Pupillary Responses and Vital Signs in Hypoglycemic Patients with Impaired Consciousness During Prehospital Care: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
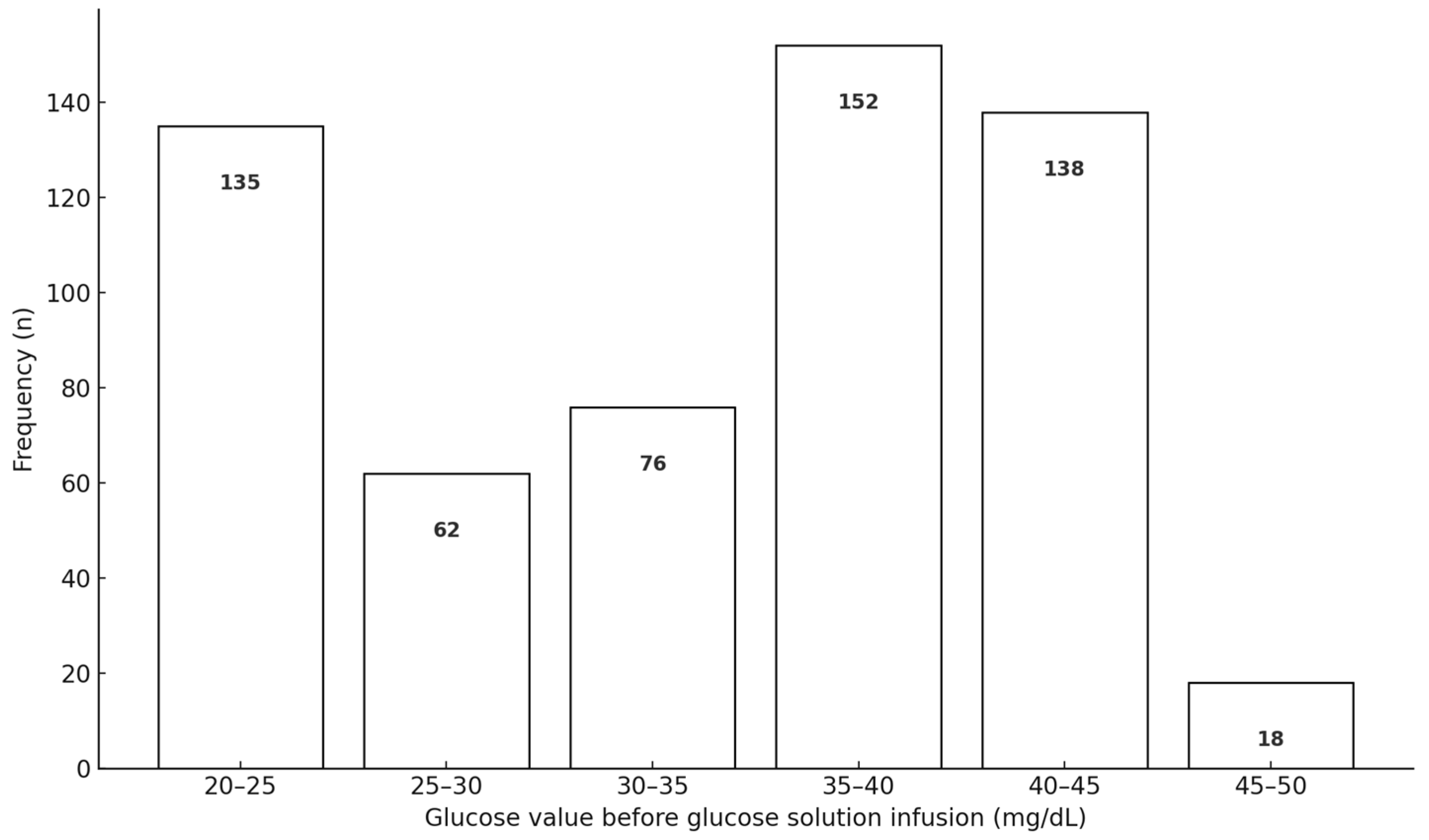
3. Results
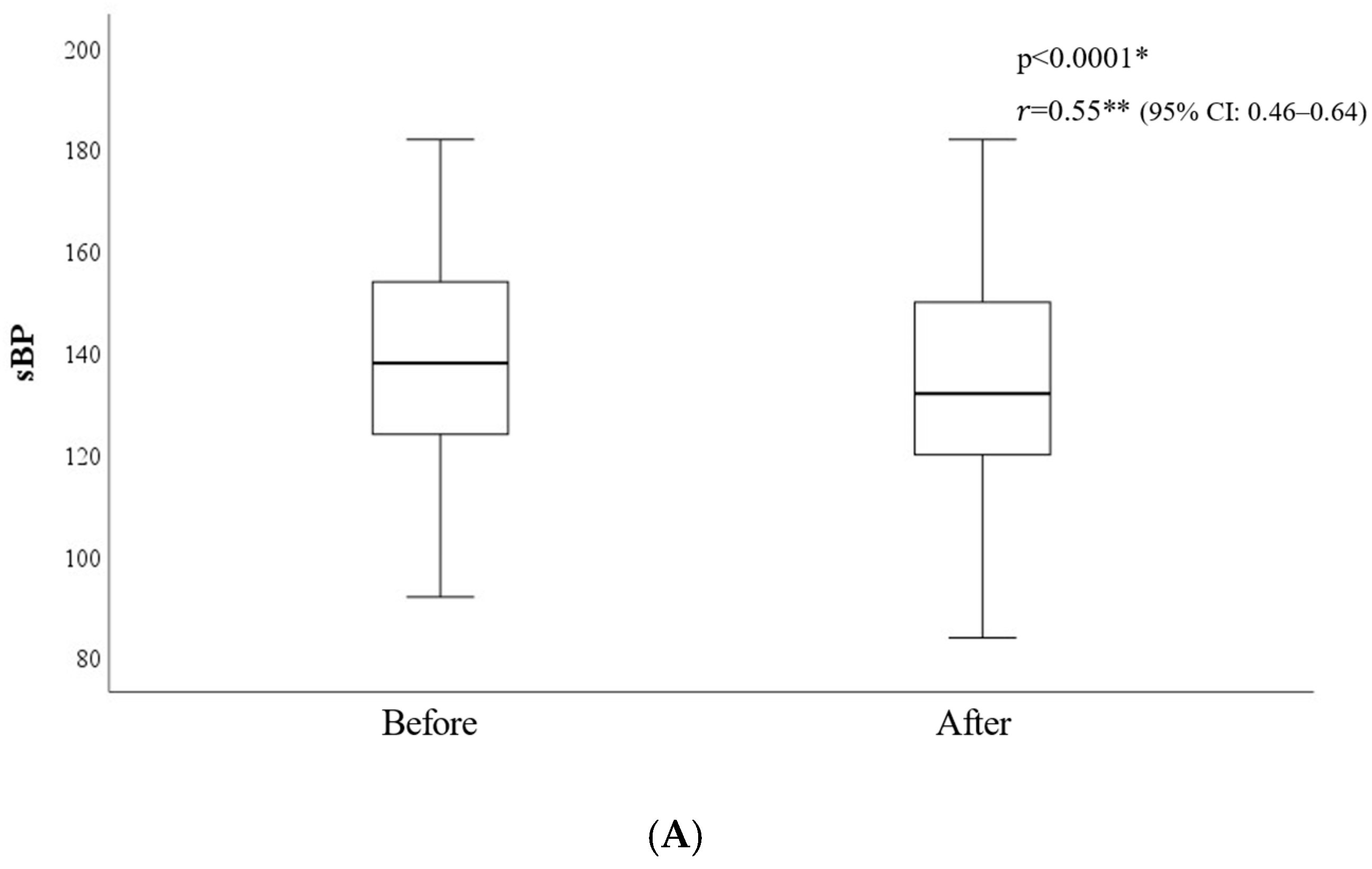
3.1. Consciousness Level and Vital Signs
3.2. Pupil Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forsberg, S.; Hojer, J.; Ludwigs, U. Prognosis in patients presenting with non-traumatic coma. J. Emerg. Med. 2012, 42, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Schneider, D. Hypoglycemia. Am. J. Med. 2014, 127, S17–S24. [Google Scholar] [CrossRef]
- Horsting, M.W.; Franken, M.D.; Meulenbelt, J.; van Klei, W.A.; de Lange, D.W. The etiology and outcome of non-traumatic coma in critical care: A systematic review. BMC Anesthesiol. 2015, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Sanello, A.; Gausche-Hill, M.; Mulkerin, W.; Sporer, K.A.; Brown, J.F.; Koenig, K.L.; Rudnick, E.M.; Salvucci, A.A.; Gilbert, G.H. Altered Mental Status: Current Evidence-based Recommendations for Prehospital Care. West. J. Emerg. Med. 2018, 19, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.; Borsook, D.; Adler, G.; Freeman, R. Stress, hypoglycemia, and the autonomic nervous system. Auton. Neurosci. 2022, 240, 102983. [Google Scholar] [CrossRef]
- Ohta, T.; Waga, S.; Handa, W.; Saito, I.; Takeuchi, K. New grading of level of disordered consiousness (author’s transl). No Shinkei Geka 1974, 2, 623–627. (In Japanese) [Google Scholar] [PubMed]
- Yumoto, T.; Naito, H.; Yorifuji, T.; Aokage, T.; Fujisaki, N.; Nakao, A. Association of Japan Coma Scale score on hospital arrival with in-hospital mortality among trauma patients. BMC Emerg. Med. 2019, 19, 65. [Google Scholar] [CrossRef]
