A Novel Concept of the “Standard Human” in the Assessment of Individual Total Heart Size: Lessons from Non-Contrast-Enhanced Cardiac CT Examinations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiovascular Risk Factors’ Evaluation Se
2.3. Coronary Artery Calcium Determination
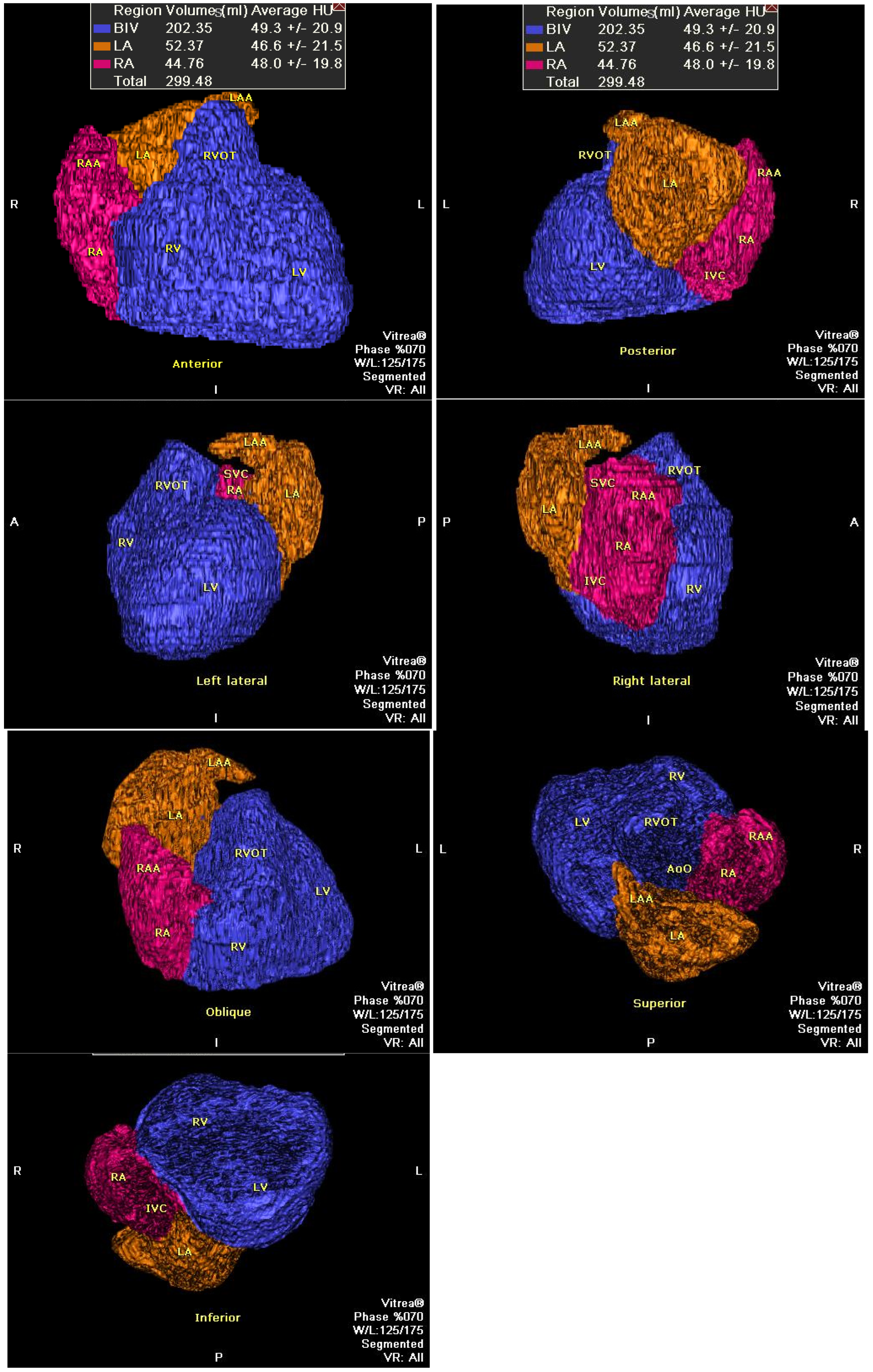
2.4. Heart Size Evaluation
2.5. Inter- and Intra-Observer Variability
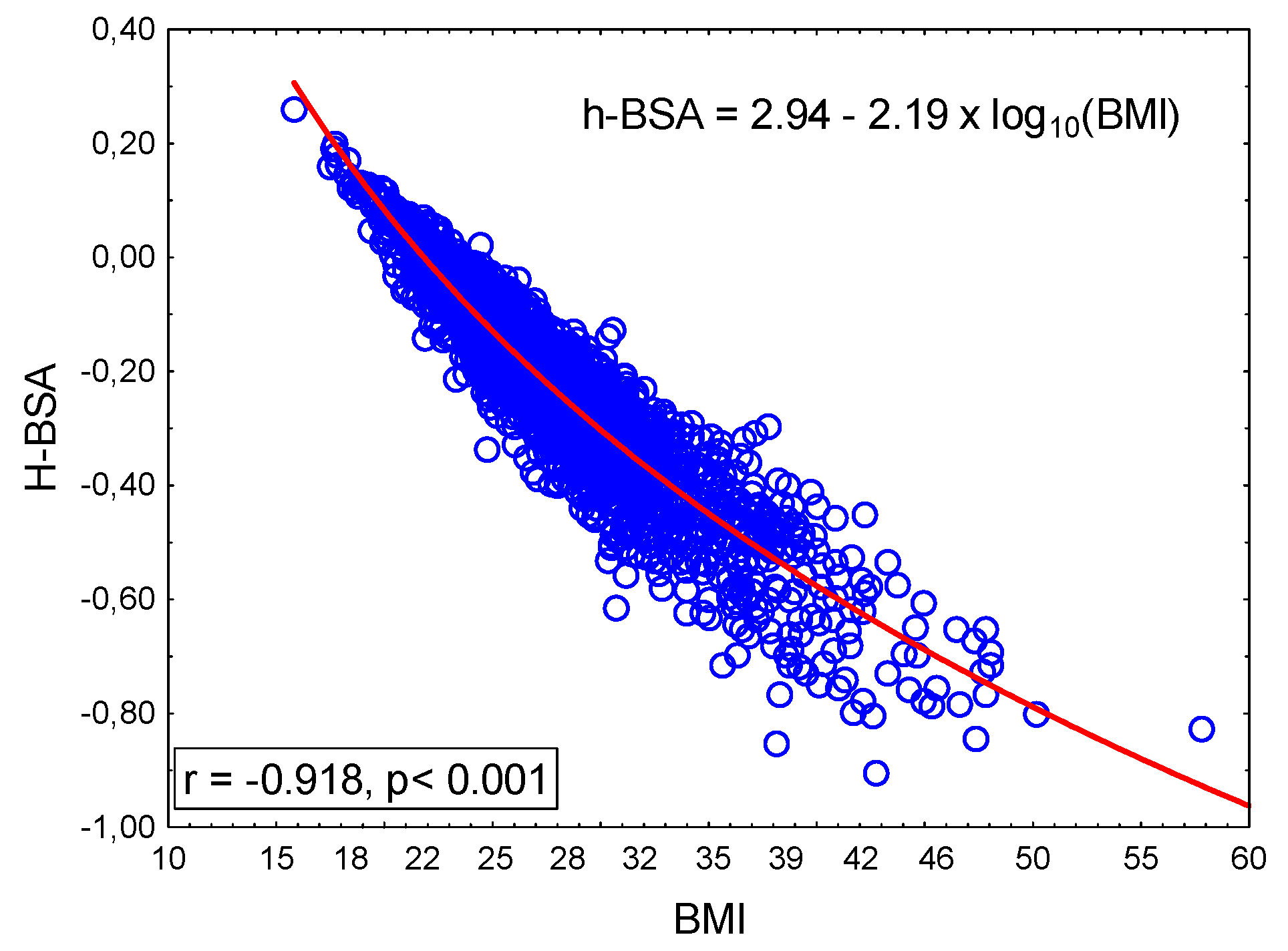
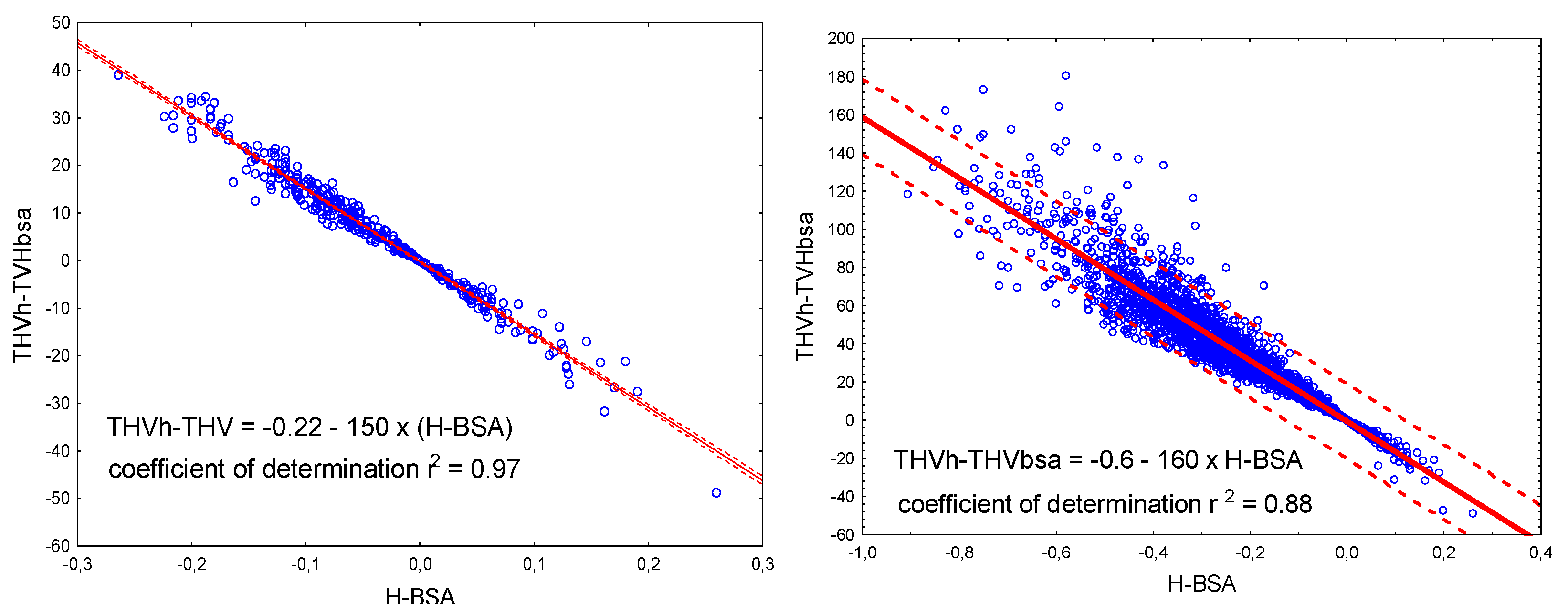
2.6. Physiological Meaning of h-BSA
2.7. Study Subgroups
2.8. Relationship Between Total Heart Volume and Height in the Reference Group
2.9. The “Standard Human”
| Case | Height [m] | Weight [kg] | BMI [kg/m2] | BSA | h-BSA | Expected THV [95% Prediction Interval] |
|---|---|---|---|---|---|---|
| A | 1.73 | 62.3 | 20.8 | 1.73 | 0 | 453 [312–595] |
| B | 48.7 | 16.3 | 1.53 | 0.2 | 409 [264–555] | |
| C | 77.5 | 25.9 | 1.93 | −0.2 | 498 [356–639] | |
| D | 89.0 | 29.8 | 2.07 | −0.34 | 529 [387–671] |
2.10. Individual-Oriented Approach
2.11. Statistical Analysis
2.12. Ethics
3. Results
3.1. Total Heart Volume in the Reference and Other Subgroups
3.2. THV Relations with Body Mass Index and CV Risk
3.3. Prevalence of Abnormal THV
4. Discussion
4.1. Main Result
4.2. Methodical Issues
4.3. Patient Selection Special Criteria
4.4. Limitations
4.5. Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badeer, H.S. Development of cardiomegaly: A unifying hypothesis explaining the growth of muscle fibers, blood vessels and collagen of heart. Cardiology 1972, 57, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, F.; Almahmeed, W.; Bays, H.; Cuevas, A.; Di Angelantonio, E.; le Roux, C.W.; Sattar, N.; Sun, M.C.; Wittert, G.; Pinto, F.J.; et al. Obesity and cardiovascular disease: Mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur. J. Prev. Cardiol. 2022, 29, 2218–2237. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B. Incidence and epidemiology of heart failure. Heart Fail. Rev. 2000, 5, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.L.; Horwich, T.B.; Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011, 8, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Hummel, S.L.; Konerman, M.C. Epidemiology, diagnosis, pathophysiology, and initial approach to heart failure with preserved ejection fraction. Cardiol. Clin. 2022, 40, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, G.A.; Fishbein, M.C. When is Having a Big Heart a Problem? Acad. Forensic Pathol. 2011, 1, 188–193. [Google Scholar] [CrossRef]
