Central Airway Carcinoid Tumorlets Following Resection of a Typical Carcinoid Tumor
Abstract
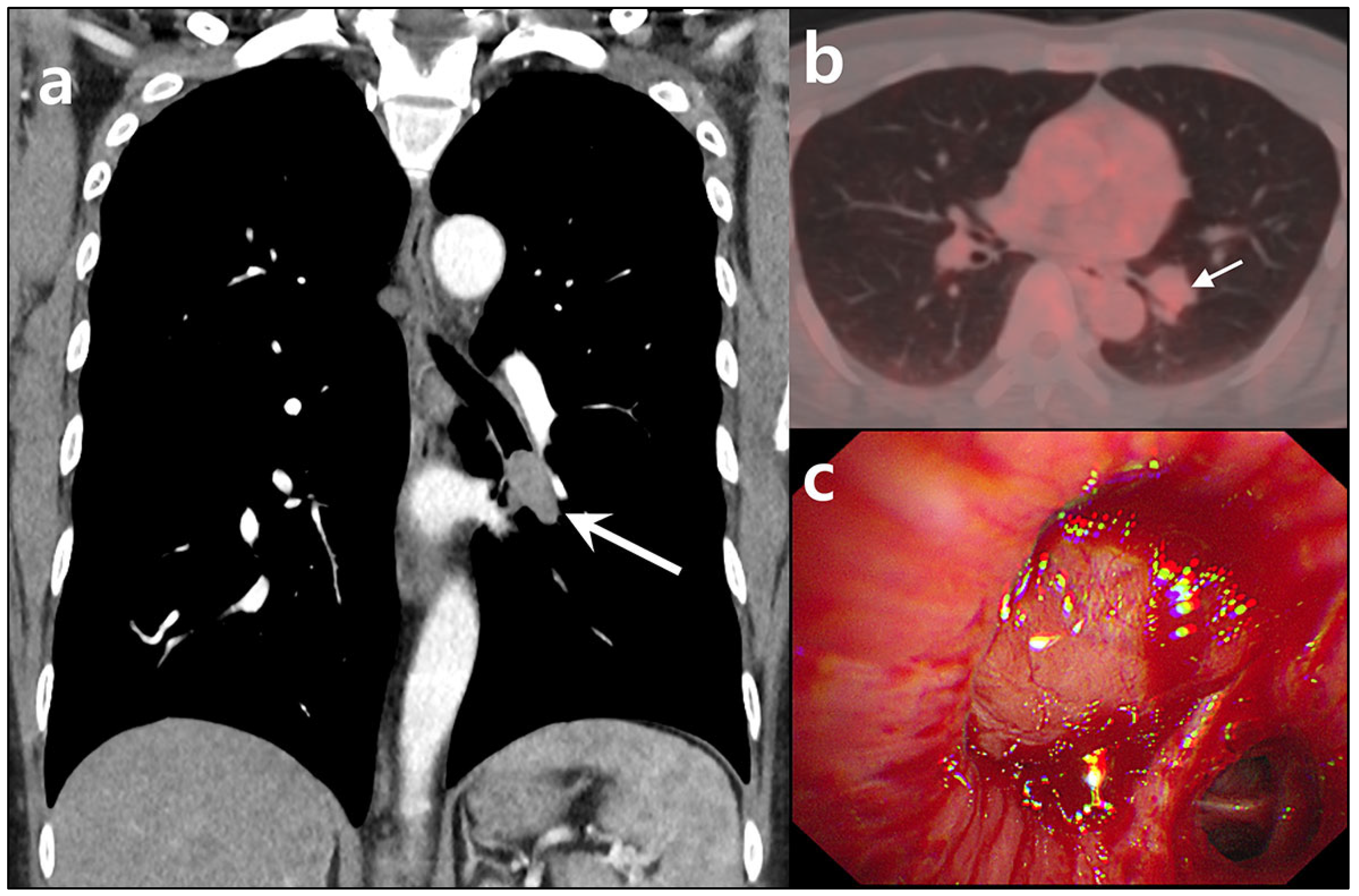

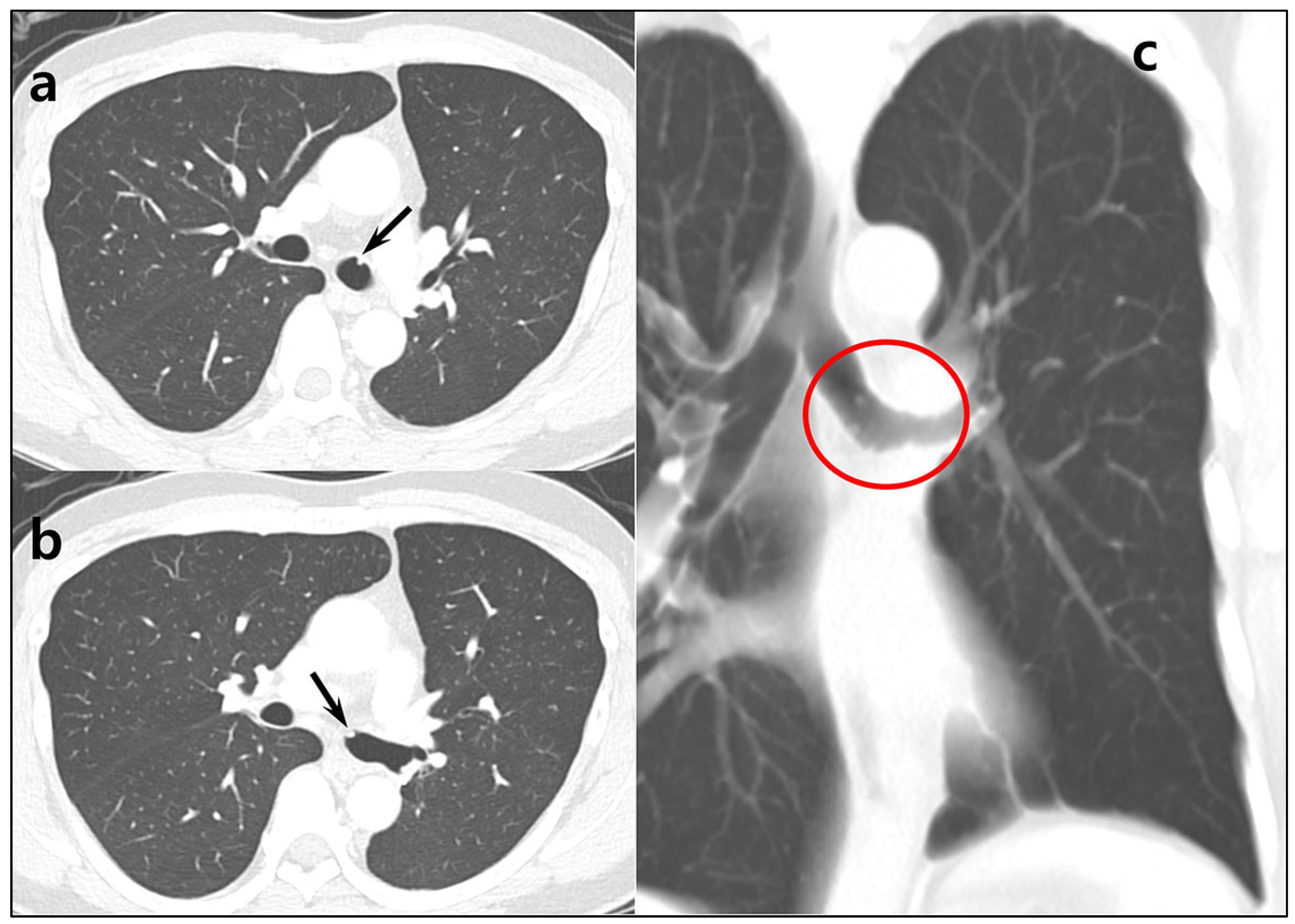

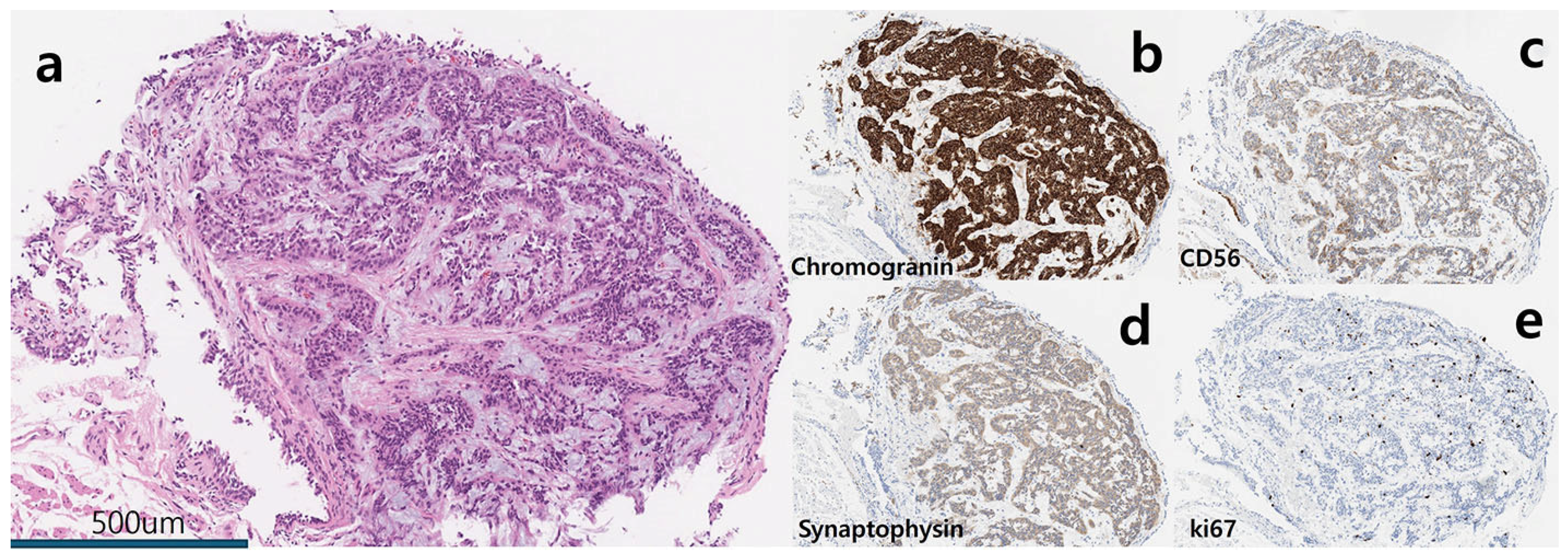
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Noguchi, M.; Furukawa, K.T.; Morimoto, M. Pulmonary neuroendocrine cells: Physiology, tissue homeostasis and disease. Dis. Model. Mech. 2020, 13, dmm046920. [Google Scholar] [CrossRef]
- Granberg, D.; Juhlin, C.C.; Falhammar, H.; Hedayati, E. Lung Carcinoids: A Comprehensive Review for Clinicians. Cancers 2023, 15, 5440. [Google Scholar] [CrossRef] [PubMed]
- Rekhtman, N. Lung neuroendocrine neoplasms: Recent progress and persistent challenges. Mod. Pathol. 2022, 35 (Suppl. S1), 36–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Hwang, G.; Pancirer, D.; Hornbacker, K.; Codima, A.; Lui, N.S.; Raj, R.; Kunz, P.; Padda, S.K. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: Clinical characteristics and progression to carcinoid tumour. Eur. Respir. J. 2022, 59, 2101058. