Association of Leptin in Sarcopenia and Bone Density in Elderly Women: An Observational Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Women aged 65 years or older.
- Agreement to undergo dual-energy X-Ray absorptiometry (DEXA) for bone and body composition assessment.
- History of femoral surgery.
- Use of osteoporosis medications within the past year.
- Current dialysis for chronic kidney disease.
- Ongoing chemotherapy for malignancy.
- Severe limitation in physical activity due to encephalopathy or major musculoskeletal disorders.
2.2. Data Collection
2.3. Group Classification
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASM | Appendicular Skeletal Muscle Mass |
| BMD | Bone Mineral Density |
| BMI | Body Mass Index |
| Fat% | Total Body Fat Percentage |
| Fat Index | Fat Mass divided by Height Squared |
| SE | Standard Error |
| KWGS | Korean Working Group on Sarcopenia |
References
- Edwards, M.; Dennison, E.; Sayer, A.A.; Fielding, R.; Cooper, C. Osteoporosis and sarcopenia in older age. Bone 2015, 80, 126–130. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Christodoulou, C.; Cooper, C. What is osteoporosis? Postgrad. Med. J. 2003, 79, 133–138. [Google Scholar] [CrossRef]
- Boschitsch, E.; Durchschlag, E.; Dimai, H. Age-related prevalence of osteoporosis and fragility fractures: Real-world data from an Austrian Menopause and Osteoporosis Clinic. Climacteric 2017, 20, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Balaskó, M.; Soós, S.; Székely, M.; Pétervári, E. Leptin and aging: Review and questions with particular emphasis on its role in the central regulation of energy balance. J. Chem. Neuroanat. 2014, 61, 248–255. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Chen, W.-L. Examining the association between serum leptin and sarcopenic obesity. J. Inflamm. Res. 2021, 14, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Giardullo, L.; Corrado, A.; Maruotti, N.; Cici, D.; Mansueto, N.; Cantatore, F.P. Adipokine role in physiopathology of inflammatory and degenerative musculoskeletal diseases. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211015034. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Ng, T.P.; Lu, Y.; Choo, R.W.M.; Tan, C.T.Y.; Nyunt, M.S.Z.; Gao, Q.; Mok, E.W.H.; Larbi, A. Dysregulated homeostatic pathways in sarcopenia among frail older adults. Aging Cell 2018, 17, e12842. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Kang, S.M.; Kang, J.-H.; Kang, S.Y.; Kim, K.K.; Kim, K.-B.; Kim, B.; Kim, S.J.; Kim, Y.-H.; Kim, J.-H. 2020 Korean Society for the Study of Obesity guidelines for the management of obesity in Korea. J. Obes. Metab. Syndr. 2021, 30, 81. [Google Scholar] [CrossRef]
- Baek, J.Y.; Jung, H.-W.; Kim, K.M.; Kim, M.; Park, C.Y.; Lee, K.-P.; Lee, S.Y.; Jang, I.-Y.; Jeon, O.H.; Lim, J.-Y. Korean Working Group on Sarcopenia guideline: Expert consensus on sarcopenia screening and diagnosis by the Korean Society of Sarcopenia, the Korean Society for Bone and Mineral Research, and the Korean Geriatrics Society. Ann. Geriatr. Med. Res. 2023, 27, 9. [Google Scholar] [CrossRef]