- Pfaffenberger, S.; Bartko, P.; Graf, A.; Pernicka, E.; Babayev, J.; Lolic, E.; Bonderman, D.; Baumgartner, H.; Maurer, G.; Mascherbauer, J. Size matters! Impact of age, sex, height, and weight on the normal heart size. Circ. Cardiovasc. Imaging 2013, 6, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, J.H., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the diagnosis and management of aortic disease: A report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Natori, S.; Lai, S.; Finn, J.P.; Gomes, A.S.; Hundley, W.G.; Jerosch-Herold, M.; Person, G.; Sinha, S.; Arai, A.; Lima, J.A.C.; et al. Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. Am. J. Roentgenol. 2006, 186 (Suppl. S2), S357–S365. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.L.; Bhat, A.; Gan, G.C.H.; Khanna, S.; Ahlenstiel, G.; Negishi, K.; Tan, T.C. The impact of body mass index on cardiac structure and function in a cohort of obese patients without traditional cardiovascular risk factors. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 19, 200211. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Cheong, B.Y.C.; Wilson, J.M.; Spann, S.J.; Pettigrew, R.I.; Preventza, O.A.; Muthupillai, R. Coronary artery calcium scoring: An evidence-based guide for primary care physicians. J. Intern. Med. 2021, 289, 309–324. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2022, 29, 5–115. [Google Scholar] [CrossRef]
- Miles, B.; Theng, B.; Zeinoddini, A.; Saleem, A. The potential impact of computed tomography coronary calcium score screening on patients with dyslipidemia. Proc. (Bayl. Univ. Med. Cent.) 2023, 36, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Madaj, P.; Budoff, M.J. Risk stratification of non-contrast CT beyond the coronary calcium scan. J. Cardiovasc. Comput. Tomogr. 2012, 6, 301–307. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Lehmann, N.; Möhlenkamp, S.; Pundt, N.; Dykun, I.; Roggenbuck, U.; Kälsch, H. Noncoronary measures enhance the predictive value of cardiac CT above traditional risk factors and CAC score in the general population. JACC Cardiovasc. Imaging 2016, 9, 1177–1185. [Google Scholar] [CrossRef]
- Bernardini, F.; Gelfusa, M.; Celeski, M.; Coletti, F.; Nusca, A.; De Stefano, D.; Grigioni, F. Beyond the calcium score: What additional information from a CT scan can assist in cardiovascular risk assessment? Appl. Sci. 2022, 13, 241. [Google Scholar] [CrossRef]