- Nakajima, M.; Okada, Y.; Sonoo, T.; Goto, T. Development and Validation of a Novel Method for Converting the Japan Coma Scale to Glasgow Coma Scale. J. Epidemiol. 2023, 33, 531–535. [Google Scholar] [CrossRef]
- Schwerin, D.L.; Svancarek, B. EMS diabetic protocols for treat and release. In StatPearls; Internet; StatPearls Publishing: Treasure Island, FL, USA, 2023; p. 2025. [Google Scholar] [PubMed]
- Lin, L.; Wu, Y.; Chen, Z.; Huang, L.; Wang, L.; Liu, L. Severe Hypoglycemia Contributing to Cognitive Dysfunction in Diabetic Mice Is Associated with Pericyte and Blood-Brain Barrier Dysfunction. Front. Aging Neurosci. 2021, 13, 775244. [Google Scholar] [CrossRef]
- Lee, A.K.; Rawlings, A.M.; Lee, C.J.; Gross, A.L.; Huang, E.S.; Sharrett, A.R.; Coresh, J.; Selvin, E. Severe hypoglycaemia, mild cognitive impairment, dementia and brain volumes in older adults with type 2 diabetes: The Atherosclerosis Risk in Communities (ARIC) cohort study. Diabetologia 2018, 61, 1956–1965. [Google Scholar] [CrossRef]
- Christou, M.A.; Christou, P.A.; Kyriakopoulos, C.; Christou, G.A.; Tigas, S. Effects of Hypoglycemia on Cardiovascular Function in Patients with Diabetes. Int. J. Mol. Sci. 2023, 24, 9357. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.W.; Majeed, M.S.; Kirresh, O. Non-Diabetic Hypoglycemia. 2023 Jul 17. In StatPearls; Internet; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Cryer, P.E.; Davis, S.N.; Shamoon, H. Hypoglycemia in diabetes. Diabetes Care 2003, 26, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Gradwohl-Matis, I.; Pann, J.; Schmittinger, C.A.; Brunauer, A.; Dankl, D.; Duenser, M.W. Prolonged bilateral reactive miosis as a symptom of severe insulin intoxication. Am. J. Case Rep. 2015, 16, 1–3. [Google Scholar] [CrossRef][Green Version]
- Satoshi, K.; Inagaki, A.; Miura, N. Clinical features of three cases of hypoglycemic coma with residual central nervous system sequelae. Diabetes 2006, 49, 267–273. [Google Scholar]