- Zoico, E.; Zamboni, M.; Adami, S.; Vettor, R.; Mazzali, G.; Tosoni, P.; Bissoli, L.; Bosello, O. Relationship between leptin levels and bone mineral density in the elderly. Clin. Endocrinol. 2003, 59, 97–103. [Google Scholar] [CrossRef]
- Pasco, J.A.; Henry, M.J.; Kotowicz, M.A.; Collier, G.R.; Ball, M.J.; Ugoni, A.M.; Nicholson, G.C. Serum leptin levels are associated with bone mass in nonobese women. J. Clin. Endocrinol. Metab. 2001, 86, 1884–1887. [Google Scholar] [CrossRef] [PubMed]
- Motyl, K.J.; Rosen, C.J. Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie 2012, 94, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W. Leptin, bone mass, and the thrifty phenotype. J. Bone Miner. Res. 2004, 19, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Roman, E.A.; Reis, D.; Romanatto, T.; Maimoni, D.; Ferreira, E.A.; Santos, G.A.; Torsoni, A.S.; Velloso, L.A.; Torsoni, M.A. Central leptin action improves skeletal muscle AKT, AMPK, and PGC1α activation by hypothalamic PI3K-dependent mechanism. Mol. Cell. Endocrinol. 2010, 314, 62–69. [Google Scholar] [CrossRef]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Pan, W.-T.; Lee, Y.; Kakuma, T.; Zhou, Y.-T.; Unger, R.H. The role of leptin resistance in the lipid abnormalities of aging. FASEB J. 2001, 15, 108–114. [Google Scholar] [CrossRef]
- Kao, T.-W.; Peng, T.-C.; Chen, W.-L.; Chi, Y.-C.; Chen, C.-L.; Yang, W.-S. Higher serum leptin levels are associated with a reduced risk of sarcopenia but a higher risk of dynapenia among older adults. J. Inflamm. Res. 2021, 14, 5817–5825. [Google Scholar] [CrossRef]
- Lana, A.; Valdés-Bécares, A.; Buño, A.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Serum leptin concentration is associated with incident frailty in older adults. Aging Dis. 2017, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J. Role of adipose tissue in body-weight regulation: Mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 2000, 59, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, A.-F.; O’Connor, S.; Morin, S.N.; Gibbs, J.C.; Willie, B.M.; Jean, S.; Gagnon, C. Association between obesity and risk of fracture, bone mineral density and bone quality in adults: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252487. [Google Scholar] [CrossRef] [PubMed]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9, 228S–233S. [Google Scholar] [CrossRef]
- Lee, D.G.; Bae, J.H. Fatty infiltration of the multifidus muscle independently increases osteoporotic vertebral compression fracture risk. BMC Musculoskelet. Disord. 2023, 24, 508. [Google Scholar] [CrossRef]
- Pérez-López, F.; Ara, I. Fragility fracture risk and skeletal muscle function. Climacteric 2016, 19, 37–41. [Google Scholar] [CrossRef]
| Variable | Total (n = 79) | Sarcopenia (n = 20) | Non-Sarcopenia (n = 59) | p-Value |
|---|---|---|---|---|
| Age(year) | 78.6 ± 5.7 | 79.7 ± 5.4 | 78.2 ± 5.8 | 0.208 |