- Tay, S.Y.; Chang, P.Y.; Lao, W.T.; Lin, Y.C.; Chung, Y.H.; Chan, W.P. The proper use of coronary calcium score and coronary computed tomography angiography for screening asymptomatic patients with cardiovascular risk factors. Sci. Rep. 2017, 7, 17653. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Picard, M.H.; Triulzi, M.O.; Thomas, J.D.; Newell, J.; King, M.E.; Weyman, A.E. New perspectives in the assessment of cardiac chamber dimensions during development and adulthood. J. Am. Coll. Cardiol. 1992, 19, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef] [PubMed]
- Bruns, S.; Wolterink, J.M.; Takx, R.A.P.; van Hamersvelt, R.W.; Sucha, D.; Viergever, M.A.; Leiner, T.; Isgum, I. Deep learning from dual-energy information for whole-heart segmentation in dual-energy and single-energy non-contrast-enhanced cardiac CT. Med. Phys. 2020, 47, 5048–5060. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.R.; Abadi, S.; Solomonica, A.; Mutlak, D.; Aronson, D.; Agmon, Y.; Lessick, J. Left-sided cardiac chamber evaluation using single-phase mid-diastolic coronary computed tomography angiography: Derivation of normal values and comparison with conventional end-diastolic and end-systolic phases. Eur. Radiol. 2016, 26, 3626–3634. [Google Scholar] [CrossRef]
- Raman, S.V.; Shah, M.; McCarthy, B.; Garcia, A.; Ferketich, A.K. Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am. Heart J. 2006, 151, 736–744. [Google Scholar] [CrossRef]
- Mao, S.S.; Li, D.; Vembar, M.; Gao, Y.; Luo, Y.; Lam, F.; Syed, Y.S.; Liu, C.; Woo, K.; Flores, F.; et al. Model-based automatic segmentation algorithm accurately assesses the whole cardiac volumetric parameters in patients with cardiac CT angiography: A validation study for evaluating the accuracy of the workstation software and establishing the reference values. Acad. Radiol. 2014, 21, 639–647. [Google Scholar] [CrossRef]
- Shalmon, T.; Arbel, Y.; Granot, Y.; Ziv-Baran, T.; Chorin, E.; Shmilovich, H.; Havakuk, O.; Berliner, S.; Carrillo Estrada, M.; Aviram, G. Cardiac gated computed tomography angiography discloses a correlation between the volumes of all four cardiac chambers and heart rate in men but not in women. Women’s Health Rep. 2020, 1, 393–401. [Google Scholar] [CrossRef]
- Fuchs, A.; Mejdahl, M.R.; Kühl, J.T.; Stisen, Z.R.; Nilsson, E.J.P.; Køber, L.V.; Nordestgaard, B.G.; Kofoed, K.F. Normal values of left ventricular mass and cardiac chamber volumes assessed by 320-detector computed tomography angiography in the Copenhagen General Population Study. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1009–1017. [Google Scholar] [CrossRef]