- Piot, V.M.; Verrijcken, A.; Vanhoof, M.; Mertens, I.; Soetens, F. Full neurological recovery after extreme hypoglycemia during intensive insulin therapy: A case report. J. Diabetes Sci. Technol. 2012, 6, 973–977. [Google Scholar] [CrossRef]
- Unger, J.; Parkin, C. Recognition, prevention, and proactive management of hypoglycemia in patients with type 1 diabetes mellitus. Postgrad. Med. 2011, 123, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Bakatselos, S.O. Hypoglycemia unawareness. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. S1), S92–S96. [Google Scholar] [CrossRef]
- Senthilkumaran, M.; Zhou, X.F.; Bobrovskaya, L. Challenges in Modelling Hypoglycaemia-Associated Autonomic Failure: A Review of Human and Animal Studies. Int. J. Endocrinol. 2016, 2016, 9801640. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Yamamoto-Honda, R.; Kajio, H.; Kishimoto, M.; Noto, H.; Hachiya, R.; Kimura, A.; Kakei, M.; Noda, M. Vital signs, QT prolongation, and newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes Care 2014, 37, 217–225. [Google Scholar] [CrossRef]
- Mizu, D.; Matsuoka, Y.; Huh, J.Y.; Ariyoshi, K. High fever or hypotension predicts non-hypoglycemia in patients with impaired consciousness in prehospital settings. Acute Med. Surg. 2021, 8, e637. [Google Scholar] [CrossRef]
- Sudhakar, P. Commentary: Recognizing pupillary dysfunction in diabetic autonomic neuropathy. Indian. J. Ophthalmol. 2019, 67, 1320–1321. [Google Scholar] [CrossRef] [PubMed]
- Pittasch, D.; Lobmann, R.; Behrens-Baumann, W.; Lehnert, H. Pupil signs of sympathetic autonomic neuropathy in patients with type 1 diabetes. Diabetes Care 2002, 25, 1545–1550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wakasugi, A.; Hanawa, T. Establishing an objective method for assessment the effects of Kampo medicine. J. Trad. Med. 2008, 25, 1–5. [Google Scholar] [CrossRef]
