Tips for Hepatologist Referral of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease with Alanine Aminotransferase Levels ≤ 30 U/L
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Procedure
2.3. Liver Biopsy and Histological Analysis
2.4. Statistical Analyses
3. Results
3.1. Comparison of Patients with MASLD and Fibrosis Stages 3–4 vs. 0–2 with ALT Levels ≤ 30 U/L
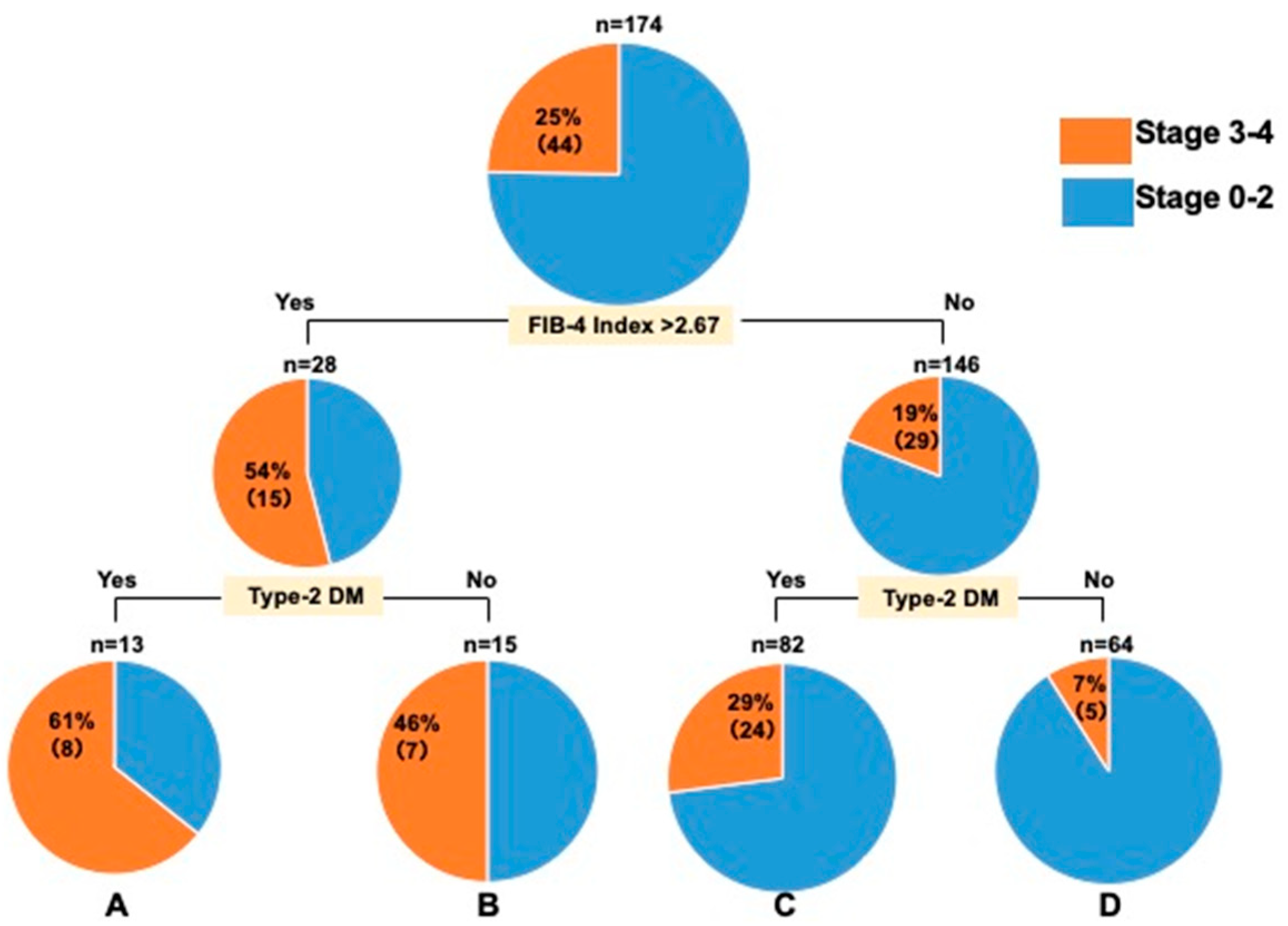
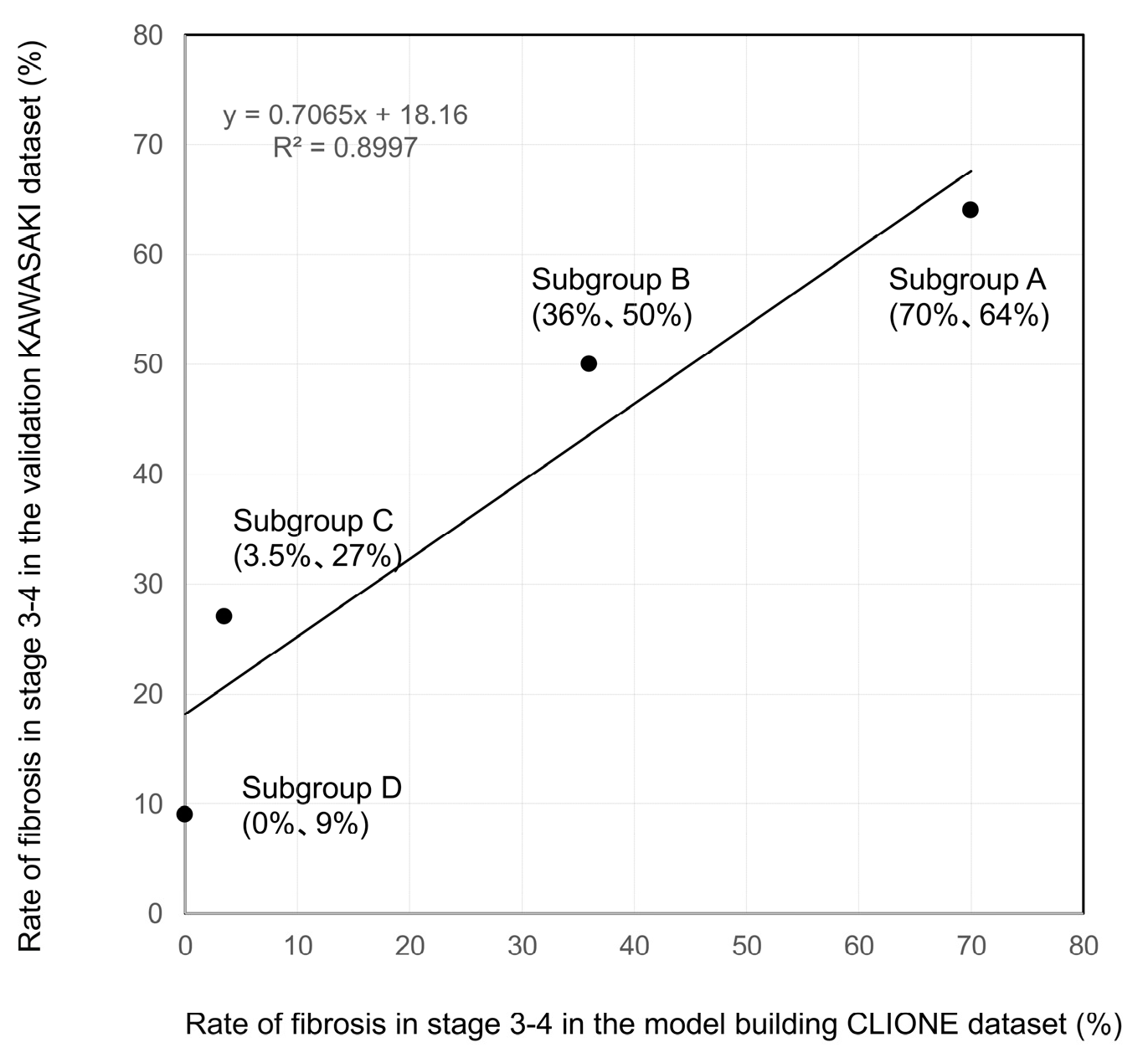
3.2. Decision-Tree Analysis of Stage ≥ 3 in Patients with MASLD with ALT Levels ≤ 30 U/L
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol. Res. 2021, 51, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kawaguchi, M.; Kawaguchi, T.; Yoshiji, H. Profiles associated with significant hepatic fibrosis consisting of alanine aminotransferase >30 U/L, exercise habits, and metabolic dysfunction-associated steatotic liver disease. Hepatol. Res. 2024, 54, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J.; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Ueno, Y.; Hiasa, Y.; Nishikawa, H.; Hige, S.; Takikawa, Y.; Taniai, M.; Ishikawa, T.; Yasui, K.; Takaki, A.; et al. The transition in the etiologies of hepatocellular carcinoma-complicated liver cirrhosis in a nationwide survey of Japan. J. Gastroenterol. 2021, 56, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chan, C.; Lee, H.W.; Huang, C.; Chen, Y.J.; Liu, P.C.; Lu, S.N.; Chuang, W.L.; Huang, J.F.; Yu, M.L.; et al. Influence of nonalcoholic fatty liver disease with increased liver enzyme levels on the risk of cirrhosis and hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2023, 21, 960–969.e1. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Wilson, L.A.; Cummings, O.W.; Clark, J.M.; Loomba, R.; Hameed, B.; Abdelmalek, M.F.; Dasarathy, S.; Neuschwander-Tetri, B.A.; Kowdley, K.; et al. Histologic findings of advanced fibrosis and cirrhosis in patients with nonalcoholic fatty liver disease who have normal aminotransferase levels. Am. J. Gastroenterol. 2019, 114, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Laouenan, C.; Vallet-Pichard, A.; Vidal-Trécan, T.; Manchon, P.; Paradis, V.; Roulot, D.; Gault, N.; Boitard, C.; Terris, B.; et al. High prevalence of NASH and advanced fibrosis in type 2 diabetes: A prospective study of 330 outpatients undergoing liver biopsies for elevated ALT, using a low threshold. Diabetes Care 2023, 46, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]


| All Patients | CLIONE Study | Kawasaki Study | |
|---|---|---|---|
| N = 289 | N = 115 | N = 174 | |
| Age, years | 62 (18–82) | 63 (28–78) | 61 (18–82) |
| Male sex % | 36 | 29 | 39 |
| Stage, 0/1/2/3/4 | 78/97/51/48/15 | 40/39/17/13/6 | 38/58/34/35/9 |
| Lobular inflammation, 0/1/2/3 | 33/202/48/6 | 9/85/18/3 | 24/117/30/3 |
| Steatosis, 0/1/2/3 | 26/205/50/8 | 2/97/11/5 | 24/108/39/3 |
| Ballooning, 0/1/2 | 147/119/23 | 56/46/13 | 91/73/10 |
| Type-2 DM (yes/no, %) | 151/138 (52) | 56/59 (49) | 95/79 (54) |
| Hypertension (yes/no, %) | 130/159 (45) | 65/50 (56) | 65/109 (37) |
| Dyslipidemia (yes/no, %) | 206/83 (71) | 65/50 (56) | 141/33 (81) |
| ALT (U/L) | 22 (9–30) | 24 (14–30) | 21 (9–29) |
| AST (U/L) | 23 (12–77) | 24 (14–77) | 23 (12–60) |
| γ-GTP (U/L) | 32 (9–440) | 35 (9–440) | 30 (11–294) |
| Total cholesterol (mg/dL) | 191 (90–307) | 194 (117–273) | 190 (90–307) |