- Juneau, D.; Erthal, F.; Clarkin, O.; Alzahrani, A.; Alenazy, A.; Hossain, A.; Inacio, R.I.; Dwivedi, G.; Dick, A.J.; Rybicki, F.J.; et al. Mid-diastolic left ventricular volume and mass: Normal values for coronary computed tomography angiography. J. Cardiovasc. Comput. Tomogr. 2017, 11, 135–140. [Google Scholar] [CrossRef]
- Massalha, A.; Almufleh, A.; Walpot, J.; Ratnayake, I.; Qureshi, R.; Abbass, T.; Pena, E.; Inacio, J.; Rybicki, F.J.; Small, G.; et al. Reference values for mid-diastolic right ventricular volume in a population referred for cardiac computed tomography: An additional diagnostic value to cardiac computed tomography. J. Cardiovasc. Comput. Tomogr. 2020, 14, 226–232. [Google Scholar] [CrossRef]
- Daniel, K.R.; Bertoni, A.G.; Ding, J.; Johnston, S.; Budoff, M.J.; Bluemke, D.A.; Carr, J.J. Comparison of methods to measure heart size using noncontrast-enhanced computed tomography: Correlation with left ventricular mass. J. Comput. Assist. Tomogr. 2008, 32, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Pagali, S.R.; Hamirani, Y.S.; Chen, A.; Cheu, G.; Gao, Y.; Li, D.; Mao, S. Sex-specific biatrial volumetric measurements obtained with use of multidetector computed tomography in subjects with and without coronary artery disease. Tex. Heart Inst. J. 2014, 41, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.Y.; Devereux, R.B.; Roman, M.J.; Meng, J.; Jow, V.M.; Jacobs, A.; Weinsaft, J.W.; Shaw, L.J.; Berman, D.S.; Callister, T.Q.; et al. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: Mean values among healthy adults free of hypertension and obesity. JACC. Cardiovasc. Imaging 2008, 1, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.; Budoff, M.J.; Shaw, L.J.; Khosa, F.; Rumberger, J.A.; Berman, D.; Callister, T.; Raggi, P.; Blumenthal, R.S.; Nasir, K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc. Imaging 2009, 2, 692–700. [Google Scholar] [CrossRef]
- Jacob, A.J.; Abdelkarim, O.; Zook, S.; Kragholm, K.H.; Gupta, P.; Cocker, M.; Giraldo, J.R.; Doherty, J.O.; Schoebigner, M.; Schwemmer, C.; et al. AI-based, automated chamber volumetry from gated, non-contrast CT. J. Cardiovasc. Comput. Tomogr. 2023, 17, 336–340. [Google Scholar] [CrossRef]
- Naghavi, M.; Reeves, A.; Atlas, K.; Zhang, C.; Budoff, M.; Henschke, C.I.; Yankelevitz, D.F.J. AI-enabled automated cardiac chambers volumetry in non-contrast ECG-gated cardiac scans vs. non-contrast non-gated lung scans. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.2765. [Google Scholar] [CrossRef]
