VSS-VER | 25.73 | 18.80 | 7.75 | 8.33 | 0.02 * |
| Questionnaire | PPPD | PPPD | p-Value | ||
|---|---|---|---|---|---|
| Without AD (N = 8) | With AD (N = 6) | ||||
| M | SD | M | SD | ||
| BSQ (T1) | 2.05 | 0.59 | 2.81 | 0.62 | 0.04 * |
| KC-P | KC-A | PPPD | ANX | |||
|---|---|---|---|---|---|---|
| Questionnaire | p-Value | p-Value | p-Value | Effect Size r | p-Value | Effect Size r |
| STAI-S | 0.57 | 0.14 | 0.44 | −0.07 | <0.00 * | −0.22 |
| STAI-T | 0.91 | 0.33 | 0.24 | −0.25 | 0.61 | −0.09 |
| ACQ | 0.67 | <0.01 * | 0.31 | −0.22 | <0.05 * | −0.35 |
| BCQ | 0.31 | 0.02 * | 0.24 | −0.25 | 0.01 * | −0.49 |
| MI-A | 0.78 | 0.03 * | 0.10 | −0.35 | 0.20 | −0.23 |
| MI-B | 0.66 | 0.12 | 0.31 | −0.22 | 0.08 | −0.31 |
| VSS-AA | 0.12 | 0.61 | 0.26 | −0.24 | 0.03 * | −0.47 |
| VSS-VER | 0.08 | 0.65 | 0.18 | −0.28 | 0.01 * | −0.38 |
| VHQ | 0.53 | - | 0.03 * | −0.46 | - | - |
| BDI-II | 0.83 | 0.02 * | 0.22 | −0.26 | 0.04 * | −0.37 |
| TAS-overall | 1.00 | 0.71 | 0.65 | −0.10 | 0.53 | −0.11 |
| TAS-DIF | 0.16 | 0.36 | 0.48 | −0.15 | 0.30 | −0.18 |
| TAS-DDF | 0.78 | 0.87 | 1.00 | 0.00 | 0.69 | −0.07 * |
| IPQ-TL a/c | 0.40 | 0.92 | 0.14 | −0.32 | 0.48 | −0.12 |
| IPQ-TL c | 0.85 | 0.62 | 0.86 | −0.04 | 0.72 | −0.06 |
| IPQ-CON | 0.68 | 0.77 | 0.18 | −0.29 | 0.12 | −0.27 |
| IPQ-PC | 0.89 | 0.84 | 0.04 * | −0.43 | <0.01 * | −0.52 |
| IPQ-CC | 0.42 | 0.95 | 0.32 | −0.21 | 0.02 * | −0.42 |
| IPQ-COH | 0.35 | 0.76 | 0.50 | −0.14 | <0.01 * | −0.53 |
| IPQ-ER | 0.50 | 0.76 | 0.10 | −0.34 | 0.03 * | −0.38 |
| ANX (N = 8) | PPPD (N = 9) | HC (N = 20) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROI | M | SD | p-Value | M | SD | p-Value | M | SD | p-Value | |
| AMY+ | T1 | 876.00 | 689.14 | 0.06 * | 796.00 | 627.90 | 0.52 | 540.00 | 490.80 | 0.65 |
| T2 | 468.36 | 585.75 | 648.00 | 615.18 | 773.52 | 1778.28 | ||||
| HIP+ | T1 | 1691.46 | 1552.80 | 0.02 ** | 1415.50 | 1525.01 | 0.52 | 1020.13 | 951.51 | 0.20 |
| T2 | 1039.36 | 1374.97 | 955.64 | 836.17 | 613.78 | 737.50 | ||||
| THA+ | T1 | 5302.92 | 3651.63 | 0.01 ** | 4506.50 | 3499.92 | 0.77 | 3672.30 | 3957.79 | 0.60 |
| T2 | 3278.45 | 3133.10 | 3536.91 | 2918.98 | 2710.96 | 3232.71 | ||||
| CG+ | T1 | 5023.08 | 4328.03 | 0.33 | 4723.00 | 5731.77 | 0.68 | 4445.91 | 5743.18 | 0.74 |
| T2 | 3864.18 | 3712.94 | 5459.00 | 4990.92 | 2703.68 | 3590.26 | ||||
| IFG+ | T1 | 7136.50 | 4620.76 | 0.03 ** | 8518.00 | 5122.95 | 0.37 | 4797.17 | 3623.65 | 0.30 |
| T2 | 4457.36 | 3359.08 | 7732.00 | 4829.74 | 4057.95 | 4143.26 | ||||