- Tokuda, Y.; Nakazato, N.; Stein, G.H. Pupillary evaluation for differential diagnosis of coma. Postgrad. Med. J. 2003, 79, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Baskaran, M.; Rukmini, A.V.; Nongpiur, M.E.; Htoon, H.; Cheng, C.Y.; Perera, S.A.; Gooley, J.J.; Aung, T.; Milea, D. Factors influencing the pupillary light reflex in healthy individuals. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1353–1359. [Google Scholar] [CrossRef]
- Kolacko, S.; Predovic, J.; Kokot, A.; Bosnar, D.; Brzovic-Saric, V.; Saric, B.; Balog, S.; Milanovic, K.; Ivastinovic, D. Do Gender, Age, Body Mass and Height Influence Eye Biometrical Properties in Young Adults? A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 11719. [Google Scholar] [CrossRef]
- Cherng, Y.G.; Baird, T.; Chen, J.T.; Wang, C.A. Background luminance effects on pupil size associated with emotion and saccade preparation. Sci. Rep. 2020, 10, 15718. [Google Scholar] [CrossRef]
- Daguet, I.; Bouhassira, D.; Gronfier, C. Baseline Pupil Diameter Is Not a Reliable Biomarker of Subjective Sleepiness. Front. Neurol. 2019, 10, 108. [Google Scholar] [CrossRef]
- Belliveau, A.P.; Somani, A.N.; Dossani, R.H. Pupillary Light Reflex. In StatPearls; Internet [Updated 2023 Jul 25]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537180/ (accessed on 25 April 2025).
- Gross, J.R.; McClelland, C.M.; Lee, M.S. An approach to anisocoria. Curr. Opin. Ophthalmol. 2016, 27, 486–492. [Google Scholar] [CrossRef]
- Martin, T.J. Horner Syndrome: A Clinical Review. ACS Chem. Neurosci. 2018, 9, 177–186. [Google Scholar] [CrossRef]
- Sarao, M.S.; Elnahry, A.G.; Sharma, S. Adie Syndrome. In StatPearls; Internet [Updated 2023 Jul 4]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531471/ (accessed on 25 April 2025).