- Naghavi, M.; Reeves, A.; Budoff, M.; Li, D.; Atlas, K.; Zhang, C.; Atlas, T.; Roy, S.K.; Henschke, C.I.; Wong, N.D.; et al. AI-enabled cardiac chambers volumetry in coronary artery calcium scans (AI-CACTM) predicts heart failure and outperforms NT-proBNP: The multi-ethnic study of Atherosclerosis. J. Cardiovasc. Comput. Tomogr. 2024, 18, 392–400. [Google Scholar] [CrossRef]
- Kay, F.U.; Abbara, S.; Joshi, P.H.; Garg, S.; Khera, A.; Peshock, R.M. Identification of high-risk left ventricular hypertrophy on calcium scoring cardiac computed tomography scans: Validation in the DHS. Circ. Cardiovasc. Imaging 2020, 13, e009678. [Google Scholar] [CrossRef]
- Han, D.; Shanbhag, A.; Miller, R.J.; Kwok, N.; Waechter, P.; Builoff, V.; Newby, D.E.; Dey., D.; Berman., D.S.; lomka, P. AI-derived left ventricular mass from noncontrast cardiac CT: Correlation with contrast CT angiography and CMR. J. Am. Coll. Cardiol. Adv. 2024, 3, 101249. [Google Scholar] [CrossRef]
- Miller, R.J.H.; Killekar, A.; Shanbhag, A.; Bednarski, B.; Michalowska, A.M.; Ruddy, T.D.; Einstein, A.J.; Newby., D.E.; Lemley, M.; Pieszko, K.; et al. Predicting mortality from AI cardiac volumes mass and coronary calcium on chest computed tomography. Nat. Commun. 2024, 15, 2747. [Google Scholar] [CrossRef]
- Chuang, M.L.; Danias, P.G.; Riley, M.F.; Hibberd, M.G.; Manning, W.J.; Douglas, P.S. Effect of increased body mass index on accuracy of two-dimensional echocardiography for measurement of left ventricular volume, ejection fraction, and mass. Am. J. Cardiol. 2001, 87, 371–374. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Mean/Proportion |
|---|---|
| Age (years) | 63 ± 11 |
| Sex (females, F/males, M) | 1503/802 |
| Risk factors (count) | 1.7 (0–4) |
| Smoking | 494 (21%) |
| Systemic arterial hypertension (SAH) | 1728 (75%) |
| High lipids (HL) | 1226 (53%) |
| Type 2 diabetes mellitus (t2DM | 458 (20%) |
| Body mass index (BMI, kg/m2) | 28.6 ± 4.9 |
| Normal | 579 (25%) |
| Overweight | 923 (40%) |
| Obese | 803 (35%) |
| Coronary artery calcium score (CACS) > 0 | 1463 (63%) |
| Sex | BMI Category | n | Age | BMI | BSA |
|---|---|---|---|---|---|
| F | 0 | 418 | 62 ± 10 | 22.87 ± 1.72 | 1.65 ± 0.11 |
| 1 | 575 | 66 ± 10 | 27.44 ± 1.44 | 1.79 ± 0.11 | |
| 2 | 510 | 64 ± 10 | 34.18 ± 3.87 | 1.99 ± 0.15 a | |
| M | 0 | 161 | 62 ± 12 | 23.37 ± 1.72 | 1.86 ± 0.13 |
| 1 | 348 | 62 ± 11 | 27.59 ± 1.41 | 2.03 ± 0.14 | |
| 2 | 293 | 60 ± 11 | 33.44 ± 3.26 | 2.22 ± 0.16 b |
| Parameters | Mean Difference | ICC | LOA | Pearson’s r |
|---|---|---|---|---|
| THV0 vs. THV1 (n = 45) | −15.6 mL | 0.996 | −51.0–19.8 | 0.992 |
| −3.1% | −9.4–3.2 | |||
| THVa vs. THVb (n = 125) | −1.7 mL | 0.996 | −33.2–29.8 | 0.991 |
| −0.4% | −6.5–5.6 |