| INS+ | T1 | 3005.50 | 2516.00 | 0.04 ** | 3775.90 | 3940.90 | 0.86 | 2541.17 | 3639.86 | 0.90 |
| T2 | 1473.27 | 1147.92 | 4273.36 | 4222.17 | 1444.18 | 1882.31 | ||||
| SMG+ | T1 | 6858.75 | 4937.74 | 0.16 | 8553.20 | 6467.69 | 0.77 | 6692.17 | 5124.60 | 0.68 |
| T2 | 5115.18 | 3313.07 | 9803.82 | 5868.81 | 4996.69 | 4668.07 | ||||
| Questionnaire | ANX | p-Value | |||
|---|---|---|---|---|---|
| Pre (N = 9) | Post (N = 9) | ||||
| M | SD | M | SD | ||
| AMY+ | 322.22 | 450.33 | 1035.78 | 715.36 | 0.006 * |
| Questionnaire | PPPD | p-Value | |||
|---|---|---|---|---|---|
| Pre (N = 9) | Post (N = 9) | ||||
| M | SD | M | SD | ||
| HIP+ | 1415.50 | 1525.01 | 955.64 | 836.17 | 0.009 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maywald, M.; Pogarell, O.; Chrobok, A.; Levai, S.; Keeser, D.; Tschentscher, N.; Rauchmann, B.-S.; Stöcklein, S.; Ertl-Wagner, B.; Papazov, B.; et al. Diagnostics and Group Therapy in Patients with Persistent Postural-Perceptual Dizziness and Anxiety Disorder: Biomarkers and Neurofunctional Correlates of Underlying Treatment Effects. Diagnostics 2025, 15, 1729. https://doi.org/10.3390/diagnostics15141729
Maywald M, Pogarell O, Chrobok A, Levai S, Keeser D, Tschentscher N, Rauchmann B-S, Stöcklein S, Ertl-Wagner B, Papazov B, et al. Diagnostics and Group Therapy in Patients with Persistent Postural-Perceptual Dizziness and Anxiety Disorder: Biomarkers and Neurofunctional Correlates of Underlying Treatment Effects. Diagnostics. 2025; 15(14):1729. https://doi.org/10.3390/diagnostics15141729
Chicago/Turabian StyleMaywald, Maximilian, Oliver Pogarell, Agnieszka Chrobok, Susanne Levai, Daniel Keeser, Nadja Tschentscher, Boris-Stephan Rauchmann, Sophia Stöcklein, Birgit Ertl-Wagner, Boris Papazov, and et al. 2025. "Diagnostics and Group Therapy in Patients with Persistent Postural-Perceptual Dizziness and Anxiety Disorder: Biomarkers and Neurofunctional Correlates of Underlying Treatment Effects" Diagnostics 15, no. 14: 1729. https://doi.org/10.3390/diagnostics15141729
APA StyleMaywald, M., Pogarell, O., Chrobok, A., Levai, S., Keeser, D., Tschentscher, N., Rauchmann, B.-S., Stöcklein, S., Ertl-Wagner, B., Papazov, B., Paolini, M., & Karch, S. (2025). Diagnostics and Group Therapy in Patients with Persistent Postural-Perceptual Dizziness and Anxiety Disorder: Biomarkers and Neurofunctional Correlates of Underlying Treatment Effects. Diagnostics, 15(14), 1729. https://doi.org/10.3390/diagnostics15141729