| BMI (kg/m2) | 24.2 ± 3.6 | 22.8 ± 2.7 | 25.1 ± 3.6 | 0.003 ** |
| ASM (kg/m2) | 5.12 ± 0.84 | 4.73 ± 0.62 | 5.24 ± 0.86 | 0.005 ** |
| Grip Strength (kg) | 17.3 ± 5.1 | 14.7 ± 4.2 | 18.2 ± 5.2 | 0.003 ** |
| Femur Neck BMD (g/cm2) | 0.69 ± 0.11 | 0.66 ± 0.10 | 0.70 ± 0.11 | 0.094 |
| Leptin (ng/mL) | 23.1 ± 17.2 | 18.1 ± 14.5 | 25.2 ± 17.8 | 0.120 |
| Compression Fracture (N) | 38 (48.1%) | 24 (30.4%) | 14 (17.7%) |
| Variable | Obese (Mean ± SD) | Non-Obese (Mean ± SD) | p-Value |
|---|---|---|---|
| Fat Index | 11.6 ± 1.99 | 8.24 ± 1.76 | <0.001 *** |
| Leptin (ng/mL) | 31.8 ± 29.6 | 14.8 ± 10.5 | <0.001 *** |
| ASM (kg) | 5.37 ± 0.59 | 4.91 ± 0.46 | <0.001 *** |
| Grip Strength (kg) | 17.8 ± 4.63 | 16.8 ± 5.04 | 0.383 |
| Femur Neck BMD (g/cm2) | 0.599 ± 0.09 | 0.577 ± 0.06 | 0.259 |
| Variable | BMI (r, 95% CI) | Fat Index (r, 95% CI) | Total Fat % (r, 95% CI) | ASM (r, 95% CI) | Grip Strength (r, 95% CI) | Femur Neck BMD (r, 95% CI) |
|---|---|---|---|---|---|---|
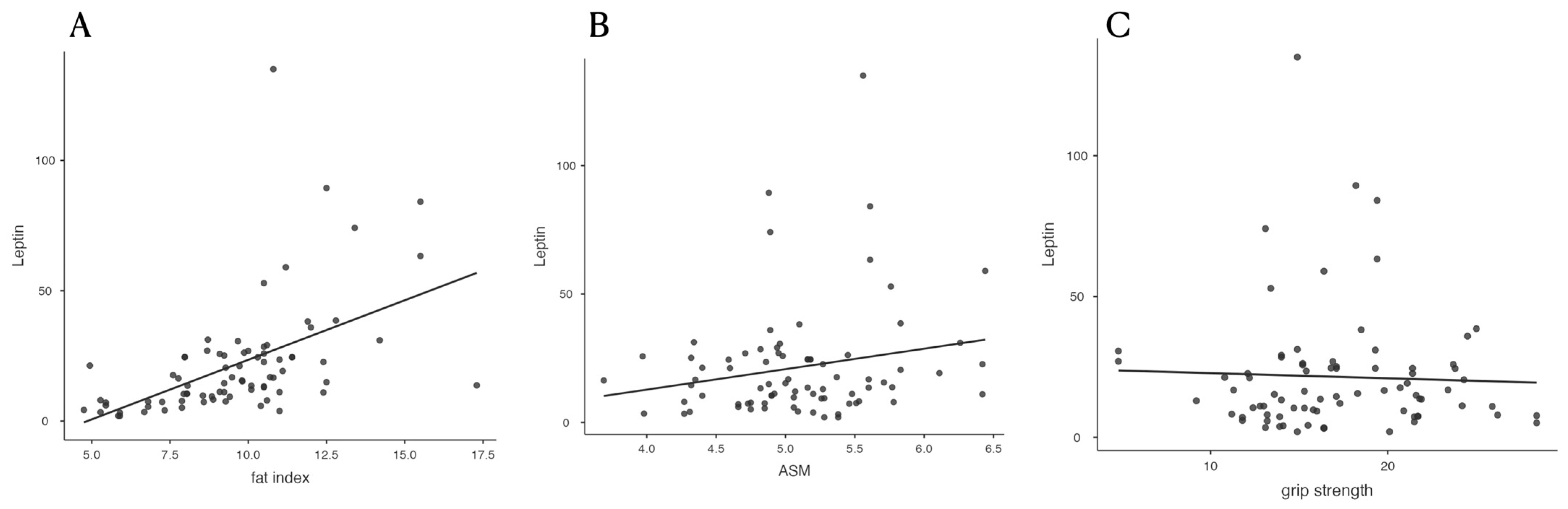
| Leptin | 0.486 *** (0.297–0.638) | 0.518 *** (0.335–0.663) | 0.526 *** (0.345–0.669) | 0.206 (−0.016–0.409) | −0.041 (−0.260–0.182) | −0.195 (−0.027–0.399) |
| BMI | — | 0.892 *** (0.836–0.930) | 0.755 *** (0.641–0.837) | 0.529 *** (0.348–0.671) | 0.222 * (0.001–0.422) | 0.339 * (0.128–0.521) |
| Fat Index | 0.892 *** (03.836–0.930) | — | 0.902 *** (0.851–0.937) | 0.468 *** (0.276–0.625) | 0.247 * (0.027–0.444) | 0.365 *** (0.157–0.543) |
| Total Fat % | 0.755 *** (0.641–0.837) | 0.902 *** (0.851–0.937) | — | 0.144 (−0.079–0.354) | 0.211 (−0.010–0.413) | 0.293 *** (0.077–0.483) |
| ASM | 0.529 *** (0.348–0.671) | 0.468 *** (0.276–0.625) | 0.144 (−0.079–0.354) | — | 0.275 * (0.066–0.474) | 0.283 * (0.066–0.474) |
| Grip Strength | 0.222 * (0.001–0.422) | 0.247 * (0.027–0.444) | 0.211 (−0.010–0.413) | 0.275 * (0.066–0.474) | — | 0.185 (−0.037–0.390) |
| Predictor | Estimate | SE | Z | p-Value |
|---|---|---|---|---|
| Leptin | −0.0203 | 0.0132 | −1.537 | 0.124 |
| ASM | −0.8340 | 0.4360 | −1.920 | 0.055 |
| Grip Strength | −0.1010 | 0.0503 | −2.000 | 0.046 * |
| Femur Neck BMD | −10.4000 | 3.6000 | −2.890 | 0.004 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.G.; Lee, J.H. Association of Leptin in Sarcopenia and Bone Density in Elderly Women: An Observational Analysis. Diagnostics 2025, 15, 1620. https://doi.org/10.3390/diagnostics15131620
Lee DG, Lee JH. Association of Leptin in Sarcopenia and Bone Density in Elderly Women: An Observational Analysis. Diagnostics. 2025; 15(13):1620. https://doi.org/10.3390/diagnostics15131620
Chicago/Turabian StyleLee, Dong Gyu, and Jong Ho Lee. 2025. "Association of Leptin in Sarcopenia and Bone Density in Elderly Women: An Observational Analysis" Diagnostics 15, no. 13: 1620. https://doi.org/10.3390/diagnostics15131620
APA StyleLee, D. G., & Lee, J. H. (2025). Association of Leptin in Sarcopenia and Bone Density in Elderly Women: An Observational Analysis. Diagnostics, 15(13), 1620. https://doi.org/10.3390/diagnostics15131620