| Sex | Female | Male | ||||
| Risk | Low | Low | ||||
| BMI | Normal | Overweight | Obesity | Normal | Overweight | Obesity |
| h-BSA | −0.022 ± 0.072 | −0.177 ± 0.061 | −0.372 ± 0.106 | −0.116 ± 0.072 | −0.274 ± 0.070 | −0.427 ± 0.094 |
| Risk | High | High | ||||
| BMI | Normal | Overweight | Obesity | Normal | Overweight | Obesity |
| h-BSA | −0.028 ± 0.068 | −0.177 ± 0.061 | −0.385 ± 0.125 | −0.114 ± 0.074 | −0.284 ± 0.070 | −0.487 ± 0.119 |
| Significance | p < 0.0001 | p < 0.0001 | ||||
| BMI Class | Risk | Sex | N | THV | THV/BSA | THV/h |
|---|---|---|---|---|---|---|
| 0 | 0 | F | 285 | 405.3 ± 73.0 | 246.2 ± 42.1 | 249.3 ± 43.1 |
| 0 | 0 | M | 68 | 506.9 ± 89.5 c | 271.7 ± 40.8 c | 290.0 ± 46.2 c |
| 0 | 1 | F | 133 | 431.4 ± 108.9 | 262.5 ± 64.9 | 266.6 ± 65.0 |
| 0 | 1 | M | 93 | 541.3 ± 115.3 c | 290.4 ± 58.4 c | 309.0 ± 63.6 c |
| 1 | 0 | F | 286 | 427.3 ± 69.9 | 237.9 ± 37.0 | 263.5 ± 40.8 |
| 1 | 0 | M | 118 | 525.1 ± 75.1 c | 260.1 ± 37.4 c | 300.4 ± 42.7 c |
| 1 | 1 | F | 289 | 452.1 ± 97.2 | 253.1 ± 52.9 | 280.7 ± 59.1 |
| 1 | 1 | M | 230 | 627.7 ± 148.2 c | 306.6 ± 66.0 c | 356.2 ± 78.6 c |
| 2 | 0 | F | 215 | 451.6 ± 68.4 | 227.3 ± 32.1 | 279.2 ± 40.5 |
| 2 | 0 | M | 49 | 536.4 ± 72.4 c | 248.2 ± 32.8 c | 308.8 ± 40.9 c |
| 2 | 1 | F | 295 | 513.9 ± 124.4 | 257.3 ± 56.2 | 319.3 ± 74.8 |
| 2 | 1 | M | 244 | 652.9 ± 152.6 c | 291.7 ± 64.0 c | 372.9 ± 84.4 c |
| Entire population | 2305 | 499.4 ± 134.7 | 260.8 ± 56.4 | 299.0 ± 72.6 | ||
| Sex | BMI | Risk | n | THVs (mL) | THVm (mL) | THVex (mL) | THV (m-ex. mL) | THVh-THVBSA (mL) |
|---|---|---|---|---|---|---|---|---|
| F | 0 | 0 | 285 | 406.0 ± 1.7 | 405.3 ± 6.3 | 410.9 ± 2.5 | −5.6 ± 5.9 | 3.1 ± 1.0 |
| F | 0 | 1 | 133 | 402.1 ± 2.4 | 431.4 ± 9.3 b | 408.4 ± 3.6 | 23.0 ± 8.7 c | 4.1 ± 1.4 |
| F | 1 | 0 | 286 | 403.8 ± 1.7 | 427.3 ± 6.3 | 442.9 ± 2.4 | −15.7 ± 5.9 | 25.7 ± 1.0 |
| F | 1 | 1 | 289 | 399.0 ± 1.7 | 452.1 ± 6.3 c | 438.0 ± 2.4 | 14.1 ± 5.9 c | 27.6 ± 1.1 a |
| F | 2 | 0 | 215 | 402.3 ± 1.9 | 451.6 ± 7.3 | 484.5 ± 2.8 | −32.9 ± 6.8 | 51.9 ± 1.1 |
| F | 2 | 1 | 295 | 398.1 ± 1.6 | 513.9 ± 6.2 c | 483.3 ± 2.4 | 30.6 ± 5.8 c | 62.0 ± 1.0 c |
| M | 0 | 0 | 68 | 460.0 ± 3.4 | 506.9 ± 12.9 | 485.8 ± 5.0 | 21.1 ± 12.1 | 18.2 ± 2.0 |
| M | 0 | 1 | 93 | 463.2 ± 2.9 | 541.3 ± 11.1 a | 488.4 ± 4.3 | 52.9 ± 10.3 a | 18.6 ± 1.7 |
| M | 1 | 0 | 118 | 462.5 ± 2.6 | 525.1 ± 9.8 | 523.0 ± 3.8 | 2.2 ± 9.2 | 40.3 ± 1.5 |
| M | 1 | 1 | 230 | 466.7 ± 1.9 | 627.7 ± 7.0 c | 529.6 ± 2.7 | 98.2 ± 6.6 c | 49.6 ± 1.1 c |