- George, A.S.; Abraham, A.P.; Nair, S.; Joseph, M. The Prevalence of Physiological Anisocoria and its Clinical Significance—A Neurosurgical Perspective. Neurol. India 2019, 67, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, S.A.; Brown, P.M.; Fox, C.; Sonksen, P.H. Pupillary signs in diabetic autonomic neuropathy. Br. Med. J. 1978, 2, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.; Eustace, P.; de Jesus, V. Pupillary autonomic denervation with increasing duration of diabetes mellitus. Br. J. Ophthalmol. 2001, 85, 1225–1230. [Google Scholar] [CrossRef]
- Isotani, H.; Fukumoto, Y. Reversibility of autonomic nerve function in relation to rapid improvement of glycemic control. Horm. Metab. Res. 2000, 32, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Schwingshandl, J.; Borkenstein, M. Effect of actual glycaemia on pupillary function in children with type 1 diabetes. Diabetes Res. Clin. Pract. 1994, 23, 163–168. [Google Scholar] [CrossRef]


| Consciousness Level Assessment by JCS | Convert JCS to GCS | |
|---|---|---|
| 0 | Alert | 15 |
| 1. Single-digit (I) | Awake without any stimuli | |
| 1 Almost fully conscious but not normal | 15 | |
| 2 Unable to recognize time, place, and person | 14 | |
| 3 Unable to recall name or date of birth | 13 | |
| 2. Double-digits (II) | Arousable by some stimulus but reverts to the previous state if stimulus stops | |
| 10 Arousable by being spoken to | 12 | |
| 20 Arousable by loud voice | 12 | |
| 30 Arousable only by repeated mechanical stimuli | 9 | |
| 3. Triple-digits (III) | Unarousable by any forceful stimuli | |
| 100 Unarousable but responds to avoid the stimuli | 7 | |
| 200 Unarousable but responds with slight movements, including decerebrate or decorticate postures | 6 | |
| 300 Does not respond at all | 3 |
| Before | After | p-Value | Effect Size b | |
|---|---|---|---|---|
| Level of Consciousness | ||||
| Alert | 0/583 (0) | 0/583 (0) | ||
| JCS 1-digit [Y/N (%)] JCS1 JCS2 JCS3 | 0/583 (0) | 583/583 (100) 485/583 98/583 1/583 | <0.001 * | r = 0.92 |
| JCS 2-digit [Y/N (%)] JCS10 JCS20 JCS30 | 431/583 (73.9) 94/431 71/431 266/431 | 0/583(0) | ||
| JCS3-digit [Y/N (%)] JCS100 JCS200 JCS300 | 152/583 (26.1) 31/152 121/152 0/152 | 0/583 (0) | ||
| Median GCS (IQR) *** | 9 (7,9) | 15 (15,15) | <0.001 * | r = 0.88 |
| Pupil responses | ||||
| Median pupil size L (IQR) (mm) | 2 (2, 3) | 3 (3, 3) | <0.0001 * | r = 0.81 |
| Median pupil size R (IQR) (mm) | 2 (2,3) | 3 (3, 3) | <0.0001 * | r = 0.81 |
| Existence of miosis L | 395/583 (67.8) | 126/583 (21.6) | <0.0001 ** | V = 0.18 |
| Existence of miosis R | 394/582 (67.7) | 120/581 (20.7) | <0.0001 ** | V = 0.16 |
| Existence of anisocoria [Y/All (%)] | 44/582 (7.6) | 6/582 (1.0) | <0.0001 ** | V = 0.32 |
| Light reflex abnormality (L) [Y/All (%)] | 488/583(83.7) | 430/582 (73.8) | <0.0001 ** | V = 0.21 |
| Light reflex abnormality (R) [Y/All (%)] | 491/583(84.2) | 438/583(75.1) | <0.0001 ** | V = 0.24 |
| ECG monitor | ||||
| Sinus tachycardia a (%) [Y/All (%)] | 158/583 (27.1) | 130/583 (22.3) | <0.0001 ** | V = 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, J.; Kinoshita, K.; Iguchi, U.; Kuwana, T. Pupillary Responses and Vital Signs in Hypoglycemic Patients with Impaired Consciousness During Prehospital Care: A Retrospective Observational Study. Diagnostics 2025, 15, 1487. https://doi.org/10.3390/diagnostics15121487
Yamaguchi J, Kinoshita K, Iguchi U, Kuwana T. Pupillary Responses and Vital Signs in Hypoglycemic Patients with Impaired Consciousness During Prehospital Care: A Retrospective Observational Study. Diagnostics. 2025; 15(12):1487. https://doi.org/10.3390/diagnostics15121487
Chicago/Turabian StyleYamaguchi, Junko, Kosaku Kinoshita, Umefumi Iguchi, and Tsukasa Kuwana. 2025. "Pupillary Responses and Vital Signs in Hypoglycemic Patients with Impaired Consciousness During Prehospital Care: A Retrospective Observational Study" Diagnostics 15, no. 12: 1487. https://doi.org/10.3390/diagnostics15121487
APA StyleYamaguchi, J., Kinoshita, K., Iguchi, U., & Kuwana, T. (2025). Pupillary Responses and Vital Signs in Hypoglycemic Patients with Impaired Consciousness During Prehospital Care: A Retrospective Observational Study. Diagnostics, 15(12), 1487. https://doi.org/10.3390/diagnostics15121487