| Platelet counts (104/µL) | 20.5 (3.4–46) | 21.1 (3.4–46) | 20.3 (6.6–37) |
| Albumin (g/dL) | 4.2 (2.5–5.4) | 4.2 (2.6–5.1) | 4.2 (2.5–5.4) |
| HbA1c (%) | 6 (3.5–12) | 6 (3.5–9.2) | 6 (3.9–12) |
| FBS (mg/dL) | 102 (66–300) | 103 (81–249) | 101 (66–300) |
| FIB-4 Index | 1.4 (0.3–12.4) | 1.42 (0.38–12.4) | 1.48 (0.30–7.3) |
| Stages 0–2 | Stages 3–4 | p-Value | |
|---|---|---|---|
| N = 226 | N = 63 | ||
| Age, years | 60 (18–77) | 67 (33–82) | <0.0001 |
| Male sex (%) | 37 | 26 | 0.1128 |
| Stage, 0/1/2/3/4 | 78/97/51/0/0 | 0/0/0/48/15 | <0.0001 |
| Lobular inflammation, 0/1/2/3 | 33/171/19/3 | 0/31/29/3 | <0.0001 |
| Steatosis, 0/1/2/3 | 18/164/39/5 | 8/41/11/3 | 0.468 |
| Ballooning, 0/1/2 | 132/82/12 | 15/37/11 | <0.0001 |
| Type-2 DM (yes/no, %) | 104/122(46) | 47/16 (74) | <0.0001 |
| HT (yes/no, %) | 89/137(39) | 41/22 (65) | 0.0003 |
| Dyslipidemia (yes/no, %) | 162/64(71) | 44/19 (69) | 0.776 |
| ALT (U/L) | 22 (10–30) | 21 (9–29) | 0.3547 |
| AST (U/L) | 23 (12–77) | 27 (15–69) | <0.0001 |
| γ-GTP (U/L) | 31 (9–440) | 36 (13–228) | 0.2024 |
| Total cholesterol (mg/dL) | 197 (90–307) | 180 (102–279) | 0.0008 |
| Platelet counts (104/µL) | 21 (7.5–46) | 16.2 (3.4–40.3) | <0.0001 |
| Albumin (g/dL) | 4 (2.5–5.4) | 4 (2.8–4.8) | <0.0001 |
| HbA1c (%) | 5.9 (3.5–12) | 6.2 (4.3–8.6) | <0.0001 |
| FBS (mg/dL) | 100 (66–300) | 110 (70–195) | 0.0444 |
| FIB-4 Index | 1.3 (0.3–7.3) | 2.58 (0.85–12.4) | <0.0001 |
| Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Age ≥ 65 years | 2.3 | 1.29–4.27 | 0.0052 |
| Type-2 DM (yes) | 3 | 1.55–5.80 | 0.0013 |