| M | 2 | 0 | 49 | 457.3 ± 4.0 | 536.4 ± 15.2 | 551.6 ± 5.9 | −15.3 ± 14.3 | 60.6 ± 2.4 |
| M | 2 | 1 | 244 | 462.5 ± 1.8 | 652.9 ± 6.8 c | 570.2 ± 2.7 | 82.8 ± 6.4 c | 81.2 ± 1.1 c |
| Sex | BMI Class | CV Risk | THV Expected | THV Abnormal | N |
|---|---|---|---|---|---|
| F | normal | low | 274 (96.1) | 11 (3.9) | 285 |
| high | 114 (85.7) | 19 (14.3) *** | 133 | ||
| total | 388 (92.8) | 30 (7.2) | 418 | ||
| overweight | low | 280 (91.2) | 6 (8.8) | 286 | |
| high | 247 (85.5) | 42 (14.5) *** | 289 | ||
| total | 527 (91.7) | 48 (8.4) | 575 | ||
| obese | low | 212 (98.6) | 3 (1.4) | 215 | |
| high | 228 (77.3) | 67 (22.7) *** | 295 | ||
| total | 440 (86.3) | 70 (13.7) | 510 | ||
| M | normal | low | 62 (91.2) | 6 (8.8) | 68 |
| high | 71 (76.3) | 22 (23.7) | 93 | ||
| total | 133 (82.6) | 28 (17.4) * | 161 | ||
| overweight | low | 109 (92.4) | 9 (7.6) | 118 | |
| high | 143 (62.2) | 87 (37.8) *** | 230 | ||
| total | 252 (72.4) | 96 (27.6) | 348 | ||
| obese | low | 48 (98.0) | 1 (2.0) | 49 | |
| high | 163 (66.8) | 81 (33.2) *** | 244 | ||
| total | 211 (72.0) | 82 (28.0) | 293 | ||
| Column total | 1951 (84.6) | 354 (15.4) | 2305 | ||
| THVUL ≤ 0 | n | THVUL > 0 | n | % > 0 | p < ¶ | |
|---|---|---|---|---|---|---|
| All | 461 ± 93 | 1951 | 709 ± 136 | 354 | 15.4 | 0.001 |
| F | 428 ± 74 | 1335 | 635 ± 102 | 148 | 6.3 | 0.001 |
| M | 536 ± 87 # | 596 | 763 ± 133 # | 206 | 20.3 *** | 0.001 |
| Low | 443 ± 82 | 985 | 582 ± 54 | 36 | 3.5 | 0.001 |
| High | 480 ± 100 # | 966 | 724 ± 135 # | 318 | 24.8 *** | 0.001 |
| Normal BP | 449 ± 88 | 509 | 686 ± 100 | 68 | 11.8 | 0.001 |
| High BP | 466 ± 94 # | 1442 | 715 ± 143 | 286 | 16.6 ** | 0.001 |
| T2DM Absent | 460 ± 92 | 1569 | 709 ± 143 | 278 | 15.5 | 0.001 |
| T2DM Present | 468 ± 97 | 382 | 712 ± 112 | 76 | 21.5 | 0.001 |
| <10 AU | 453 ± 91 | 1007 | 717 ± 146 | 123 | 10.9 | 0.001 |
| ≥10 AU | 469 ± 94 | 1065 | 707 ± 133 | 231 | 17.8 *** | 0.001 |
| Group/Subgroup | Parameter | THVUL ≤ 0 | THVUL > 0 |
|---|---|---|---|
| All n = 2305 | n = 2047 | n = 258 | |
| THV/h | 281 ± 51 | 438 ± 70 *** | |
| THV/BSA | 247 ± 55 | 369 ± 55 *** | |
| THVh-THVBSA | 34 ± 25 | 69 ± 38 *** | |
| F n = 1503 | n = 1408 | n = 95 | |
| THV/h | 268 ± 46 | 417 ± 63 *** | |
| THV/BSA | 239 ± 38 | 358 ± 55 *** | |
| THVh-THVBSA | 29 ± 24 | 59 ± 43 *** | |
| M n = 802 | n = 639 | n = 163 | |
| THV/h | 311 ± 47 | 450 ± 71 *** | |
| THV/BSA | 264 ± 36 | 375 ± 54 *** | |
| THVh-THVBSA | 46 ± 25 | 75 ± 35 *** | |
| Low risk n = 1021 | n = 1011 | n = 10 | |
| THV/h | 270 ± 46 | 377 ± 16 *** | |
| THV/BSA | 242 ± 38 | 346 ± 12 *** | |