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.S.; Akin, O.; Berger, D.M.; Zakowski, M.F.; Panicek, D.M. Pulmonary tumorlets: CT findings. Am. J. Roentgenol. 2004, 183, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.C.; Thomas, C.F.; Jett, J.R., Jr.; Swensen, S.J.; Myers, J.L. Significance of multiple carcinoid tumors and tumorlets in surgical lung specimens: Analysis of 28 patients. Chest 2007, 131, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Maki, Y.; Okada, K.; Nakamura, R.; Hirano, Y.; Fujiwara, T.; Yamasaki, R.; Ichimura, K.; Matsuura, M. A case of multiple lung carcinoid tumors localized in the right lower lobe. Respir. Med. Case Rep. 2022, 38, 101679. [Google Scholar] [CrossRef]
- De Giorgi, U.; Fanini, F.; Amadori, D.; Cancellieri, A.; Fiorentini, G.; Poletti, V.; Barzon, L.; Masi, G. Tumorlets in familial history of bronchopulmonary carcinoid. J. Thorac. Oncol. 2011, 6, 1613–1614. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Tegeltija, D.; Popovic, A.; Lecic, S.K.; Lovrenski, A. An Unusual Finding of Tumorlet Carcinoid and Endobronchial Hamartoma in the Lobar Bronchus. Int. J. Morphol. 2022, 40, 990–994. [Google Scholar] [CrossRef]
- Pandey, A.; Bargunam, P.; Saini, M.; Kundu, S.; Sharma, M. Carcinoid tumorlet/typical carcinoid of the lung: An incidental bronchoscopic finding and cytological dilemma. J. Assoc. Chest Physicians 2024, 12, 33–39. [Google Scholar] [CrossRef]
- Ferolla, P.; Daddi, N.; Urbani, M.; Semeraro, A.; Ribacchi, R.; Giovenali, P.; Ascani, S.; De Angelis, V.; Crino, L.; Puma, F.; et al. Tumorlets, multicentric carcinoids, lymph-nodal metastases, and long-term behavior in bronchial carcinoids. J. Thorac. Oncol. 2009, 4, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.L.; Frilling, A.; de Herder, W.W.; Hörsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Miyanaga, A.; Masuda, M.; Motoi, N.; Tsuta, K.; Nakamura, Y.; Nishijima, N.; Watanabe, S.I.; Asamura, H.; Tsuchida, A.; Seike, M.; et al. Whole-exome and RNA sequencing of pulmonary carcinoid reveals chromosomal rearrangements associated with recurrence. Lung Cancer 2020, 145, 85–94. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, K.; Jeon, K.N.; Heo, I.R.; An, H.J.; Song, D.H. Central Airway Carcinoid Tumorlets Following Resection of a Typical Carcinoid Tumor. Diagnostics 2025, 15, 1651. https://doi.org/10.3390/diagnostics15131651
Bae K, Jeon KN, Heo IR, An HJ, Song DH. Central Airway Carcinoid Tumorlets Following Resection of a Typical Carcinoid Tumor. Diagnostics. 2025; 15(13):1651. https://doi.org/10.3390/diagnostics15131651
Chicago/Turabian StyleBae, Kyungsoo, Kyung Nyeo Jeon, I Re Heo, Hyo Jung An, and Dae Hyun Song. 2025. "Central Airway Carcinoid Tumorlets Following Resection of a Typical Carcinoid Tumor" Diagnostics 15, no. 13: 1651. https://doi.org/10.3390/diagnostics15131651
APA StyleBae, K., Jeon, K. N., Heo, I. R., An, H. J., & Song, D. H. (2025). Central Airway Carcinoid Tumorlets Following Resection of a Typical Carcinoid Tumor. Diagnostics, 15(13), 1651. https://doi.org/10.3390/diagnostics15131651