| Hypertension (yes) | 2 | 1.09–3.78 | 0.0263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawanaka, M.; Fujii, H.; Iwaki, M.; Hayashi, H.; Toyoda, H.; Oeda, S.; Hyogo, H.; Morishita, A.; Munekage, K.; Kawata, K.; et al. Tips for Hepatologist Referral of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease with Alanine Aminotransferase Levels ≤ 30 U/L. Diagnostics 2025, 15, 1591. https://doi.org/10.3390/diagnostics15131591
Kawanaka M, Fujii H, Iwaki M, Hayashi H, Toyoda H, Oeda S, Hyogo H, Morishita A, Munekage K, Kawata K, et al. Tips for Hepatologist Referral of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease with Alanine Aminotransferase Levels ≤ 30 U/L. Diagnostics. 2025; 15(13):1591. https://doi.org/10.3390/diagnostics15131591
Chicago/Turabian StyleKawanaka, Miwa, Hideki Fujii, Michihiro Iwaki, Hideki Hayashi, Hidenori Toyoda, Satoshi Oeda, Hideyuki Hyogo, Asahiro Morishita, Kensuke Munekage, Kazuhito Kawata, and et al. 2025. "Tips for Hepatologist Referral of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease with Alanine Aminotransferase Levels ≤ 30 U/L" Diagnostics 15, no. 13: 1591. https://doi.org/10.3390/diagnostics15131591
APA StyleKawanaka, M., Fujii, H., Iwaki, M., Hayashi, H., Toyoda, H., Oeda, S., Hyogo, H., Morishita, A., Munekage, K., Kawata, K., Tsutsumi, T., Sawada, K., Maeshiro, T., Tobita, H., Yoshida, Y., Naito, M., Araki, A., Arakaki, S., Kawaguchi, T., ... Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD). (2025). Tips for Hepatologist Referral of Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease with Alanine Aminotransferase Levels ≤ 30 U/L. Diagnostics, 15(13), 1591. https://doi.org/10.3390/diagnostics15131591