| THVh-THVBSA | 28 ± 24 | 30 ± 21 NS | |
| High risk n = 1284 | n = 1036 | n = 248 | |
| THV/h | 293 ± 45 | 441 ± 70 *** | |
| THV/BSA | 252 ± 39 | 370 ± 57 *** | |
| THVh-THVBSA | 41 ± 27 | 71 ± 38 *** | |
| Normal BP n = 577 | n = 533 | n = 44 | |
| THV/h | 273 ± 50 | 426 ± 44 *** | |
| THV/BSA | 247 ± 40 | 370 ± 53 *** | |
| THVh-THVBSA | 26 ± 23 | 57 ± 36 *** | |
| High BP n = 1728 | n = 1514 | n = 214 | |
| THV/h | 284 ± 51 | 441 ± 74 *** | |
| THV/BSA | 247 ± 39 | 370 ± 55 *** | |
| THVh-THVBSA | 37 ± 26 | 71 ± 38 *** | |
| T2DM Absent n = 1728 | n = 1514 | n = 214 | |
| THV/h | 284 ± 51 | 441 ± 74 *** | |
| THV/BSA | 247 ± 39 | 369 ± 55 *** | |
| THVh-THVBSA | 37 ± 26 | 72 ± 39 *** | |
| T2DM Present n = 458 | n = 399 | n = 59 | |
| THV/h | 286 ± 53 | 435 ± 56 *** | |
| THV/BSA | 242 ± 38 | 358 ± 39 *** | |
| THVh-THVBSA | 44 ± 27 | 77 ± 37 *** | |
| <10 AU n = 1009 | n = 920 | n = 89 | |
| THV/h | 276 ± 50 | 442 ± 71 *** | |
| THV/BSA | 245 ± 39 | 372 ± 58 *** | |
| THVh-THVBSA | 31 ± 26 | 71 ± 39 *** | |
| ≥10 AU n = 1296 | n = 1127 | n = 169 | |
| THV/h | 286 ± 51 | 436 ± 69 *** | |
| THV/BSA | 249 ± 39 | 368 ± 53 *** | |
| THVh-THVBSA | 37 ± 25 | 69 ± 38 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosnowski, M.; Parma, Z.; Syzdół, M.; Brożek, G.; Harpula, J.; Tendera, M.; Wojakowski, W. A Novel Concept of the “Standard Human” in the Assessment of Individual Total Heart Size: Lessons from Non-Contrast-Enhanced Cardiac CT Examinations. Diagnostics 2025, 15, 1502. https://doi.org/10.3390/diagnostics15121502
Sosnowski M, Parma Z, Syzdół M, Brożek G, Harpula J, Tendera M, Wojakowski W. A Novel Concept of the “Standard Human” in the Assessment of Individual Total Heart Size: Lessons from Non-Contrast-Enhanced Cardiac CT Examinations. Diagnostics. 2025; 15(12):1502. https://doi.org/10.3390/diagnostics15121502
Chicago/Turabian StyleSosnowski, Maciej, Zofia Parma, Marcin Syzdół, Grzegorz Brożek, Jan Harpula, Michał Tendera, and Wojciech Wojakowski. 2025. "A Novel Concept of the “Standard Human” in the Assessment of Individual Total Heart Size: Lessons from Non-Contrast-Enhanced Cardiac CT Examinations" Diagnostics 15, no. 12: 1502. https://doi.org/10.3390/diagnostics15121502
APA StyleSosnowski, M., Parma, Z., Syzdół, M., Brożek, G., Harpula, J., Tendera, M., & Wojakowski, W. (2025). A Novel Concept of the “Standard Human” in the Assessment of Individual Total Heart Size: Lessons from Non-Contrast-Enhanced Cardiac CT Examinations. Diagnostics, 15(12), 1502. https://doi.org/10.3390/diagnostics15121502





